Combining Quantitative Food-intake Assays and Forcibly Activating Neurons to Study Appetite in Drosophila
Summary
Quantitative food-intake assays with dyed food provide a robust and high-throughput means to evaluate feeding motivation. Combining the food consumption assay with thermogenetic and optogenetic screens is a powerful approach to investigate the neural circuits underlying appetite in adult Drosophila melanogaster.
Abstract
Food consumption is under the tight control of the brain, which integrates the physiological status, palatability, and nutritional contents of the food, and issues commands to start or stop feeding. Deciphering the processes underlying the decision-making of timely and moderate feeding carries major implications in our understanding of physiological and psychological disorders related to feeding control. Simple, quantitative, and robust methods are required to measure the food ingestion of animals after experimental manipulation, such as forcibly increasing the activities of certain target neurons. Here, we introduced dye-labeling-based feeding assays to facilitate the neurogenetic study of feeding control in adult fruit flies. We review available feeding assays, and then describe our methods step-by-step from setup to analysis, which combine thermogenetic and optogenetic manipulation of neurons controlling feeding motivation with dye-labeled food intake assay. We also discuss the advantages and limitations of our methods, compared with other feeding assays, to help readers choose an appropriate assay.
Introduction
Quantifying the amount of food ingested is important for evaluating multiple aspects of feeding controls by the brain in responding to the internal needs (such as hunger states) and external factors (such as food quality and palatability)1,2,3,4,5,6,7,8,9. In recent years, the efforts of deciphering the neural substrates of feeding control in Drosophila lead to the development of multiple assays to directly quantify the amount of food ingested or serve as an indicator of feeding motivation10,11,12,13,14,15,16.
The CApillary FEeder (CAFE) assay12,13 was developed to measure the amount of consumption of liquid food in a glass microcapillary. The CAFE assay is highly sensitive and reproducible17 and simplifies the measurement of food consumption, especially for quantifying long-term feeding18. However, this assay requires the flies to climb to the tip of the microcapillary and feed upside-down, which is not suitable for all strains. Additionally, because the flies to be tested using the CAFE assay have to be reared on liquid food, the effect of these rearing conditions on metabolism status or the potential malnutrition remains to be determined.
The Proboscis Extension Response (PER) assay11,14 counts the frequency of proboscis extension responses toward gentle touches of food drops. PER assay proved as an excellent way to evaluate feeding motivation of individual fly and asses the influence of palatability and content of food18,19. However, it is not a direct quantification of intake amount.
Recently, a semi-automatic method, the manual feeding assay (MAFE)15, was developed. In MAFE, a single immobilized fly is fed manually with a microcapillary containing food. Given that proboscis extension responses and food consumption can be monitored simultaneously, MAFE is suitable for assessing nutrient values and the effects of pharmacological manipulation. However, immobilizing a fly might negatively impact its behavioral performance, including feeding.
Additionally, fly Proboscis and Activity Detector (FlyPAD)10 was developed to automatically quantify feeding behavior. Using machine vision methods, FlyPAD records physical interactions between a fly and food to quantify the frequency and duration of proboscis extensions as an indicator of feeding motivation. FlyPAD provides a high-throughput approach to monitor the feeding behaviors of a free-moving fly, although the sensitivity and robustness of this system remains to be further confirmed by more studies12.
Labeling strategies are frequently used to estimate food ingestion in flies. It is common to label food with chemical tracers and, after feeding, measure the amount of ingested tracer to calculate the quantity of food intake. Radioactive tracers16,17,20,21,22,23,24,25 allow for the detection through the cuticle without homogenization of the flies. This method provides remarkably low variability and high sensitivity18, and is feasible for long-term study of food intake. However, the availability of usable radioisotopes and different rates of absorbance and excretion should be taken into consideration when working with this assay.
Labeling and tracing food intake with non-toxic food colors is a safer and simpler alternative2,3,26,27,28. Flies are homogenized after feeding with food containing soluble and non-absorbable dyes, and the amount of the ingested dye is later quantified using a spectrophotometer3,24,28,29. The labeling strategy is easy to perform and provides high efficiency, but with a caveat. The volume of food intake estimated from the ingested dye is smaller than the actual volume because excretion begins as early as 15 min after flies start feeding17. Additionally, the assay assesses food ingestion typically within a 60-min period, which is only suitable for investigation of short-term feeding behavior24,28. Moreover, multiple internal and external factors, such as genotype17, gender17, mated state17, rearing density30, circadian rhythm31,32, and food quality3,8,16, influence food intake. Therefore, the feeding duration might need to be adjusted according to specific experimental conditions. Besides facilitating the quantification of food intake, food colors are also used to assess food choices2,19,27, and to visualize the meniscus in a microcapillary in CAFE assay12.
Here, we introduce a protocol combined manipulation of neuronal activity with dye-labeling approach. This strategy has been proved useful in our neurogenetic study on feeding control in adult fruit flies24. The visual scoring method allows for a quick estimation of food consumption; thus, it is useful for screening through a large number of strains in a timely fashion. The candidates from the screen are then analyzed in detail using a colorimetric method to provide objective and precise quantification in additional study.
Besides the feeding assays, we also describe the thermogenetic27,33,34,35 and optogenetic36 methods of forcibly activating target neurons in Drosophila. To activate neurons by thermogenetic operation is simple and convenient with Drosophila Transient Receptor Potential Ankyrin 1 (dTRPA1), which is a temperature- and voltage-gated cation channel that increases neuronal excitability when the ambient temperature rises above 23 °C33,37; however, testing animals at high temperatures might produce adverse effects on behavior. Another effective approach to activate neurons in Drosophila is using optogenetics with CsChrimson36, which is a red-shifted variant of channelrhodopsin that increases the excitability of neurons when exposed to light. Optogenetics offers higher temporal resolution and lesser disturbance to behaviors than thermogenetics. Combining the quantitative measurement of food intake with the manipulation of neuronal activity represents an effective approach for studying the neural mechanisms of feeding.
We describe in detail the preparation of the feeding chamber and the flies to be tested. Using Taotie-Gal4 flies as a model24, we describe activating neurons by thermogenetics and optogenetics. Two assays of quantification of food consumption with dye-labeled food are also described in the protocol.
Protocol
1. Preparing the feeding chamber
Note: The feeding chamber for dye-labeling feeding assay consists of two parts: the outside container (as a cover) and the inside container (as food source).
- Modify the outside container from a glass vial for culturing Drosophila (with an internal diameter of 31.8 mm and a height of 80 mm) (Figure 1A, 1C). Cover the bottom of the container with a layer of 1% agarose (5 mL) to keep the feeding setup properly humidified and serve as a soft substrate for the flies to walk upon (Figure 1B). The container thereby provides a controlled but naturalistic setting carrying with minimal stress or disturbance to the flies.
- Prepare the outside container. Suspend 0.5 g of agarose in 50 mL of double distilled water, heat with frequent agitation, and boil to dissolve completely on a magnetic stirrer with a heat plate.
- When the 1% agarose cools to about 60 °C, transfer 5 mL of 1% agarose to an empty outside container (Figure 1B), and let the container stay open until cooled down to room temperature to yield an agarose pad.
- Prepare dye-labeled food. Composition of the dye-labeled food varies according to different experimental purposes. Here is an example of preparing the dye-labeled food containing only sucrose.
- Add 0.5 g of agarose to 50 mL of double distilled water, heat with frequent agitation, and boil to dissolve completely on a magnetic stirrer with a heat plate.
- When the above 1% agarose cools to about 60 °C, add 1.71 g of sucrose to the agarose to obtain 100 mM sucrose.
- Add 0.25 g of blue dye (erioglaucine disodium) to the agarose solution to obtain 0.5% (w/v) dye-labeled food.
- Prepare the inside container. Put the empty inside containers on a flat horizontal surface, then add 750 µL of blue dye-labeled food to individual food container, let it solidify for at least 5 min.
Note: The inside container is a small plastic dish with an internal diameter of 13.6 mm and a height of 7.5 mm (Figure 1A, 1C). It is filled with dye-labeled food, and is then positioned at the center of the agarose pad of the outside container. - Transfer a filled inside container into a prepared outside container and position it at the center of the agarose pad. Let the feeding chambers stay open at room temperature for 40 min until the wall of the outside container is free of condensation.
Note: The dry wall is necessary to prevent the flies from being stuck onto the wall by contacting water drops. - Prepare feeding chambers with dye-free food (same composition but without dye) for background subtraction.
2. Preparation of flies
Note: Adult female flies 5 to 10 day after eclosion are normally used for feeding assays. It is recommended to Prepare flies of different genotypes, including genetic controls, and to test in parallel. 20 flies (from one feeding chamber) generate one data point.
- Consider the population density for rearing adult flies. Typically, control the population density by varying the number of parental flies according to the strains and the dimensions of culture bottles. As an example, transfer 15 males and 30 females of Canton-S to a culture bottle (with a diameter of 31.8 mm and a height of 80 mm) and let the females to lay eggs for one day then remove the adult flies.
- Prepare flies for thermogenetic activation.
- Use the Gal4/UAS system38 to express dTRPA133 in various neurons by crossing UAS-dTrpA1 flies with different GAL4 driver lines (Figure 2B).
- Keep the adult flies on standard food at 22 °C, 60% relative humidity, and a 12 h/12 h light-dark cycle.
- Two days prior to the feeding assay, sort and collect flies on a CO2 fly pad under a stereo microscope into a fresh food vial and keep a density of 20 flies per vial.
- Prepare flies for optogenetic activation.
- Use the Gal4/UAS system38 to express CsChrimson36 in different neurons by crossing UAS-CsChrimson flies with different GAL4 driver lines.
- Collect newly eclosed (within 24 h) flies and transfer them into a vial with standard food containing 400 µM all-trans-retinal.
- To prevent premature activation, wrap the rearing vials with aluminum foil and then put them into a dark box to protect flies from unwanted light stimulation. Keep the flies at 25 °C, 60% humidity in the dark for 4 – 6 days.
- Two days prior to the feeding assay, sort female flies on CO2 fly pad under a stereo microscope. Collect 20 flies into one food vial and keep them in the dark prior to the feeding test.
- Conduct all operations under dim light as quickly as possible to avoid the effects caused by ambient light. Use a LED lamp placed far away from the working area, the intensity at the working area is 5 µW/cm2.
Note: Use starved Canton-S flies as a positive control for the feeding assay. Deprive these flies of food and maintain on 1% agarose for 24 h prior to the feeding test. The levels of food consumption of these flies help evaluate the day-to-day fluctuations.
3. Thermogenetic activation
- Conduct all feeding experiments at the same time of the day, around Zeitgeber time 4 – 631,32, to minimize variations due to circadian rhythms.
- Prepare an incubator (or a small room with a temperature-controlled heater) as the heating environment.
- Before behavioral experiments, equilibrate the prepared feeding chambers at the experimental temperature (30 °C) for 2 h to ensure the temperature is relative uniform in the feeding chamber.
- To start the feeding process, carefully transfer 20 flies from their home vial into a preheated feeding chamber by gentle tapping. Continue the feeding process at 30 °C for 60 min ( Figure 3A) .
Note: Frequent agitations, such as opening and closing the incubator door, adversely affect the feeding behavior, thus should be minimized. - Cease feeding at 60 min by freezing the feeding chambers at -80 °C (or -20 °C) for 30 min.
Note: Freezing helps to stop feedings in all chambers simultaneously. Freezing flies at -80 °C serves two purposes, for euthanizing the flies and for storage until homogenization for up to one week. - For the temperature control group, transfer 20 flies into a room temperature feeding chamber with dye-colored food to start the feeding process at 22 °C for 60 min.
- Cease feeding of the temperature control group by freezing the chambers at -80 °C (or -20 °C) for 30 min.
4. Optogenetic activation
- Conduct all feeding experiments at the same time of the day, around Zeitgeber time 4 – 631,32, to minimize variations due to circadian rhythms.
- Carefully transfer 20 flies from one vial into a feeding chamber by gentle tapping, and then put the chamber sideways on the illumination setup (Figure 4A, 4B).
- For optogenetic manipulation, use an array of orange LEDs (607 nm) as the source of light stimulation, and fix a horizontal plexiglass plate above the LEDs to support the feeding chambers during illumination. Keep the setup in an incubator at 25 °C with 60% humidity (Figure 4A, 4B).
- Stimulate the genetic control flies in parallel with the Taotie>CsChrimson flies, but in different feeding chambers.
- Continue the feeding process for 60 min with back illumination by the orange light.
- Determine the optimal illumination intensities for optogenetics experimentally36. For the Taotie>CsChrimson flies, an intensity of 2.2 mW/mm2 induces paralysis (cessation of locomotion and loss of postural control), therefore, use an intermediate intensity of 0.1 mW/mm2 to stimulate the Taotie>CsChrimson flies during the feeding test.
- Cease the feeding by freezing feeding chambers at -80 °C (or -20 °C) for 30 min.
5. Visual estimation of food consumption
Note: The visual scoring approach provides a semi-quantitative estimation of food consumptions. Although it is not as objective as the optical method, this method allows for going through a large number of fly strains in a timely fashion, making it suitable for the first round of a genetic screen to quickly narrow down the list of candidates for further tests.
- After the feeding process, anesthetize and score the flies on a CO2 fly pad under a stereo microscope. Give individual score to each fly based on the volume and intensity of dye-colored food in its abdomen (Figure 2A).
NOTE: Criteria for visual estimation: the score system has 4 levels of food intake corresponding to different volumes and intensities of colored food in the abdomen.- Score flies without detectable dye-colored food a score of 0; give those with faint (barely detectable) blue dye or with dye-colored food occupying less than one third of the volume of the abdomen a score of 1.
- Score flies with intense dye-colored food occupying one-half of the abdomen a score of 2.
- Score those with intense dyed content occupying greater than half of the abdomen a score of 3 (Figure 2A).
- Calculate the proportion of flies with different scores in each experimental condition (Figure 2B).
6. Colorimetric quantification of food consumption
- After cessation of feeding, pour out all 20 flies from a feeding chamber onto a piece of weighing paper, then transfer them into a 1.5 mL tube using a soft brush.
Note: Do not take all of the chambers out of -80 °C storage at the same time to avoid flies sticking to the chamber wall because of water condensation on the cold chamber. - Add 500 µL of PBST to the tube and homogenize the flies using a grinding mill at 60 Hz for 5 s.
- Confirm sufficient homogenization by observation when there are no detectable fragments of body parts.
- Spin the tubes with homogenates for 30 min at 13,000 rpm to clear the debris.
- After centrifugation, transfer 100 µL of supernatant to a well of a 96-well plate.
- After all samples are loaded, put the plate into a plate reader to measure the absorbance of the samples at 630 nm.
- To remove the background absorbance, subtract the absorbance of the supernatants from flies fed on food without dye from the absorbance of the supernatant from the blue food-fed flies.
Note: This step is optional, depending on the compositions of the food used. When a fly food (without the dye) generates absorbance at 630 nm, it is necessary to perform this step to eliminate the background.
Representative Results
Thermogenetic screen.
Abnormally increased appetite causes elevated food intake, regardless of physiological needs. We utilized this scheme to design a high-throughput behavioral screen to obtain genetic handles of neurons related to hunger and satiated states (Figure 1). The screen yielded Taotie-Gal424. When the Taotie-Gal4 neurons were forcibly activated at 30 °C, the Taotie>TrpA1 flies ingested larger quantities of food than the controls (Figure 2, Figure 3).
Visually scoring the food consumption.
Because some neurotransmitters and neuropeptides have been indicated in the regulation of feeding behavior, we have tested the corresponding GAL4 lines, including NPF20, sNPF39, octopamine21, dopamine27,40, serotonin41,42, AKH35, and Dilp27,8,35. To quantify the feeding response, we visually inspected and scored flies with various amounts of detectable dye in the gut (Figure 2A). After activation of Taotie neurons, about 58% of Taotie>dTrpA1 flies exhibited strong feeding behaviors, and 23% of these showed mild feeding behaviors (Figure 2B). In contrast, only marginal feeding behaviors were observed in flies with other neurons activated via dTrpA1 (Figure 2B).
Colorimetric quantification of food intake.
To quantify food intake with high precision and objectivity, we measured the absorbance of fly extraction at a wavelength specific to the added dye in the food2,27,28. To correlate an absorbance value with the volume of food-intake, a standard curve was obtained by measuring the absorbance of the sample solutions (the same buffer for homogenizing the flies, PBST) mixed with different amounts of dyes). As our results demonstrate, acute activated Taotie-GAL4 neurons by TRPA1 dramatically increased food intake compared with genetic control and the temperature control during the same test period (Figure 3B), suggesting that the Taotie-GAL4 labeled neurons participate in regulation of food intake of adult Drosophila.
Optogenetic activation to promote food intake in Drosophila.
We used UAS-CsChrimson to activate Taotie neurons by illumination with an orange light36 (Figure 4A, 4B). When Taotie>CsChrimson flies were stimulated by LED lights at 607 nm, they ingested significantly more food than controls (Figure 4C). Furthermore, the amounts of ingested food correlated well with the intensities of stimulation light (Figure 4D). Thus, besides thermogenetics with dTrpA1, optogenetic activation with CsChrimson in Taotie neurons also promotes feeding motivation in satiated flies.
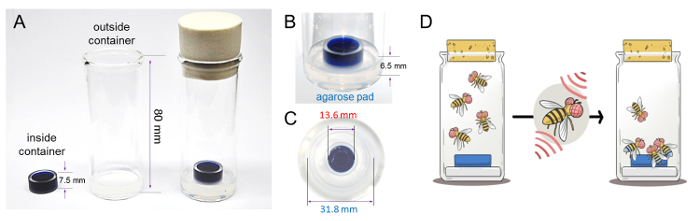
Figure 1. A feeding paradigm for analyzing appetite in adult flies. (A) The feeding chamber contains an inside container filled with dye-colored food and an outside container padded with 1% agarose. From left to right, the inside container, the outside container, and the complete feeding chamber. (B) The inside container sits on top of the agarose pad at the bottom of the outside container. (C) Top view of the feeding chamber. (D) A schematic showing the "appetite screen" experiment. Normal satiated flies rarely ate food (left vial), while forcibly activating certain feeding control neurons caused satiated flies to ingest additional food, thus exhibiting dye-colored food in the abdomen (right vial). Please click here to view a larger version of this figure.
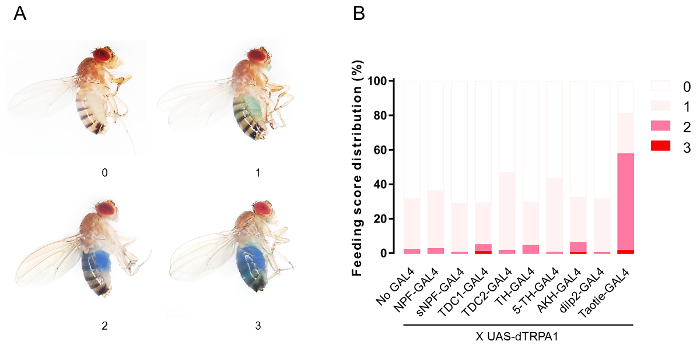
Figure 2. Visual estimation of food ingestion. (A) Exemplar images to show the criteria for scoring food content in the abdomen. (B) The distribution of feeding scores in flies with indicated neurons being activated by dTrpA1 at 30 °C. No-Gal4: UAS-dTrpA1 (genetic control); NPF-GAL4: neuropeptide F positive neurons; sNPF-GAL4: short neuropeptide F positive neurons; TDC1-GAL4 and TDC2-GAL4: octopamine neurons; TH-GAL4: dopamine neurons; 5-HT: serotonin neurons; AKH: adipokinetic hormone positive neurons; dip2: insulin-like peptide 2 positive neurons; Taotie-GAL4: Taotie-GAL4 labels neurons (n = 120 flies per condition). Please click here to view a larger version of this figure.
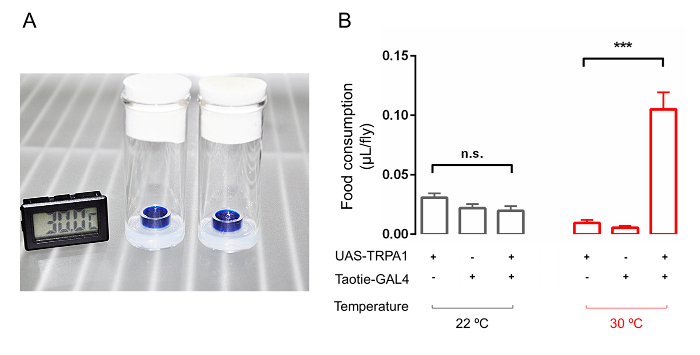
Figure 3. Thermogenetic-activation of Taotie neurons increases food consumption in adult flies. (A) An image shows the setup of the thermogenetic activation experiment. The feeding test was performed in an incubator at 30 °C. (B) Food consumption by colorimetric quantification in satiated flies tested at either 30 °C or 22 °C for 1 h. The amount of food consumption of a single fly was calculated from that of 20 flies in one feeding chamber (n = 6). n.s. indicates not significant (p >0.05); ***p <0.001. One-way ANOVA followed with Tukey's post hoc test was used to analyze multiple comparisons. Error bars indicate mean ± SEM. Please click here to view a larger version of this figure.
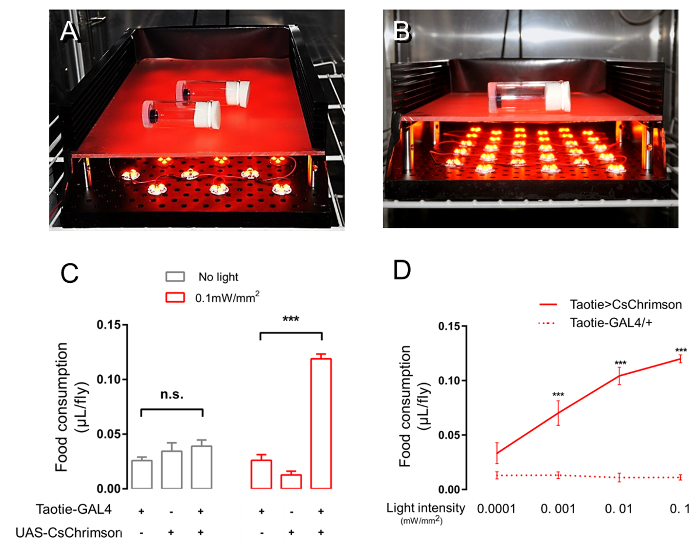
Figure 4. Optogenetic-activation of Taotie neurons increases the amount of food intake in an intensity-dependent manner. (A,B) Two views of the setup for optogenetic activation. The feeding chambers were laid sideways on a supporting plate and illuminated from the bottom by an array of orange LEDs. The front side of the illumination box was removed to show the inside LEDs. The behavioral experiments were performed inside of an incubator at 25 °C, 60% RH. (C) Food consumption of satiated flies of indicated genotypes when tested under optogenetic illumination or in the dark for 1 h (n = 6). (D) Food ingestion by satiated Taotie>CsChrimson flies was correlated with the illumination intensity (n = 6). n.s. indicates not significant (p >0.05); ***p <0.001, One-way ANOVA followed with Tukey's post hoc test was used to analyze multiple comparisons, Student's t-test for two group comparisons. Error bars indicate mean ± SEM. Please click here to view a larger version of this figure.
Discussion
This report focuses on the technical process of dye-labeling feeding assays of food consumption in the context of thermogenetic and optogenetic activation to manipulate neurons controlling feeding. This simple and reliable protocol will help to elucidate the function of candidate neurons in feeding control, to measure the food preference of flies, and to identify novel players in the feeding control circuits via feeding-based genetic screens24.
The dye labeling strategy is feasible in investigating short-term feeding behaviors. The dye, erioglaucine disodium, dissolved in and ingested with the food, is a critical indicator of food consumption. Deshpande et al. demonstrated that the blue dye itself showed no influence on feeding when measured by both the CAFE assay and the radioisotope labeling food intake assay17. Similarly, our PER results (stimulating the fly's leg with dye-labeled or dye-free 100 mM sucrose solution) indicated that flies exhibited no significant difference in their response toward dye-labeled or dye-free food. However, there are still no clear data regarding whether the dye is tasteless in Drosophila.
Besides the critical steps indicated in the protocol, pay particular attention to the general status of the flies to be tested. Besides being reared and maintained at a non-overcrowded density, the flies should be handled gently, and avoid unnecessary shocks or stresses, in order to produce consistent feeding results.
The feeding paradigm presented here has certain limitations. First, our methods quantify food consumption over a 60 min period. To evaluate food intakes over longer periods, such as a day, a different method, such as CAFE, would be needed. Second, compared with quantification methods using radioisotopes, the colorimetric method is less sensitive and difficult to resolve differences when amounts of food consumption are low. Third, considering that some flies would begin to discharge as early as 15 min after the start of feeding, this protocol actually measures the net result of food intake and excretion in a population17. Fourth, the colorimetric quantification of food intake described here was for a fly group, estimation of food ingestion of a single fly depends on other feeding assays, such as radioisotope labeling, the MAFE assay, or FlyPAD. Nevertheless, certain advantages of colorimetric quantification of food intake, such as being a simple, stable, visible, and high-throughput method, make it a prime choice for quantification of large numbers of strains, especially for feeding-based genetic screens and the follow-up studies.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by National Basic Research Program of China (2012CB825504), National Natural Science Foundation of China (91232720 and 9163210042), Chinese Academy of Sciences (CAS) (GJHZ201302 and QYZDY-SSW-SMC015), Bill and Melinda Gates Foundation (OPP1119434), and 100-Talents Program of CAS to Y. Zhu.
Materials
| UAS-CsChrimson | Bloomintoon | 55135 | |
| UAS-dTrpA1 | Bloomintoon | 26263 | |
| TDC1-GAL4 | Bloomintoon | 9312 | |
| TDC2-GAL4 | Bloomintoon | 9313 | |
| sNPF-GAL4 | Provided by Z. Zhao | ||
| NPF-GAL4 | Provided by Y. Rao | ||
| TH-GAL4 | Provided by Y. Rao | ||
| 5-HT-GAL4 | Provided by Y. Rao | ||
| AKH-GAL4 | Provided by Y. Rao | ||
| dip2-GAL4 | Provided by Y. Rao | ||
| Taotie-GAL4 | Provided by J. Carlson | ||
| Agarose | Biowest | G-10 | |
| Sucrose | Sigma | S7903 | |
| Erioglaucine disodium salt | Sigma | 861146 | |
| all-trans-retinal | Sigma | R2500 | stored in darkness |
| Triton X-100 | Amresco | 9002-93-01 | |
| Fly food | 1 L food contains: 77.7 g corn meal, 32.19 g yeast, 5 g agar, 0.726 g CaCl2, 31.62 g sucrose, 63.2 g glucose, 2 g potassium sorbate, pH | ||
| 1x PBS buffer | 1 L 1X PBS contains: 8 g Nacl, 0.2 g Kcl, 1.44 g Na2HPO4, 0.24 g KH2PO4, pH 7.4 | ||
| PBST buffer | 1X PBS with 1% Triton X-100 | ||
| Grinding mill | Shang Hai Jing Xin | Tissuelyser-24 | |
| Incubator | Ning Bo Jiang Nan | HWS-80 | |
| Magnetic stirrer with a heat plate | Chang Zhou Bo Yuan | CJJ 78-1 | |
| Spectrometer | Thorlabs | CCS200/M | |
| Microplate Spectrophotometer | Thermo Scientific | Multiskan GO Type: 1510, REF 51119200 | |
| Fluorescence stereo microscope | Leica | M205FA | |
| Stereo microscope | Leica | S6E | |
| Outside container | Jiang Su Hai Men | glass vial with a diameter of 31.8 mm and a height of 80 mm (inside dimension) | |
| Inside container | Beijing Yi Ran machinery factory | plastic dish with a diameter of 13.6 mm and a height of 7.5 mm (inside dimension) | |
| 1.5 mL Eppendorf tubes | Hai Men Ning Mong | ||
| 96 well plate | Corning Incorporated | Costar 3599 | |
| LEDs | Xin Xing Yuan Guangdian | 607 nm, 3W | https://item.taobao.com/item.htm?id=20158878058 |
References
- Gao, Q., Horvath, T. L. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 30, 367-398 (2007).
- Bjordal, M., Arquier, N., Kniazeff, J., Pin, J. P., Leopold, P. Sensing of amino acids in a dopaminergic circuitry promotes rejection of an incomplete diet in Drosophila. Cell. 156 (3), 510-521 (2014).
- Edgecomb, R. S., Harth, C. E., Schneiderman, A. M. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 197, 215-235 (1994).
- Miyamoto, T., Slone, J., Song, X., Amrein, H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 151 (5), 1113-1125 (2012).
- Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S., Schwartz, M. W. Central nervous system control of food intake and body weight. Nature. 443 (7109), 289-295 (2006).
- Pool, A. H., Scott, K. Feeding regulation in Drosophila. Curr Opin Neurobiol. 29, 57-63 (2014).
- Soderberg, J. A., Carlsson, M. A., Nassel, D. R. Insulin-Producing Cells in the Drosophila Brain also Express Satiety-Inducing Cholecystokinin-Like Peptide, Drosulfakinin. Front Endocrinol (Lausanne). 3, 109 (2012).
- Stafford, J. W., Lynd, K. M., Jung, A. Y., Gordon, M. D. Integration of taste and calorie sensing in Drosophila. J Neurosci. 32 (42), 14767-14774 (2012).
- Wu, Q., Zhang, Y., Xu, J., Shen, P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci U S A. 102 (37), 13289-13294 (2005).
- Itskov, P. M., et al. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat Commun. 5, 4560 (2014).
- Mair, W., Piper, M. D., Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 3 (7), e223 (2005).
- Diegelmann, S., et al. The CApillary FEeder Assay Measures Food Intake in Drosophila melanogaster. J Vis Exp. (121), (2017).
- Ja, W. W., et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 104 (20), 8253-8256 (2007).
- Shiraiwa, T., Carlson, J. R. Proboscis extension response (PER) assay in Drosophila. J Vis Exp. (3), e193 (2007).
- Qi, W., et al. A quantitative feeding assay in adult Drosophila reveals rapid modulation of food ingestion by its nutritional value. Mol Brain. 8, 87 (2015).
- Ja, W. W., et al. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc Natl Acad Sci U S A. 106 (44), 18633-18637 (2009).
- Deshpande, S. A., et al. Quantifying Drosophila food intake: comparative analysis of current methodology. Nat Methods. 11 (5), 535-540 (2014).
- Deshpande, S. A., et al. Acidic Food pH Increases Palatability and Consumption and Extends Drosophila Lifespan. J Nutr. 145 (12), 2789-2796 (2015).
- Dus, M., Min, S., Keene, A. C., Lee, G. Y., Suh, G. S. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 108 (28), 11644-11649 (2011).
- Shen, P., Cai, H. N. Drosophila neuropeptide F mediates integration of chemosensory stimulation and conditioning of the nervous system by food. J Neurobiol. 47 (1), 16-25 (2001).
- Yang, Z., et al. Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proc Natl Acad Sci U S A. 112 (16), 5219-5224 (2015).
- Ramdya, P., Schneider, J., Levine, J. D. The neurogenetics of group behavior in Drosophila melanogaster. J Exp Biol. 220 (Pt 1), 35-41 (2017).
- Sanchez-Alcaniz, J. A., Zappia, G., Marion-Poll, F., Benton, R. A mechanosensory receptor required for food texture detection in Drosophila. Nat Commun. 8, 14192 (2017).
- Zhan, Y. P., Liu, L., Zhu, Y. Taotie neurons regulate appetite in Drosophila. Nat Commun. 7, 13633 (2016).
- Yu, Y., et al. Regulation of starvation-induced hyperactivity by insulin and glucagon signaling in adult Drosophila. Elife. 5, (2016).
- Wood, J. G., et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 430 (7000), 686-689 (2004).
- Inagaki, H. K., et al. Visualizing Neuromodulation In Vivo: TANGO-Mapping of Dopamine Signaling Reveals Appetite Control of Sugar Sensing. Cell. 148 (3), 583-595 (2012).
- Wong, R., Piper, M. D., Wertheim, B., Partridge, L. Quantification of food intake in Drosophila. PLoS One. 4 (6), e6063 (2009).
- Sen, R., et al. Moonwalker Descending Neurons Mediate Visually Evoked Retreat in Drosophila. Curr Biol. 27 (5), 766-771 (2017).
- Ewing, L. S., Ewing, A. W. Courtship of Drosophila melanogaster in large observation chambers: the influence of female reproductive state. Behaviour. 101 (1), 243-252 (1987).
- Chatterjee, A., Tanoue, S., Houl, J. H., Hardin, P. E. Regulation of gustatory physiology and appetitive behavior by the Drosophila circadian clock. Curr Biol. 20 (4), 300-309 (2010).
- Xu, K., Zheng, X., Sehgal, A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 8 (4), 289-300 (2008).
- Hamada, F. N., et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 454 (7201), 217-255 (2008).
- Viswanath, V., et al. Ion channels – Opposite thermosensor in fruitfly and mouse. Nature. 423 (6942), 822-823 (2003).
- Yu, Y., et al. Regulation of starvation-induced hyperactivity by insulin and glucagon signaling in adult Drosophila. Elife. 5, (2016).
- Klapoetke, N. C., et al. Independent optical excitation of distinct neural populations. Nat Methods. 11 (3), 338-346 (2014).
- Viswanath, V., et al. Opposite thermosensor in fruitfly and mouse. Nature. 423 (6942), 822-823 (2003).
- Brand, A. H., Perrimon, N. Targeted Gene-Expression as a Means of Altering Cell Fates and Generating Dominant Phenotypes. Development. 118 (2), 401-415 (1993).
- Lee, K. S., You, K. H., Choo, J. K., Han, Y. M., Yu, K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 279 (49), 50781-50789 (2004).
- Marella, S., Mann, K., Scott, K. Dopaminergic Modulation of Sucrose Acceptance Behavior in Drosophila. Neuron. 73 (5), 941-950 (2012).
- Albin, S. D., et al. A Subset of Serotonergic Neurons Evokes Hunger in Adult Drosophila. Current Biology. 25 (18), 2435-2440 (2015).
- Ro, J., et al. Serotonin signaling mediates protein valuation and aging. eLife. 5, e16843 (2016).

