Ex Vivo Hepatic Perfusion Through the Portal Vein in Mouse
Summary
The protocol describes a straightforward method of resectioning an intact mouse liver for metabolic studies through portal vein perfusion.
Abstract
Metabolic diseases such as diabetes, pre-diabetes, non-alcoholic fatty liver disease (NAFLD), and nonalcoholic steatohepatitis (NASH) are becoming increasingly common. Ex vivo liver perfusions allow for a comprehensive analysis of liver metabolism using nuclear magnetic resonance (NMR), in nutritional conditions that can be rigorously controlled. As in silico simulations remain a primarily theoretical means of assessing hormone actions and the effects of pharmaceutical intervention, the perfused liver remains one of the most valuable test beds for understanding hepatic metabolism. As these studies guide basic insights into hepatic physiology, results must be accurate and reproducible. The greatest factor in the reproducibility of ex vivo hepatic perfusion is the quality of surgery. Therefore, we have introduced an organized and streamlined method to perform ex vivo mouse liver perfusions in the context of in situ NMR experiments. We also describe a unique application and discuss common issues encountered in these studies. The overall purpose is to provide an uncomplicated guide to a technique we have refined over several years that we deem the golden standard for obtaining reproducible results in hepatic resections and perfusions in the context of in situ NMR experiments. The distance to the center of the field for the magnet as well as the inaccessibility of the tissue to intervention during the NMR experiment makes our methods novel.
Introduction
Ex vivo perfusions are crucial in the study of hepatic metabolism, and perfusion through the portal vein is the standard for these studies. In order to study hepatic metabolism in isolation, the liver must be resected from the body to avoid complications arising from metabolism in other organs (i.e., whole-body metabolism) and to exert control over hormone availability (insulin, glucagon, etc.). This approach can be essential for understanding the effects of diseases such as diabetes, NAFLD, and NASH on hepatic metabolism as well as mechanisms of drug action. This article serves as a guide to hepatic resection and perfusion. We have developed a streamlined procedure to perform these metabolic liver studies with sufficient rigor and reproducibility. If the surgery is not performed correctly, there is pronounced variability in the metabolic data obtained. We describe an organized method to perform portal vein catheterization and liver resection in the context of metabolic studies in situ in a nuclear magnetic resonance (NMR) spectrometer, as described in the literature1,2,3,4,5.
Currently, there is no literature describing an ex vivo hepatic perfusion using a glass column within an NMR. Nor is there a video or text publication providing a clear example of how to perform the procedure with the mouse liver, specifically, demonstrating how to catheterize the portal vein, resect a liver, transfer, and hang the liver onto a glass column. As the genetically modified mouse is ubiquitously used for studying liver metabolism, this is an essential procedure that deserves a complete description. Liver perfusion surgeries are not new, but this article is a gold standard method accompanied by a video demonstrating the technical excellence described in this paper to aid everyone interested in this procedure. The method presented here would be best applied to real-time metabolism to detect the function and turnover of metabolites in disease models.
This method uses a 100 cm water-jacketed glass column, which allows the liver to hang at the bottom of the cannula encapsulated by perfusate inside an NMR tube. Heated water in the glass jacket is used to control perfusate temperature. A thin layer oxygenator is pressurized with 95%/5% O2/CO2 for pH control. By using three separate pumps, the perfusate column height is set, which provides constant pressure to the liver. Flow rates are not controlled beyond the application of constant pressure (Figure 1). To confirm the liver is appropriately functioning, oxygen measurements are taken along with flow rates. In our hands, this set of preconditions leads to highly repeatable NMR experiments for the assessment of liver metabolic function.
Protocol
Experiments involving mice were handled in compliance with the University of Florida Institutional Animal Care and Use Committee (protocol number #201909320). The mouse strain used was C57BL/6J; all mice were male. This method is generally applicable for studies using other standard mouse strains as well. This surgery is optimally performed by two individuals working together.
1. Initial set-up
- Perfuse livers with perfusate containing Krebs-Henseleit electrolytes6(25 mM NaHCO3, 112 mM NaCl, 4.7 mM KCl, 1.2 mM each of MgSO4, KH2PO4, and 0.5 mM sodium-EDTA, 1.25 mM CaCl2), 6 mM sodium lactate, 0.6 mM sodium pyruvate, 0.2 mM [U-13C] sodium propionate, 10% (v/v) D2O, and 0.63 mM mixed fatty acids (containing palmitic acid (22.1% of total), palmitoleic acid (5.2%), stearic acid (2.7%), oleic acid (27%), linoleic acid (37.7%), γ-linolenic acid (2.4%), and decosahexanoic acid (2.8%)) along with 2% (w/v) bovine serum albumin. Set final pH of perfusate to 7.3 using HCl (and NaOH, if necessary).
2. Pre-surgery set-up
- Assemble two 1 mL syringes with a 23G needle which are 19.05 mm long. Fill one syringe with 0.01 mL of 1000 units/mL heparin and 0.19 mL of saline (0.9% (w/v) NaCl in water; Table 1).
- Fill the second syringe with 0.2 mL of 2% lidocaine and 0.6 mL of 0.9% saline (Table 1). In another 1 mL syringe with a 27 G 38.1 mm needle, fill with the perfusate and maintain at 37 °C.
3. Perfusate column set-up
- Set the glass bottle containing 500 mL of perfusate into the water bath (Figure 1B). Turn the water bath on and set the temperature to ~42 °C. The higher temperature in the water bath enables maintaining 37 °C in the perfusion column.
- Once the water heats up to 42 °C, turn on the two pumps to circulate the perfusate from the bottle throughout the thin film oxygenator and the water-jacketed 100 cm glass column (Figure 1A-E).
- Turn on the oxygenating gas (95% oxygen and 5% carbon dioxide) to pressurize the oxygenator7 (Figure 1C). Adjust perfusate column height to achieve a flow rate of 8 mL/min with the catheter attached (see step 9 for flow rate measurement)5,8,9.
NOTE: Flow rate refers to the rate at which perfusate is being expelled by the liver.
4. Anesthetization of the mouse
- Don PPE as required by IACUC protocol and other appropriate safety guidelines.
NOTE: The following steps have been optimized for mice that are 9 – 13 weeks old. - Place the mouse in the isoflurane chamber. Turn the delivery gas to 100% oxygen, a flow rate of 1 L/min, and the isoflurane to 2%10. Wait till breathing slows down and is steady.
NOTE: For the mouse to reach a stable surgical plane, as evidenced by a slow and steady respiratory rate and lack of toe pinch reflex, the oxygen flow rate can be adjusted to ~1.5 L/min to ~3 L/min and the isoflurane concentration from 1 – 3%. The delivery rate of carrier gas and isoflurane concentration depends on the animal's age and weight and factors such as noise and light. - Disinfect the abdomen with 70% alcohol. Administer heparin through a deep subcutaneous injection in the abdominal fat layer (Figure 2). Place the mouse back into the anesthesia chamber for 10 min.
NOTE: Shaving is not required as this procedure is terminal.
5. Celiotomy
- Transfer the mouse from the anesthesia chamber to the surgery platform and place it in the supine position (Figure 3).
- Place the nose of the mouse into a nose cone and tape the paws down. Take care to not apply any strain on the neck that may lead to suffocation.
- Administer lidocaine through a subcutaneous injection bilaterally in the anterior iliac crest region11 (Figure 3). Perform a toe pinch test to confirm the absence of all pain reflexes.
- Perform celiotomy to expose the internal organs (Figure 4). Make a 3 cm wide incision (the width of the entire abdomen of the mouse).
NOTE: Width will change with the age and diet of the mouse. - Expand the incision by using a hemostat that pulls traction by clamping on the xiphoid process (Figure 4).
6. Cannulation of portal vein
- Use a cotton-tipped applicator to clear the small and large intestines covering the portal vein. Position a silk suture under the arch of the portal vein proximal to the liver (Figure 4A).
- Depending on the anatomical structure, place the second silk suture proximal or distal of the inferior mesenteric vein distal from the liver (Figure 4A)12,13. Use a 2-0 suture for both sutures.
- Once the sutures are in place, cannulate the portal vein with a 22G catheter14 (Figure 4B). When inserting the catheter, keep the bevel pointed up. Enter the portal vein at no more than a 15° angle.
- Tie the first suture past the catheter tapper. After the portal vein is cannulated, anchor the catheter 2-3 mm distal from the branch of the portal vein with the silk suture (Figure 4B).
NOTE: The assistant should roll shoulders and wrists to prevent dislodging the catheter or tearing of the portal vein. Each suture requires two knots. - Next, secure the lower portion of the catheter with the second suture. With the help of the surgical assistant, tie a knot with the suture to secure the catheter to the distal portion of the portal vein and the surrounding tissue.
7. Resection of liver post portal vein cannulation
- After the catheter is secured, insert a 1 mL syringe with a 27G needle which is 38.1 mm long into the catheter to flush blood and air bubbles.
NOTE: There is usually a backflow of blood out of the catheter from the pressure. - Use a 1 mm I.D. x 5 mm O.D. silicone tube with a fixed stopcock to couple the perfusion column (Figure 1A) to the catheter allowing the flow of the buffer into the liver marking the start of the perfusion. Start a timer at this point to mark the start of the perfusion.
- Relieve increased vascular pressure by making an incision, using scissors, into the inferior vena cava.
- Confirm flow of perfusate through the liver by observing the homogenous change in liver color from pink/red to a pale yellow. Once the flow is confirmed, excise the stomach, small intestine, large intestine, and the right kidney from surrounding tissue.
- With the help of the surgical assistant, maneuver the liver around the abdominal and thoracic cavity as the surgeon cuts through the parietal peritoneum and thoracic tissue to resect the liver
- Lastly, lift the liver upwards and cut the remaining connective tissues holding the liver in place with scissors. Slowly manipulate the liver for ease of view. Remove any fur that sticks to the liver by rinsing off with perfusate before encapsulating it within the NMR tube.
NOTE: In this procedure, only the liver is removed. All other organs are left in the body of the animal. The bile duct can be removed based on the protocol of the experiment. Although for this experiment, it was left in place.
8. Hanging the liver from the column
- Once the surgeon hands the liver and tubing to the assistant, the assistant disconnects the tubing from the catheter and column.
- Fill the catheter with perfusate until a meniscus is formed on the top of the catheter. Attach the catheter to the column for the liver to hang and perfuse.
NOTE: The bead of perfusate on the catheter provides sufficient volume for the liver to function until its connected.The catheter attached to the liver is press fitted to the bottom of the column. - Screw a 20 mm NMR tube onto the 100 cm glass column to encapsulate the liver (Figure 5). To avoid torsion of the liver and the portal vein, slowly screw on the NMR tube. If torsion does occur and flow is stopped, unscrew and rescrew the NMR tube. This will remedy the occlusion and flow will return.
- Perfuse the liver for 30-60 min based on the details of the study.
NOTE: Time is based on the perfusion experiment. For this experiment, metabolic turnover was measured within 30 min. The liver perfusion can take up to 10 min to reach a steady state. The time to steady-state starts once the inferior vena cava has been cut and hepatic flow is established.
9. Flow measurement
- Place a weigh boat on a top loading balance and zero the balance. Place the tubing from the roller pump pulling efferent perfusate from the NMR into the weigh boat and start the timer.
- Weigh the mass of liquid accumulated over 1 min which yields the flow rate of the liver. Place the tube back into the waste/collection container.
10. Oxygen Measurement
NOTE: Oxygen meter measurements were set up according to the manufacturer's instructions15.
- Place the electrode containing 20 µL of 50% KCl saturated solution on the platinum dome and place five 10 µL drops around the lower platinum ring of the electrode.
- Remove the adhesive off the cigarette paper. Lay a piece of polytetrafluoroethylene membrane over the cigarette paper.
- Place the two pieces on top of the electrode. Fit a small O-ring around the top of the electrode. Trim the paper to lay flat onto the lower platinum ring on the electrode.
NOTE: Some overhang is acceptable. It's essential to cover the silver of the electrode. - Place the larger O-ring on the electrode. Couple the electrode to the water chamber and tighten the base to hold the electrode in place. Turn on the water bath and allow to heat up to 37 °C.
- Open oxygen meter software. Click on Calibrate > Air-Saturated Water. Set stirrer speed to 75 and the temperature to 37 °C.
- Fill a 50 mL vial halfway with water and shake vigorously for 2 min. This is air-saturated water, use it as 100% standard. Fill the oxygen meter chamber with ~2 mL of water and place the two-piece stopper on.
- Click Okay on the screen and allow the signal to plateau. Once the signal has reached a plateau, click Okay. Dispose of liquid in the chamber and dry with tissue paper.
- Repeat steps 10.6-10.7 but with 200 mM sodium sulfite (0% standard). Click on Save calibration.
NOTE: No vigorous shaking is needed for the 0% standard. - During perfusion, use two 5 mL syringes. One syringe for circulating perfusate (oxygen in) and the second for efferent perfusate from the NMR tube (oxygen out).
- When drawing up perfusate for both in and out measurements, draw 3-4 mL each time.
NOTE: This column has glass tubing to allow for access into the NMR tube to withdraw the perfusate that has flowed through the liver. - Measure the circulating perfusate, first just as water in step 10.6 and dispose of in step 10.7. Repeat the same steps for the efferent perfusate.
- Perform oxygen measurements every 10 min.
Representative Results
Liver function is primarily assessed by oxygen consumption and flow rate. A flow rate of 4-8 mL/min and oxygen consumption of 1 µmol/min.g is typical. These measures will vary depending on specific experimental conditions and biological differences.
The exact amount of isoflurane used will depend on the type of anesthesia system being used as well as the environment and age/weight of the mouse. During the surgery, the isoflurane and delivery gas do not change, although some changes may be necessary depending on the specifics of the surgical area (e.g., background noise)10. When heparin is injected deep subcutaneously, the onset of action could be delayed by up to 20-40 min. A 10 min waiting period post heparin administration ensures onset of action16. Lidocaine has a 2 min onset of action11.
When inserting the catheter, keep the bevel pointed up, and enter at no more than 15° angle from the portal vein. Both sutures have two knots. The first suture must be tied past the catheter tapper. If cannulation of the portal vein is successful, the liver bleaches from the flush. As the surgeon is resecting the liver, the assistant clears the resected contents with a cotton-tipped applicator. To prevent contaminating the liver and prevent nicks to the lobes, do not cut through the stomach. Do not apply too much tension to the portal vein or liver when holding the catheter to prevent dislodging or tearing the portal vein.
The perfusion hardware setup requires extensive attention to detail (Figure 1). Heparin injections (Figure 2) are essential to the experiment. If blood coagulates, it will occlude the catheter that is inserted into the portal vein, preventing flow. The lidocaine injection (Figure 3) is to aid in desensitizing the area for pain relief. Table 1 provides a simple dosing chart for heparin and lidocaine with saline. The celiotomy and suturing (Figure 4) are essential for a successful portal vein catheterization, liver resection, and successful transfer to the perfusion rig. Flow rate and oxygen consumption measurements are vital to monitoring the liver's health and function (Figure 6). There is often a slight difference in O2 consumption between fed and fasted livers, which we attribute to increased energy demands imposed by gluconeogenesis in the fasted liver.
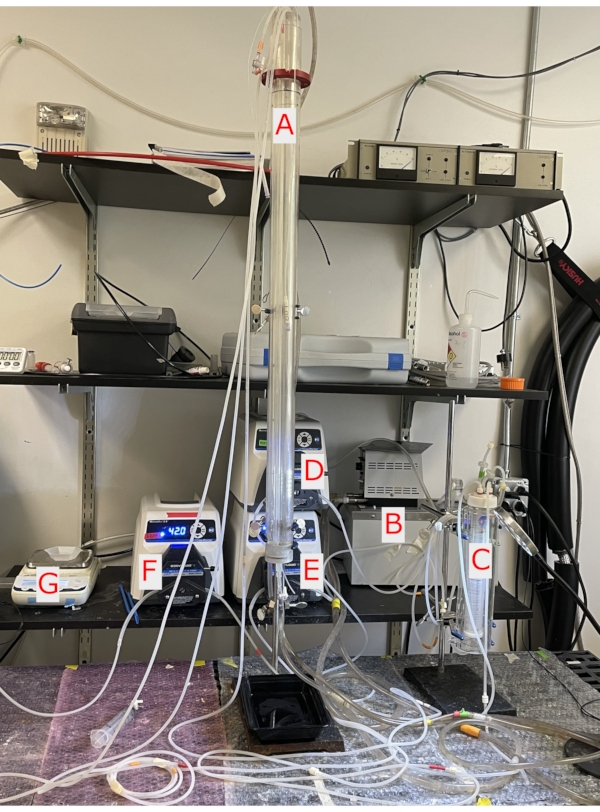
Figure 1. Perfusion column and pumps. A. A 100 cm water-jacketed glass column in which the liver hangs at the bottom. B. The water pump circulates water through the glass column and heats the perfusate. C. Glass thin layer oxygenator is pressurized with 95%/5% O2/CO2 oxygenating the perfusate. D. The ball-bearing pump circulates perfusate from the water bath into the thin layer oxygenator and the glass column. E. The ball-bearing pump circulates perfusate being delivered into the column from the delivery pump keeping perfusate oxygenated and maintaining a flow of 8 mL/min. F. The ball-bearing pump removes efferent perfusate from the NMR tube. G. Weighing scale to weigh perfusate from NMR tube to obtain flow rate of the liver. Please click here to view a larger version of this figure.
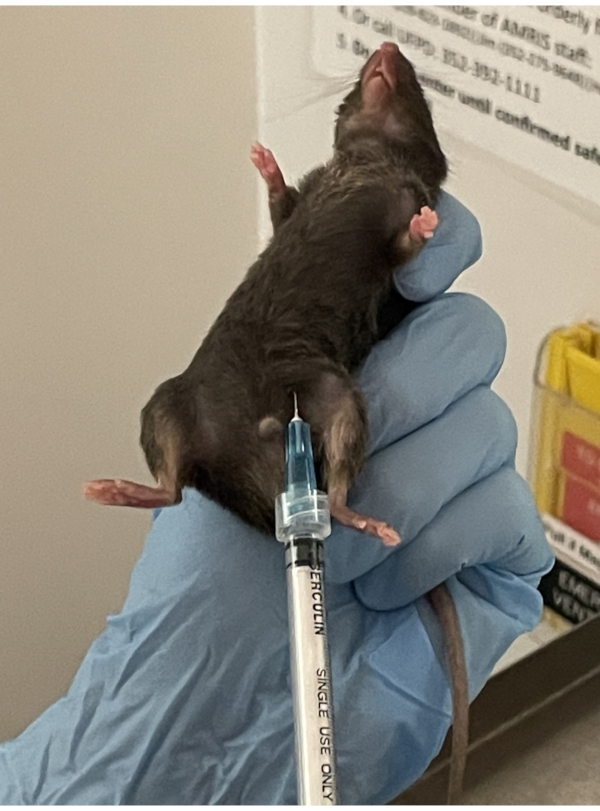
Figure 2. Heparin injection. Deep subcutaneous injection of heparin is given in the lower abdominal fat layer of the mouse. It is important when picking up the mouse, to pull the skin tight to allow the needle to penetrate the skin with ease. Please click here to view a larger version of this figure.
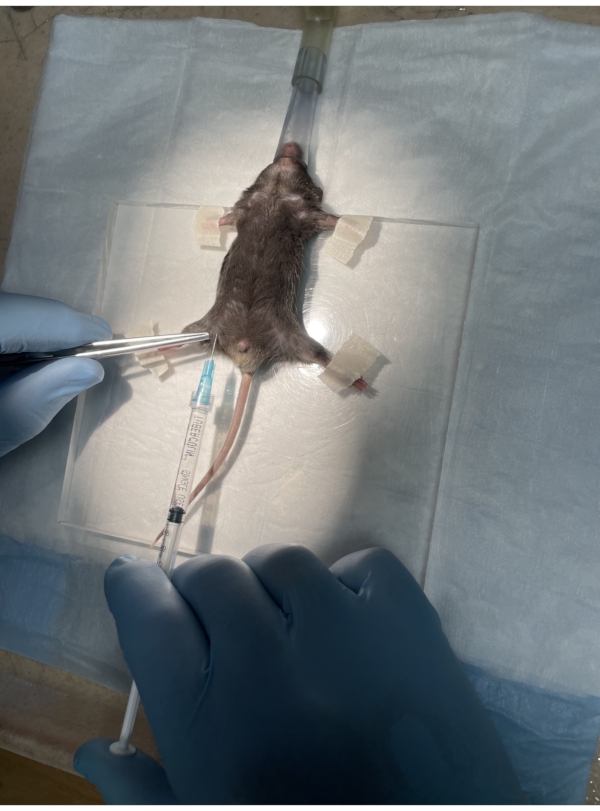
Figure 3. Lidocaine injection. The mouse is placed in the supine position on the surgical platform with its paws taped down and the nose in the nose cone. Lidocaine is administered subcutaneously in the iliac crest region. Please click here to view a larger version of this figure.
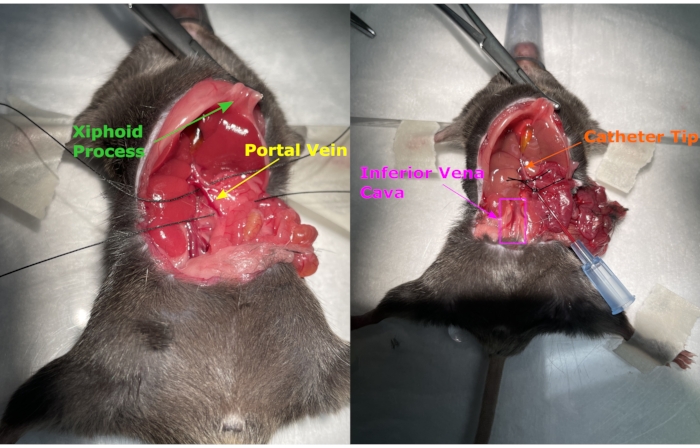
Figure 4. Celiotomy and suture. The celiotomy exposes the internal organs, and a hemostat pulls traction through the xiphoid process to help open the incision further. Two sutures are placed around the portal vein, the catheter is inserted, and the sutures are tied. Please click here to view a larger version of this figure.
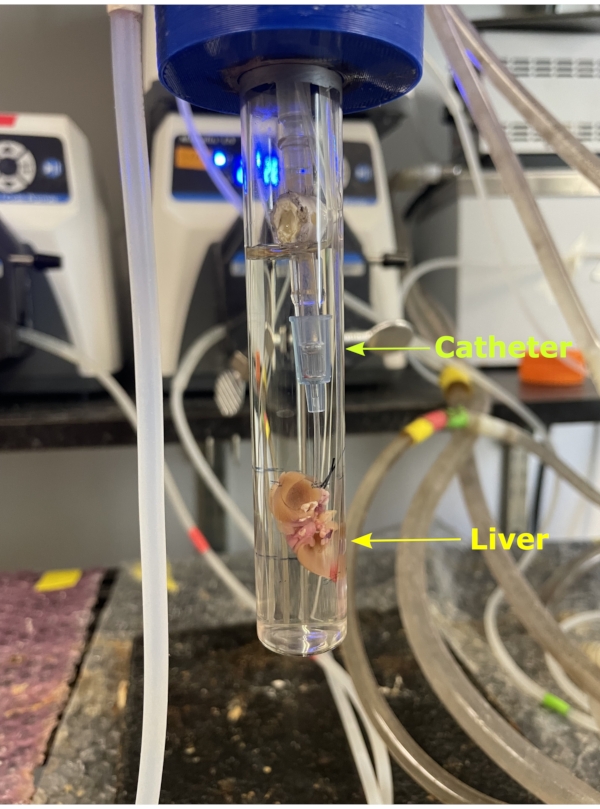
Figure 5. NMR tube. The liver removed from the body along with the catheter which is then attached to the silicon tubing attached to the glass column. The liver is hung from the column and encapsulated by the NMR tube. A 20 mm NMR tube is then carefully screwed onto the column encapsulating the liver. Please click here to view a larger version of this figure.
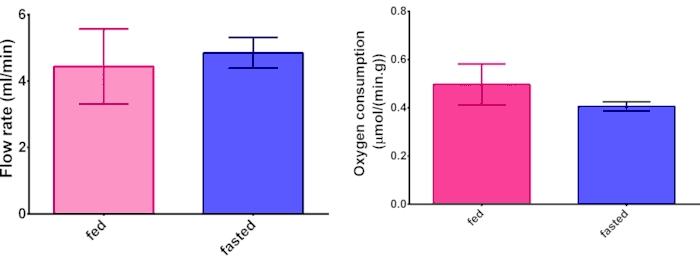
Figure 6. Oxygen consumption and flow rate. Representative data from comparing hepatic oxygen consumption and flow rate measurements between fed and fasted livers. N = 3 and error bars are standard deviation. Please click here to view a larger version of this figure.
| Heparin 1000 units/mL | Saline 0.9% | Total |
| 0.01 mL | 0.19 mL | 0.2 mL |
| Lidocaine 2% | Saline 0.9% | Total |
| 0.2 mL | 0.6 mL | 0.8 mL |
Table 1. Heparin and lidocaine dose with saline. The table displays the concentration of heparin and lidocaine and the dose of each pharmaceutical with saline.
Discussion
This surgical procedure is challenging and requires extensive practice to achieve reproducible results. Isoflurane and carrier gas should be adjusted as needed to maintain the viability of the animal through as much of the surgical procedure as possible. Environment, time of day, age, weight, and several other factors will affect anesthesia. Weight, diet, strain of mice and age could affect surgery as fat buildup can interfere with visualizing the portal vein. When taping the paws down, care must be taken to not apply any strain on the neck that may result in suffocation. Furthermore, the fattier the mouse the tighter the suture will need to be around the catheter to counteract the decreased coefficient of friction between the catheter and vein induced by the lipids. The onset of action time for heparin is essential as excessive exposure to isoflurane produces artifacts in organs17. Administration of heparin and lidocaine injections require a 23G needle 19.05 mm length, ensuring no trauma to internal organs during the injection. The suture loop must be over the taper of the catheter, or it will occlude the vein when tightened. Once the catheter is inserted there is usually a backflow of blood out of the catheter from the pressure, which is a positive sign of correct placement. The catheter may be pulled upwards for visual confirmation that the catheter tip is far enough past the first suture. Rolling the wrists and shoulders will ensure that the suture will not slide off the tapered tip when the assistant ties the suture. When transferring the liver from the silicone perfusion tubing to the column a bead of perfusate is left on top of the catheter. The perfusate bead will prevent any air bubbles from entering the catheter and going into the liver. The meniscus of perfusate on top of the catheter provides sufficient volume for the liver to function until its connected. To avoid torsion of the liver and the portal vein, the NMR tube is slowly screwed on. Additionally, the perfusion system used in this experiment does not require a pressure valve. The flow rates of the pumps are maintained such that glass perfusions column contains perfusate at a height of ~12 cm. The liver takes up Krebs buffer by gravity, negating any pressure difference between the two pumps with no effect on the flow rate of the liver. Since the liver is perfused through gravity the amount of perfusate taken up by the liver is set by the liver's natural biological activity. No data was collected for perfusion pressure as portal vein pressure is not measurable in this system.
The first incision of the celiotomy is shallow to create an opening, and subsequent cuts are deeper to avoid nicking the lobes of the liver. The overall length of the incision for the celiotomy is 3 cm for mice of this age and size but will change based on strain, age, and weight. Although the study described uses mice 9-13 weeks old, older, or younger mice can be studied as well as rats. The size of the NMR tube and the portal vein catheter would need to be changed based on the anatomical size of the liver and portal vein for the study of concern. If the portal vein is not straight, a cotton-tipped applicator can aid in manipulating the vein when inserting the catheter. Although the bile duct is not removed, if there is an experimental need for its absence the duct can be removed with fine tweezers. Excessive manipulation of the portal vein when placing sutures will cause constriction making catheter placement more difficult. Any fur that sticks to the liver is rinsed off with perfusate before press fitting the catheter to the column and encapsulating it within the NMR tube. The time frame of 30 min for perfusion can be changed from 20 min to 60 min, but all reliable data will be collected after the initial 10 min of perfusion.
A marker of successful hepatic tissue resection is after the perfusion the liver has no deformities or any other integrity issues. It is homogeneously pale yellow throughout. If the tissue was injured during surgery such as a nick it would have dark yellow spots around it. Also, if the liver was damaged from the perfusion, it would not perfuse. If the tissue experienced poor perfusion of the Krebs buffer, there would be dark yellow streaks throughout the organ as a result of starvation leading to tissue death. Another method of monitoring liver health is hepatic oxygen consumption (Figure 6). It has been shown that mice livers contain higher lipid and glycogen amounts but have similar total protein amounts, so it was expected that the hepatic oxygen consumption when normalized to liver mass would have a similar value. A third method is the NMR data of the real-time data of metabolic turnover.
The method's main limitation is terminal surgery itself. There is a substantial cost in mice, equipment, time, and personnel. Therefore, utmost care must be exercised when performing these procedures and collecting data. Biological variation within the mouse model can generate difficulty in surgery. Moreover, it is imperative to avoid optical enhancements. No optical enhancements are needed as all anatomy is visible to the naked eye. Optical enhancements increase the potential for errors to occur as the surgeon and assistant have a limited field of view, leading to knicks in the liver or unintended tension on the portal vein, causing a failure if the catheter pulls out. Proper implementation of these methods will result in > 95% surgery success rate in the C57BL/6J mouse. Another limitation to consider is the 10 min period required for the liver to reach a steady state. It is not a limitation for the study described here, nor in many others, but for any experiment warranting the initial 10 min of data this method will not suffice. The lack of the complex hormonal signature associated with whole-body metabolism also serves as a limitation, though glucagon, insulin, etc., and any combination thereof, can be added back to the perfusate.
There are several potential future applications for this technique. As more pharmaceuticals are developed for the treatment of NASH, standard methods for assessing liver energy metabolism could find wide application. As NASH is strongly associated with liver cancer, models of these cancers are also subjects for study.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by funding from the National Institutes of Health (R01-DK105346, P41-GM122698, 5U2C-DK119889). A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory's Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility, which is supported by the National Science Foundation Cooperative Agreement No. DMR-1644779 and the State of Florida.
Materials
| 1 mL Luer-Lock Single Use Sterile Disposable Syringe | N/A | N/A | Non-specific Brand |
| 100 cm Water Jacketed Glass Column | N/A | N/A | Custom Made |
| 2-0 Silk Suture | Braintree Scientific | N/A | |
| 22 Gauge Catherter 1 in. Without Safety | Terumo | SRFF2225 | |
| 23 G 0.75 in. Hypodemeric Needles | Exel International | 26407 | |
| 27 G 1.5 in. Hypodemeric Needles | Exel International | 26426 | |
| 4×4 in. Surgical Platform | N/A | N/A | Custom Made |
| 70% Alcohol Wipe | N/A | N/A | Non-specific Brand |
| Circulating Water Bath | MS Lauda | N/A | Model no longer manufactured |
| Cotton Tip Applicator | N/A | N/A | Non-specific Brand |
| Delicate Operating Scissors; Straight; Sharp-Sharp; 30mm Blade Length; 4 3/4 " | Roboz | RS-6702 | |
| Dumont #5/45 Forceps | Fine Scientific Tools | 11251-35 | |
| Dumont #7 – Fine Forceps | Fine Scientific Tools | 11274-20 | |
| Hemostats | Fine Scientific Tools | 13015-14 | |
| Heparin Sodium Injectable 1000 units/mL | RX Generics | 71288-0402-02 | |
| Isoflurane | Patterson Veterinary | 14043-0704-06 | |
| Lidocaine HCl 2% | VEDCO Inc. | 50989-0417-12 | |
| Membrane-Thin-Layer Oxygenator | Radnoti | N/A | |
| Metzenbaum Scissors; Curved; Blunt; 27 mm Blade Length; 5 " | Roboz | RS-6013 | |
| Oxygen Meter System | Hanstech Instruments Ltd. | N/A | |
| Saline 0.9% Solution | N/A | N/A | Saline is made in lab |
| Scale | N/A | N/A | Non-specific Brand |
| Variable Speed Analog Console Pump Systems | Cole Palmer | N/A | Models are custom per application |
| Weigh boats | N/A | N/A | Non-specific Brand |
References
- Ragavan, M., McLeod, M. A., Giacalone, A. G., Merritt, M. E. Hyperpolarized Dihydroxyacetone Is a Sensitive Probe of Hepatic Gluconeogenic State. Metabolites. 11 (7), 441 (2021).
- Lumata, L. Hyperpolarized (13)C Magnetic Resonance and Its Use in Metabolic Assessment of Cultured Cells and Perfused Organs. Methods in Enzymology. 561, 561-573 (2015).
- Moreno, K. X., et al. Real-time detection of hepatic gluconeogenic and glycogenolytic states using hyperpolarized [2-13C] dihydroxyacetone. The Journal of Biological Chemistry. 289 (52), 35859-35867 (2014).
- Moreno, K. X., et al. Hyperpolarized δ-[1-13C] gluconolactone as a probe of the pentose phosphate pathway. NMR in Biomedicine. 30 (6), (2017).
- Merritt, M. E., Harrison, C., Sherry, A. D., Malloy, C. R., Burgess, S. C. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proceedings of the National Academy of Sciences of the United States of America. 108 (47), 19084-19089 (2011).
- Bailey, L. E., Ong, S. D. Krebs-Henseleit solution as a physiological buffer in perfused and superfused preparations. Journal of Pharmacological Methods. 1 (2), 171-175 (1978).
- Kolwicz, S. C., Tian, R. Assessment of Cardiac Function and Energetics in Isolated Mouse Hearts Using 31P NMR Spectroscopy. Journal of Visualized Experiments: JoVE. (42), e2069 (2010).
- Hwang, G. H., et al. Protective effect of butylated hydroxylanisole against hydrogen peroxide-induced apoptosis in primary cultured mouse hepatocytes. Journal of Veterinary Science. 16 (1), 17-23 (2015).
- Bessems, M., et al. The isolated perfused rat liver: standardization of a time-honoured model. Laboratory Animals. 40 (3), 236-246 (2006).
- Beal, E. W., et al. A Small Animal Model of Ex Vivo Normothermic Liver Perfusion. Journal of Visualized Experiments: JoVE. (136), e57541 (2018).
- Collins, J. B., Song, J., Mahabir, R. C. Onset and duration of intradermal mixtures of bupivacaine and lidocaine with epinephrine. The Canadian Journal of Plastic Surgery. 21 (1), 51-53 (2013).
- . Medical Dictionary Available from: https://www.merriam-webster.com/medical (2022)
- Thorpe, D. R. . A Dissection in Color: The Rat (and the Sheep’s Brain). , (1968).
- Cabral, F., et al. Purification of Hepatocytes and Sinusoidal Endothelial Cells from Mouse Liver Perfusion. Journal of Visualized Experiments: JoVE. (132), e56993 (2018).
- . Operations Manual Setup, Installation and Maintenance Available from: https://www.chem.ucla.edu/dept/Faculty/merchant/pdf/electrode_prep_maintenance.pdf (2006)
- . Heparin Available from: https://go.drugbank.com/drugs/DB01109 (2022)
- Overmyer, K. A., Thonusin, C., Qi, N. R., Burant, C. F., Evans, C. R. Impact of anesthesia and euthanasia on metabolomics of mammalian tissues: studies in a C57BL/6J mouse model. PloS One. 10 (2), 0117232 (2015).

