Effects of Desmodium caudatum on Gastrointestinal Hormones and Intestinal Flora in Rats with Gastritis
Summary
The present protocol describes the method for analyzing intestinal flora using Illumina-based 16S rRNA gene sequencing and provides a framework for evaluating the effectiveness of herbal decoctions.
Abstract
In order to preliminarily explore the effects of Desmodium caudatum on gastritis and intestinal flora in rats, a chronic gastritis rat model was established using the classic sodium salicylate method. Eighteen SPF rats were divided into three groups: the control group (Group C), the model group (Group M), and the treatment group (Group T). Pathological sections of the gastric wall were taken from rats in each group. Furthermore, the concentrations of gastrin and malondialdehyde in the serum of rats in each group were determined by ELISA. Additionally, the effects of D. caudatum on the intestinal flora of rats with gastritis were explored through a detailed comparison of gut bacterial communities in the three groups, employing Illumina-based 16S rRNA gene sequencing. The results indicated that D. caudatum decoction could reduce the malondialdehyde content and increase the gastrin content. Moreover, D. caudatum decoction was found to enhance the diversity and abundance of intestinal flora, exerting a positive impact on the treatment of gastritis by regulating and restoring the intestinal flora.
Introduction
Chronic gastritis (CG), one of the most common clinical diseases, is characterized by chronic and persistent inflammatory changes in the gastric mucosa epithelium, which is frequently and repeatedly attacked by various pathogenic factors1. The incidence rate of CG ranks first among all types of stomach diseases, accounting for 40% to 60% of the outpatient service rate in the Department of Gastroenterology2. Moreover, the incidence rate generally increases with age, especially in individuals who are middle-aged and older3. Undoubtedly, CG significantly reduces people's quality of life, emphasizing the critical need to discover new therapeutic agents.
Numerous studies have reported that the occurrence and development of CG are linked to the secretion of gastrointestinal hormones, such as gastrin (GAS)4,5. GAS, a common gastrointestinal peptide hormone in the digestive tract, stimulates cells to secrete gastric acid by promoting the release of histamine from eosinophils. Additionally, it improves the nutrition and blood supply of the gastric mucosa, promoting the proliferation of gastric mucosa and parietal cells6. Therefore, gastrin can be utilized as an indicator to evaluate the development level of CG. Furthermore, lipid peroxidation products triggered by reactive oxides can activate inflammatory cells, leading to CG. Malondialdehyde (MDA), a lipid peroxidation marker, is a commonly used indicator to measure the degree of oxidative stress. It reflects the level of free radicals in the gastric mucosa to a certain extent. The MDA level can indicate the attack of unsaturated fatty acids in the local gastric mucosa caused by free radicals7,8.
Intestinal microecology consists of millions of microorganisms residing in the host gut, playing a vital role in maintaining host health and regulating host immunity. A healthy intestinal flora, characterized by high richness, diversity, and stable microbiota function, acts as a protective barrier against the invasion of pathogenic microorganisms by participating in metabolism. Disturbances in the intestinal flora make individuals more susceptible to acute and chronic gastrointestinal diseases9,10. In recent years, microbiota therapy for gastrointestinal diseases has progressed rapidly and demonstrated significant efficacy11. In summary, the consideration of intestinal flora is crucial in understanding and addressing gastrointestinal diseases.
As an indispensable component of the treasure trove of traditional Chinese medicine, folk medicinal materials hold great significance for clinical practice and the modernization of national medicine. The roots and the entire parts of Desmodium caudatum (Thunb.) DC. has been widely used for relieving stomach discomfort in the Beichuan Qiang area of Mianyang City for an extended period. Some articles indicate the scientific basis for treating gastrointestinal diseases with D. caudatum, citing its effects such as hemostasis, anti-oxidation, and gastrointestinal protection12,13,14. However, due to the lack of in-depth research, no clinical pharmacodynamics mechanism has been established. This article aims to study the effects of D. caudatum in treating gastritis based on gastrointestinal hormones and intestinal flora, providing a basis for its rational clinical application.
Protocol
The procedures for the care and use of animals were approved by the Ethics Committee of Mianyang Normal University, and all relevant institutional and governmental regulations concerning the ethical use of animals were strictly followed. For the present study, SPF rats (Kunming species, both male and female, weighing 180-220 g) were utilized. The animals were obtained from a commercial source (see Table of Materials). All animals were housed in a pathogen-free environment and provided ad libitum access to food.
1. Preparation of reagents
- Prepare a 5% sodium salicylate solution by weighing 2.5 g of sodium salicylate powder (see Table of Materials) with an electronic scale. Dissolve the powder in a small amount of distilled water and dilute it to a total volume of 50 mL.
- Prepare the decoction of D. caudatum (12.8 g/kg). Weigh 128 g of D. caudatum (see Table of Materials), place it into a round-bottom flask containing 1000 mL of laboratory distilled water, and concentrate it to a final volume of 100 mL.
2. Grouping and administration
- Divide the rats into different groups and intragastrically administer the required solutions.
NOTE: For the present study, 18 rats (9 males, 9 females) were divided into three groups: the control group (Group C), the model group (Group M), and the treatment group (Group T), with 6 rats in each group. The control group received saline solution, and the model group received a 5% sodium salicylate solution for 5 weeks at a dose of 10 mL/kg/day15. The treatment group was pretreated with a 5% sodium salicylate solution for 5 weeks at a dose of 10 mL/kg/day, followed by administration of the D. caudatum decoction at a dosage of 1 mL/100 g for 10 days15,16. - Collect feces from the rats in dry and sterile microcentrifuge tubes using the tail-carrying method17 on the last day of the experiment and freeze them in a liquid nitrogen ultra-low-temperature freezer.
- After the end of sampling, sacrifice the rats by cervical dislocation (following institutionally approved protocols). Open the abdominal cavity with scissors to expose the abdominal aorta.
- Insert the needle (23-25 G) almost parallel into the abdominal aorta, collect the serum, and preserve it in an ultra-low-temperature freezer. Take out the stomachs from the abdominal cavity16 and soak in a 10% formalin solution for preservation.
NOTE: In Group M, the chronic gastritis model is considered successful if the gastric mucosa is dilated and congested with regular glandular morphology15, while observing a small amount of inflammatory cell infiltration in the lamina propria.
- Insert the needle (23-25 G) almost parallel into the abdominal aorta, collect the serum, and preserve it in an ultra-low-temperature freezer. Take out the stomachs from the abdominal cavity16 and soak in a 10% formalin solution for preservation.
3. Pathological section of gastric wall
- Take the gastric wall samples from rats in each group for pathological sections. Subsequently, perform routine hematoxylin-eosin staining to observe and analyze the pathological changes18.
4. Determination of serum gastrointestinal hormones
- In the serum sample, detect the contents of gastrin (GAS) and malondialdehyde (MDA)19 using ELISA following the manufacturer's instructions (see Table of Materials). Utilize a microplate reader for the analysis.
5. Fecal total DNA extraction and PCR amplification and purification
- Perform microbial DNA extraction utilizing the commercially available DNA isolation kit (see Table of Materials) and determine the concentration and purity using UV-Vis spectrophotometer16. Subsequently, assess DNA integrity through 1% agarose gel electrophoresis18.
- Amplify the bacteria 16S rRNA gene with primers 338F (5′-ACT CCT ACG GGA GGC AGC AG-3′) and 806R (5′-GAC TAC HVG GGG GTW TCT AT-3′) using a thermocycler PCR system17. The primers are obtained from commercial sources (see Table of Materials).
NOTE: Follow the PCR program: denaturation (3 min, 95 °C), followed by 27 cycles (30 s, 95 °C; 30 s, 55 °C; 45 s, 72 °C), and a final extension (10 min, 72 °C). Conduct PCR reactions with the following components: 4 µL of 5× DNA polymerase buffer, 2 µL of 2.5 mM dNTPs, 0.8 µL of each primer (5 µM), 0.4 µL of DNA polymerase, and 10 ng of template DNA (see Table of Materials). - Extract, purify, and quantify the PCR products sequentially using 2% agarose gel, commercially available DNA gel extraction kit, and fluorometer, respectively17.
6. Sequencing
NOTE: Step 6 and step 7 are performed following previously published methods20,21.
- Utilize the Illumina platform for sequencing.
NOTE: One end of the DNA fragment complements the base of the connector embedded in the chip, securing it onto the chip. The other end randomly complements the base of another adjacent buried joint, forming a "bridge." - Perform PCR amplification to generate DNA clusters. Linearize DNA amplifiers into single strands.
- Introduce an altered DNA polymerase alongside deoxyribonucleotide triphosphates (dNTPs) featuring four distinct fluorescent markers. Synthesize only one base per cycle.
- Utilize a laser to scan the surface of the reaction plate and determine the nucleotide types polymerized during the initial reaction of each template sequence.
- Chemically cleave the "fluorescence group" and "termination group" to reestablish the 3' sticky end. Subsequently, advance to polymerizing the second nucleotide.
- Obtain the sequence of template DNA fragments by analyzing the fluorescence signal results collected in each round.
7. Processing of sequencing data
- Initially, trim the reads of raw fastq data at any site with a score < Q20 and eliminate reads with uncertain bases. Additionally, permit two mismatches in precisely matched primers. Subsequently, merge the sequences with an overlap >10 bp.
- Cluster operational taxonomic units (OTUs) with 97% similarity using UPARSE, and remove chimeric sequences with UCHIME20,21.
- Employ the RDP Classifier algorithm to analyze the taxonomy of each 16S rRNA gene sequence against the Silva (SSU123) 16S rRNA database, using a confidence threshold of 70%.
- Perform Alpha diversity analysis and Beta diversity analysis using Mothur and Qiime, respectively (see Table of Materials).
8. Statistical analysis
- Analyze the data in this study using statistical analysis software (see Table of Materials). Employ one-way analysis of variance (ANOVA) for comparisons among multiple groups and utilize the least-significant-difference (LSD) test for comparisons between two groups.
- Consider P < 0.05 as statistically significant in all comparisons. P < 0.01 is regarded as extremely significant.
Representative Results
The results of the pathological section of the stomach wall are depicted in Figure 1. In comparison to Group C, Group M exhibited mild gastric wall atrophy and mild inflammation. However, when compared to Group M, Group T showed no evident inflammation, intestinal metaplasia, or atrophy. This suggests that D. caudatum decoction can effectively improve gastritis.
The serum gastrointestinal hormone assay results are presented in Figure 2. The content of malondialdehyde (MDA) was significantly higher in Group M than in Group C. After treatment with D. caudatum decoction, it was significantly reduced. Regarding the content of gastrin (GAS), Group M showed significantly lower levels than Group C, but Group T exhibited a significant increase. These results indicate that D. caudatum decoction may regulate the level of gastrointestinal hormones to treat gastritis.
Alpha diversity analysis, as shown in Table 1 and Figure 3, reveals a significant difference between the communities of Group M and Group T. The number of bacteria in Group C and Group T is higher than that in Group M, suggesting that the number and types of intestinal bacteria in rats gradually tend to a normal level after treatment.
Species composition analysis
According to Figure 4A, the community barplot illustrates that Lactobacillus and norank_f__Muribaculaceae account for the largest proportion in Group C. Group M contains a higher abundance of Clostridium_sensu_stricto_1 and some Helicobacter. The community composition of Group T is more similar to that of Group C, with important components being Lactobacillus and Romboutsia. Additionally, the Spearman correlation heatmap (Figure 4B) combined with the circos diagram (Figure 4C) reveals significant differences in flora composition between Group C and Group M, with distinct variations in dominant flora. After treatment, Group T tends to return to a normal flora state. Furthermore, Figure 5 indicates extreme differences in community composition between Group M and Group C, with significant changes occurring in the structure and composition of intestinal flora in rats with gastritis. After treatment, there are similarities in flora composition between Group C and Group T, as well as some similarities between Group T and Group M. These results indicate that D. caudatum decoction could restore the intestinal flora of rats with gastritis to a normal or close-to-normal state.
Species difference analysis
From the multi-species comparison column chart in Figure 6A, it can be observed that Norank_f_Muribaculaceae is abundant in Group C and significantly differs from Group M and Group T. After treatment, the number of Norank_f_Muribaculaceae in Group T shows an increasing trend. Additionally, Clostridium_sensu_stricto_1, abundant in Group M, exhibits resilience in harsh environments. After treatment, the number decreases significantly, suggesting that D. caudatum decoction improves the survival environment of intestinal flora to some extent. On the other hand, the LEfSe multi-level species hierarchy tree analysis in Figure 6B indicates 44 species with significant differences between groups. Norank_f_muribaculaceae, Clostridium_sensu_stricto_1, and Romboutsia were found to be abundant in Group C, Group M, and Group T, respectively.
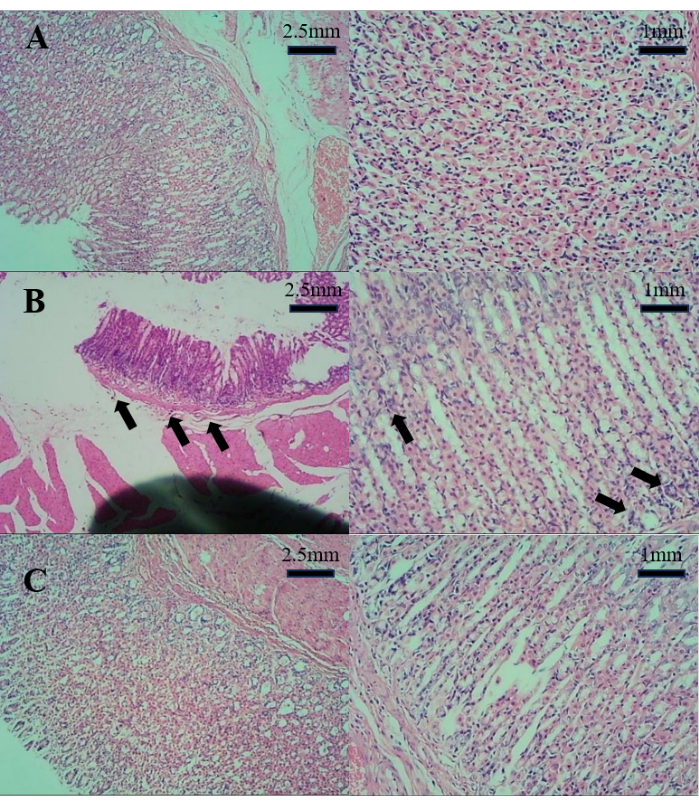
Figure 1: The results of the pathological section of the stomach wall. (A) Pathological section of the stomach in Group C. (B) Pathological section of the stomach in Group M. (C) Pathological section of the stomach in Group T. Please click here to view a larger version of this figure.
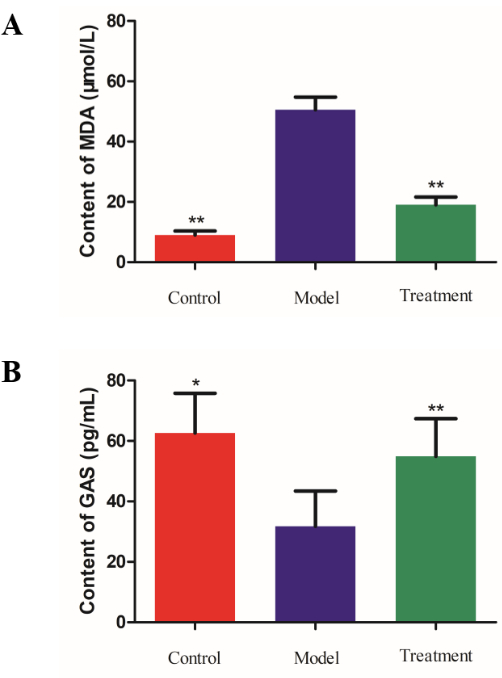
Figure 2: The contents of serum gastrointestinal hormones. (A) Bar analysis chart of MDA (malondialdehyde) determination results. (B) Bar analysis chart of GAS determination results. *P < 0.05, **P < 0.01. Please click here to view a larger version of this figure.
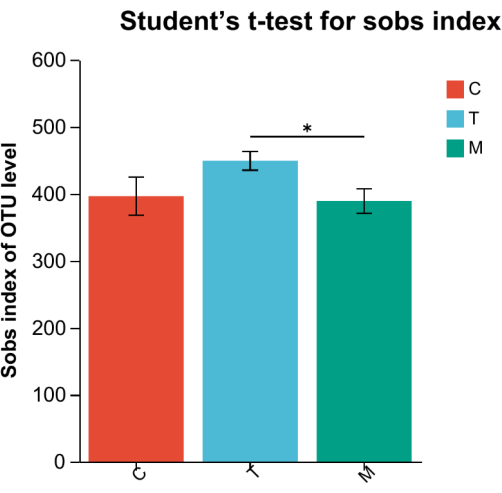
Figure 3: Column gram of diversity index T. Red bar represents Group C, blue represents Group T, while green represents Group M.*P < 0.05, **P < 0.01. Please click here to view a larger version of this figure.
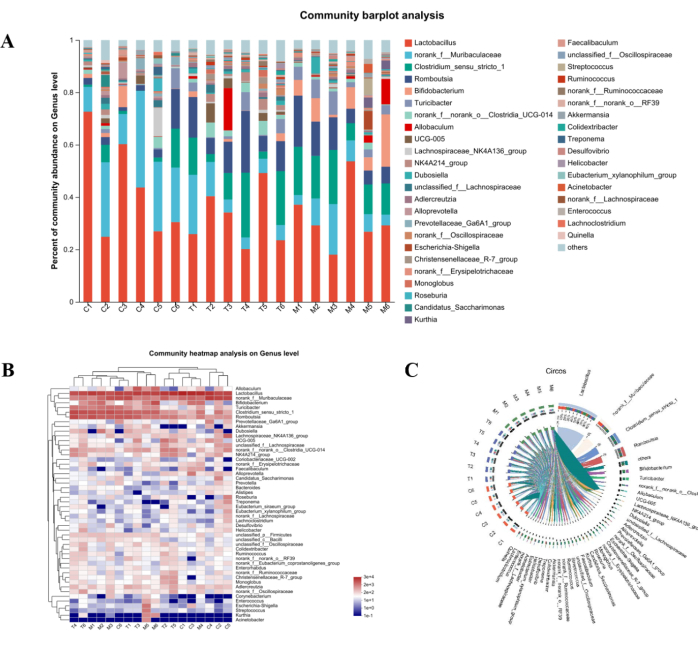
Figure 4: Community composition analysis. (A) Community histogram analysis. (B) Community heatmap analysis on the level of Genus. (C) Circos diagram analysis of relationship between samples and species. Please click here to view a larger version of this figure.
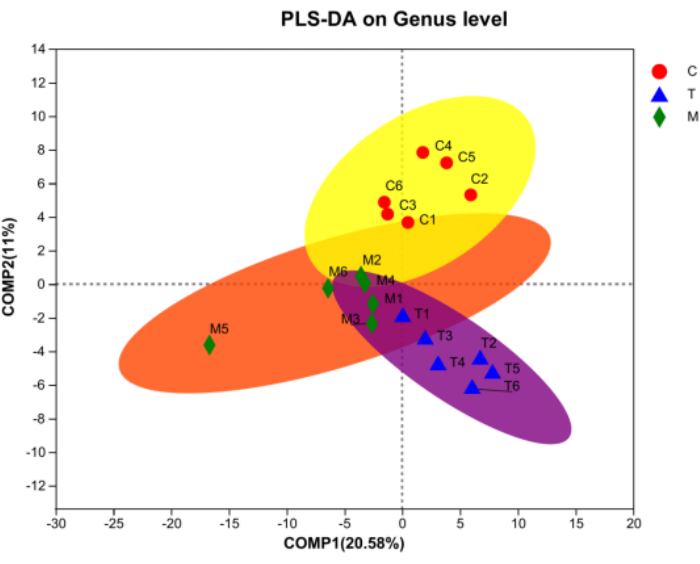
Figure 5: PLS-DA analysis on the level of Genus. Red represents Group C, blue represents Group T, and green represents Group M. Please click here to view a larger version of this figure.
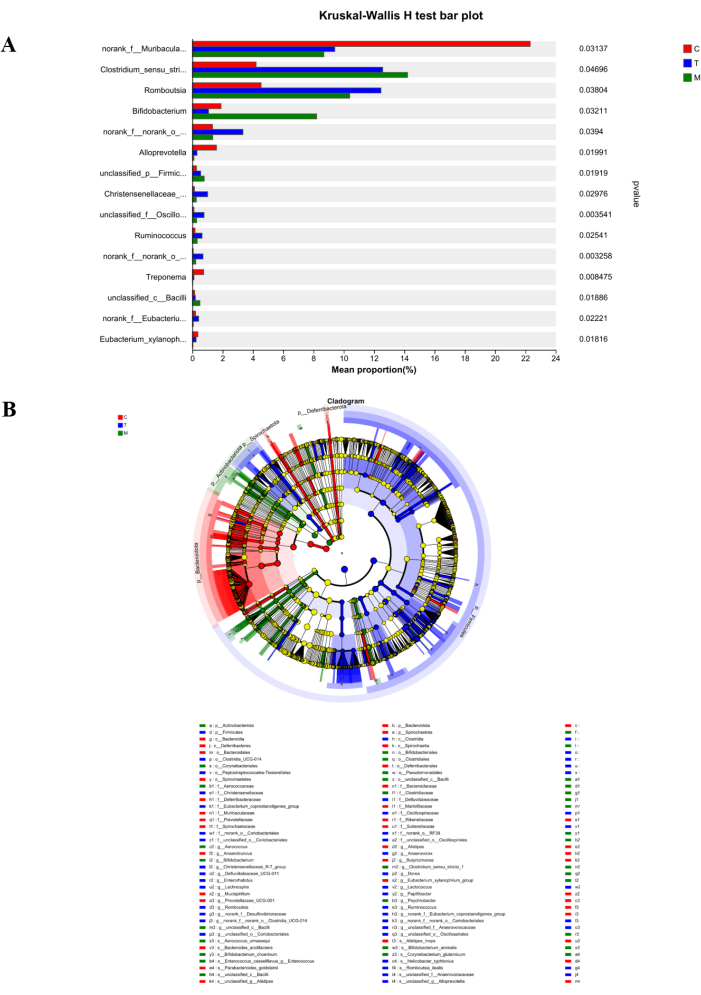
Figure 6: Species difference analysis. (A) Kruskal-Wallis H test bar plot. (B) Tree map of LefSe multi-level species. Red represents Group C, blue represents Group T, and green represents Group M. Please click here to view a larger version of this figure.
| Group | Mean value | Standard deviation |
| C | 3.5201 | 0.63641 |
| M | 3.2755 | 0.28494 |
| T | 3.5388 | 0.29945 |
Table 1: Alpha diversity index results.
Discussion
D. caudatum, a commonly used folk medicine by the Qiang nationality12, has shown significant efficacy in treating gastrointestinal diseases. With the advancement of modern pharmacological research, the imbalance of flora resulting from gastrointestinal microecology imbalance is identified as a key factor in acute and chronic gastrointestinal diseases22,23. Certain microorganisms in the intestinal tract play a crucial role in maintaining dynamic balance in the body, and hormones in the serum reflect the physiological and pathological state of the body. Therefore, the study focuses on intestinal flora and gastrointestinal hormones.
16S rRNA high-throughput gene sequencing is a primary method for detecting intestinal microorganisms. It enables the detection of microorganism types and functions in collected samples, allowing for accurate and quantitative analysis of each species of intestinal microorganisms. In recent years, 16S rRNA high-throughput gene sequencing has rapidly developed and been widely used for microbial diversity analysis in various ecological environments24,25,26.
The effects of D. caudatum on the intestinal flora of rats with gastritis were investigated through a detailed comparison of gut bacterial communities in three groups based on Illumina-based 16S rRNA gene sequencing. Modern scientific research indicates that Lactobacillus, norank_f__Muribaculaceae, Romboutsia, and Bifidobacterium are probiotics, each with specific health benefits such as reducing the risk of multiple malignant tumors, producing vitamin K with anti-inflammatory potential, promoting polysaccharide metabolism, and improving diarrhea and constipation27,28,29. Clostridium_sensu_stricto_1, a pathogenic bacterium in intestinal injury, can cause inflammation and bacteremia and is closely linked to blood and gastrointestinal tumors30. Chronic gastritis and atrophic gastritis contribute significantly to gastric tumors31.
Beta analysis indicates significant changes in the contents of Lactobacillus, norank_f__Muribaculaceae, Romboutsia, Bifidobacterium, and Clostridium_sensu_stricto_1 after treatment. The contents of Lactobacillus, norank_f__Muribaculaceae, and Romboutsia increased significantly, while Bifidobacterium and Clostridium_sensu_stricto_1 decreased significantly after D. caudatum treatment. Additionally, the community barplot suggests that the community composition of Group T is more similar to that of Group C. These results indicate that D. caudatum could improve the prevalence of gastritis in rats. However, the specific mechanisms behind the reduction in the content of the probiotic Bifidobacterium by D. caudatum require further research.
The ELISA kit method possesses high sensitivity, strong specificity, simplicity in operation, and suitability for small sample sizes32. In this study, it was utilized to detect the contents of malondialdehyde (MDA) and gastrin (GAS) in rat serum. MDA reflects the degree of lipid peroxidation, indirectly indicating the extent of cell damage. In rats with gastritis, the MDA level was significantly higher than that in normal rats, suggesting damage to gastric parietal cells. D. caudatum treatment significantly decreased the MDA level, indicating its potential in treating gastritis. Conversely, D. caudatum increased the reduced GAS level, contributing to the repair or treatment of gastritis.
However, there are limitations to both 16S rRNA high-throughput gene sequencing and the ELISA kit method. In 16S rRNA high-throughput gene sequencing, low resolution may make it challenging to distinguish strains or genera with highly similar sequences, leading to difficulties in classification at higher taxonomic levels such as genus, family, or phylum33. For the ELISA kit method, sample pretreatment steps like dilution and washing are usually required, introducing potential errors and variability34.
This study suggests that D. caudatum may treat gastritis by regulating gastrointestinal hormones and intestinal flora, providing insights into its broad clinical applications. However, future research should delve into other gastrointestinal hormones, the mechanisms of regulating intestinal flora, and the effective chemicals involved.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by the Key R&D projects of the Science and Technology Department of Sichuan Province (2020YFS0539).
Materials
| Alpha diversity analysis | Mothur 1.30.2 | ||
| AxyPrep deoxyribonucleic acid (DNA) gel extraction kit | Axygen Biosciences | AP-GX-50 | |
| Beta diversity analysis | Qiime 1.9.1 | ||
| Cryogenic refrigerator | Forma-86C ULT freezer | ||
| Desmodium caudatum | The Traditional Chinese Medicine Hospital of Beichuan Qiang Autonomous County | ||
| E.Z.N.A. soil kit | Omega Bio-tek | D5625-01 | |
| Illumina MiSeq Platform | Illumina Miseq PE300/NovaSeq PE250 | ||
| Multiskan Spectrum | spectraMax i3 | ||
| OTU clustering | Uparse 7.0.1090 | ||
| OTU statistics | Usearch 7.0 | ||
| PCR instrument | TransGen AP221-02 | ||
| PCR instrument | ABI GeneAmp 9700 | ||
| QuantiFluor-ST double-stranded DNA (dsDNA) system | Promega Corp. | ||
| Sequence classification annotation | RDP Classifier 2.11 | ||
| Sodium salicylate | Sichuan Xilong Chemical Co., Ltd | 54-21-7 | |
| SPF rats | Chengdu Dashuo Experimental Animal Co., Ltd | ||
| SPSS 18.0 | IBM |
References
- Marginean, C. M., et al. The importance of accurate early diagnosis and eradication in Helicobacter pylori infection: pictorial summary review in children and adults. Antibiotics (Basel). 12 (1), 60 (2022).
- Sipponen, P., Maaroos, H. I. Chronic gastritis. Scandinavian Journal of Gastroenterology. 50 (6), 657-667 (2015).
- Wang, D. J. Methodological quality and reporting quality evaluation of chinese medicine diagnosis and treatment guidelines for chronic gastritis in China. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 24 (7), 2776-2783 (2022).
- Burkitt, M. D., Varro, A., Pritchard, D. M. Importance of gastrin in the pathogenesis and treatment of gastric tumors. World Journal of Gastroenterology. 15 (1), 1-16 (2009).
- Hayakawa, Y., Chang, W., Jin, G., Wang, T. C. Gastrin and upper GI cancers. Current Opinion in Pharmacology. 31, 31-37 (2016).
- Zhang, C. Z., He, M. X., Jin, L. W., Liu, W. D. Effect of aluminum phosphate gel combined with atropine on patients with acute gastritis and its effect on serum gastrin and malondialdehyde levels. International Journal of Digestive Diseases. 40 (2), 137-140 (2020).
- Wang, Y. K., et al. Levels of malondialdehyde in the gastric juice: its association with Helicobacter pylori infection and stomach diseases. Helicobacter. 23 (2), e12460 (2018).
- Turkkan, E., et al. Does Helicobacter pylori-induced inflammation of gastric mucosa determine the severity of symptoms in functional dyspepsia. Journal of Gastroenterology. 44 (1), 66-70 (2009).
- Liu, Y., Cai, C., Qin, X. Regulation of gut microbiota of Astragali Radix in treating for chronic atrophic gastritis rats based on metabolomics coupled with 16S rRNA gene sequencing. Chemico-Biological Interactions. 365, 110063 (2022).
- Gai, X., et al. Heptadecanoic acid and pentadecanoic acid crosstalk with fecal-derived gut microbiota are potential non-invasive biomarkers for chronic atrophic gastritis. Frontiers in Cellular and Infection Microbiology. 12, 1064737 (2023).
- Sgambato, D., et al. Gut microbiota and gastric disease. Minerva Gastroenterologica e Dietologica. 63 (4), 345-354 (2017).
- Li, J., et al. Pharmacogenetic study of Desmodium caudatum. Anais Da Academia Brasileira De Ciencias. 91 (2), e20180637 (2019).
- Xu, Q. N., et al. Phenolic glycosides and flavonoids with antioxidant and anticancer activities from Desmodium caudatum. Natural Product Research. 35 (22), 4534-4541 (2021).
- Li, W., et al. Anti-inflammatory and antioxidant activities of phenolic compounds from Desmodium caudatum leaves and stems. Archives of Pharmacal Research. 37 (6), 721-727 (2014).
- Shao, X. H., Wang, J. G. Establishment of chronic atrophic gastritis in a rat model. Journal of Zhangjiakou Medical Collage. 19 (2), 11-13 (2002).
- Yu, C., et al. Dysbiosis of gut microbiota is associated with gastric carcinogenesis in rats. Biomedicine & Pharmacotherapy. 126, 110036 (2020).
- Chen, R., et al. Fecal metabolomics combined with 16S rRNA gene sequencing to analyze the changes of gut microbiota in rats with kidney-yang deficiency syndrome and the intervention effect of You-gui pill. Journal of Ethnopharmacology. 224, 112139 (2019).
- Li, Q., et al. Magnetic anchoring and guidance-assisted endoscopic irreversible electroporation for gastric mucosal ablation: a preclinical study in canine model. Surgical Endoscopy. 35 (10), 5665-5674 (2021).
- Xu, H., et al. Therapeutic assessment of fractions of Gastrodiae Rhizoma on chronic atrophic gastritis by 1H NMR-based metabolomics. Journal of Ethnopharmacology. 254, 112403 (2020).
- Zhang, B. N., et al. Effects of Atractylodes lancea extracts on intestinal flora and serum metabolites in mice with intestinal dysbacteriosis. Proteome Science. 21 (1), 5 (2023).
- Tian, H., et al. The therapeutic effects of Magnolia officinalis extraction on an antibiotics-induced intestinal dysbacteriosis in mice. Current Microbiology. 77 (9), 2413-2421 (2020).
- Wang, J., et al. Tumor and microecology committee of China anti-cancer association. Chinese expert consensus on intestinal microecology and management of digestive tract complications related to tumor treatment (version 2022). Journal of Cancer Research and Therapeutics. 18 (7), 1835-1844 (2022).
- Xu, W., Xu, L., Xu, C. Relationship between Helicobacter pylori infection and gastrointestinal microecology. Frontiers in Cellular and Infection Microbiology. 12, 938608 (2022).
- Johnson, J. S., et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nature Communications. 10 (1), 5029 (2019).
- Callahan, B. J., et al. High-throughput amplicon sequencing of the full-length 16S rRNA gene with single-nucleotide resolution. Nucleic Acids Research. 47 (18), e103 (2019).
- Langille, M. G., et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology. 31 (9), 814-821 (2013).
- Huang, D., et al. Characteristics of intestinal flora in patients with gastric cancer based on high throughput sequencing technology. Chinese Journal of Clinical Research. 35 (3), 303-306 (2022).
- Shi, Y., Luo, J., Narbad, A., Chen, Q. Advances in lactobacillus restoration for β-lactam antibiotic-induced dysbiosis: a system review in intestinal microbiota and immune homeostasis. Microorganisms. 11 (1), 179 (2023).
- Grigor’eva, I. N. Gallstone disease, obesity and the Firmicutes/Bacteroidetes ratio as a possible biomarker of gut dysbiosis. Journal of Personalized Medicine. 11 (1), 13 (2020).
- Thorne, G. M. Diagnosis of infectious diarrheal diseases. Infectious Disease Clinics of North America. 2 (3), 747-748 (1988).
- Banks, M., et al. British society of gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 68 (9), 1545-1575 (2019).
- Roy-Lachapelle, A., et al. Evaluation of ELISA-based method for total anabaenopeptins determination and comparative analysis with on-line SPE-UHPLC-HRMS in freshwater cyanobacterial blooms. Talanta. 223, 121802 (2021).
- Shahi, S. K., et al. Microbiota analysis using two-step PCR and next-generation 16S rRNA gene sequencing. Journal of Visualized Experiments. (152), e59980 (2019).
- Huang, R., et al. Blocking-free ELISA using a gold nanoparticle layer coated commercial microwell plate. Sensors (Basel). 18 (10), 3537 (2018).

