In Vivo Two-Photon Microscopy of Single Nerve Endings in Skin
Summary
The protocol for dynamic longitudinal imaging and selective laser lesion of nerve endings in reporter transgenic mice is presented.
Abstract
Nerve endings in skin are involved in physiological processes such as sensing1 as well as in pathological processes such as neuropathic pain2. Their close-to-surface positioning facilitates microscopic imaging of skin nerve endings in living intact animal. Using multiphoton microscopy, it is possible to obtain fine images overcoming the problem of strong light scattering of the skin tissue. Reporter transgenic mice that express EYFP under the control of Thy-1 promoter in neurons (including periphery sensory neurons) are well suited for the longitudinal studies of individual nerve endings over extended periods of time up to several months or even life-long. Furthermore, using the same femtosecond laser as for the imaging, it is possible to produce highly selective lesions of nerve fibers for the studies of the nerve fiber restructuring. Here, we present a simple and reliable protocol for longitudinal multiphoton in vivo imaging and laser-based microsurgery on mouse skin nerve endings.
Introduction
Cutaneous nerve endings undergo dynamical changes under different pathophysiological states. Nerve fibers can go through the process of degeneration and regeneration or restructuring in the course of such diseases as peripheral neuropathy2 or Morton’s neuroma3. After traumatic injury, an important part of nerve endings dynamics in skin is reinnervation of the damaged area. However, the common approach for investigation of nerve endings is ex vivo histological sectioning that lacks real-time information on the ongoing processes4. Using genetically encoded fluorescent markers, it is possible to track the nerve endings in skin of live animals, thus obtaining rich and significantly more relevant information on the structural changes. The investigation of cutaneous nerve endings is possible using conventional fluorescent microscopy, however, the strong light scattering of the skin tissue strongly undermines the quality of the data acquired5. Multiphoton microscopy allows acquisition of high-resolution images in the strongly scattering tissues due to the non-linear summation of energy of excitation light photons resulting in emission of fluorescence only from the focal point of the objective. This effect leads to a robust increase in the penetration depth and improvement of signal-to-noise ratio for the measurement in skin tissues6. Using the same laser as for imaging, it is possible to produce selective dissection of the nerve fibers7. In the following protocol we show the method of longitudinal imaging of cutaneous nerve endings in vivo in reporter transgenic mice combined with selective laser lesion using commercially available multiphoton microscope system.
Protocol
Procedures involving animal subjects have been approved by the National Animal Experiment Board, Finland.
1. Animal Preparation for Imaging
- Anesthetize a mouse by intraperitional (IP) injection of ketamine (0.08 mg per body weight) and xylazine (0.01 mg per body weight). Check the anesthesia with the rear toe pinch reflex.
- Immerse animal’s eyes in eye drops (Viscotears) to protect the eyes from dehydration.
- Put the mouse on a heating pad (Supertech) at 37 °C to prevent hypothermia.
- Clean the foot pad designated for imaging with 70% ethanol.
- Add a drop of water on the skin of the foot pad for the immersion coupling between skin and cover glass.
- Put plastic packaging material under the hind paw that is positioned under a metal ring with cover class.
- Adjust the thickness of the plastic packaging material to flatten the skin.
- Put a drop of water on the cover glass for immersion between cover glass and the objective.
2. Metal Fixator Ring Preparation
- For the stabilization of the skin utilize the metal fixator. We use Community Design-protected two-wing fixator with a metal ring (courtesy of Neurotar Ltd, Finland). Fill the syringe with superglue, put the small drop of superglue through the needle on the ring and gently spread the glue for uniform coverage of the ring surface. It is critical to put the minimal amount of glue that is needed only for uniform coverage, because excessive amount of glue may reduce the field of the view and obstruct subsequent imaging.
NOTE: Alternatively the combination of a metal bar (from beneath) and standard microscope glass slide (as a cover) could be used to flatten the skin and to ensure proper optical coupling of the assembly14. Two paper clips could be used to fix the paw between glass and metal bar surface (Figure 4). - Cover the ring with microscope cover slide (5 mm diameter, Electron Microscopy Science).
- Screw on the ring fixator to the rigid metal bar that is montaged on the motorized microscope stage.
3. Imaging Procedure
- In case of using thy1-YFP-H mice, choose the blue part of fluorescence lamp spectra to visualize nerve fibers. Find the nerve of interest in epifluorescence mode and focus on it.
- Write down the coordinates of a spot for longitudinal imaging. Use a carpal pad as a reference point. During the following imaging sessions, detect first the carpal pad and then find the same upstream big nerve fibers and go back using the saved coordinates. It is essential to position the foot pad in the same direction perpendicular to the metal bar to keep the same system of reference.
- Turn on the two-photon mode. For the thy1-YFP-H mice, it is suitable to use the 950 nm wavelength of laser radiation to visualize the nerve fibers.
- Select laser wavelength and emission channels (from 450 to 480 nm for second harmonic generation detection, from 520 to 550 nm for imaging of YFP-labeled nerves and from 580 to 630 nm for detection of YFP fluorescence and autofluorescence of the hair), start with low laser power (3-5%) to prevent photodamage and bleaching. The typical voltage on the photomultiplier tubes is 600-700 V for this kind of imaging.
- Set the desired resolution (512 x 512 or 640 x 640 for fast events measurements, 800 x 800 or 1,024 x 1,024 for morphological imaging), axial step (1-3 µm), thickness of the sample and the time intervals (1-10 min). The exposure time is in the order of 1 msec per one pixel.
- First mark the upper and lower boundaries of the imaging volume in the software.
- Acquire the reference image stack for the reference before the lesion. When needed, it is possible to record the baseline during several minutes.
- Repeatedly (at least every 10-15 min) check animal breathing rate and reflexes, when needed apply an additional amount of anesthetics.
- After imaging clean the pad with a tissue and put the mouse in a recovery box at 36 °C until it is fully awake.
NOTE: Usually, the duration of one imaging session combined with laser induced lesion (see below) does not exceed 1 hr. We recommend verifying that the animal is deeply anaesthetized every 10-15 min; this should not interfere with imaging itself but should be considered while experimental procedure is planned. The duration of the effect of a single ketamine/xylazyne dose is around 30 to 40 min, thus we recommend to apply additional ¼ to ½ dose after 25-30 min.
NOTE: It should be considered as well when the experimentation is planning that the frequency of imaging sessions for an individual animal should not exceed 1 session per 2 days to avoid adverse effects of repetitive anesthesia.
4. Laser Lesion
- After acquiring the reference image stack, use the bleaching protocol to make a micro lesion.
- Increase the laser power to 100%.
- Increase the exposure time to 100-1,000 msec per region of interest (100-1,000x more than in case of standard imaging).
- Outline the area for the lesion.
- Make the lesion by switching on the bleaching.
- Switch back to the regular imaging mode and continue with time-lapse recordings.
5. Data Processing and Analysis
- Proceed with unmixing using Spectral Unmixing plugin in ImageJ8 (Joachim Walter, http://rsbweb.nih.gov/ij/plugins/spectral-unmixing.html).
- Open the image stack and split the channels.
- Find the area free of the nerves or hair for the background measurements. Put a region of interest in that area.
- Outline carefully the part of the hair where it is clearly distinguishable from the nerves.
- Outline the nerve in the part of the image where it is separate from the hair.
- Save unmixing matrix.
- Apply measured unmixing matrix for the whole stack.
- Save the new processed image stacks in *.tif format.
Representative Results
Using the described technique it is possible to track the same fiber after the lesion and to study the degradation of the damaged nerve endings (Figure 1). Acquisition of the stack with the thickness of 120-150 µm is usually proper for the repetitive imaging during several days in order to keep the whole fiber in the field of view.
The lesion typically can be produced concisely when the plastic packaging material is adjusted to flatten the skin, so the collagen layers appear with uniform intensity on the image (Figure 2). The nerve endings above the collagen layer are the most suitable to produce the precise lesion.
In case the skin is not flattened, the image may occur to be blurred (Figure 3). The intensity in this situation can still be suitable for morphological investigations of the nerve fibers but the laser lesion procedure would be hindered.
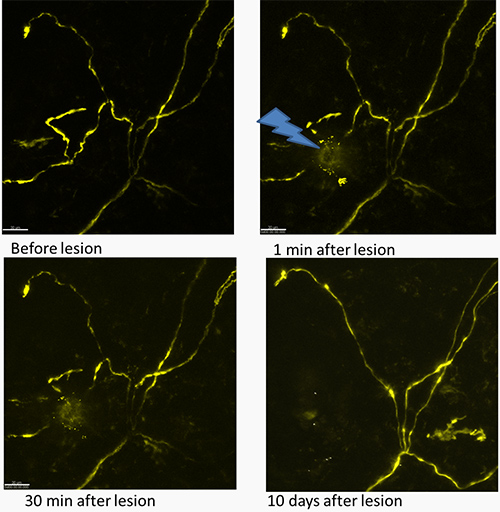
Figure 1. Time track of the effect of laser lesion on the nerve fiber presented as maximum projection images. The upper images show the fiber before and directly after the lesion, the lower panel shows the same fiber after 30 min and 10 days (left and right panels, correspondingly). There are some YFP expressing structures of non-neuronal nature that appear 10 days after lesion. These structures are likely to be keratinocytes that are known to express Thy1 gene transiently under traumatic conditions. The YFP fluorescence of nerve fibers is shown in yellow. An arrow marks the region of the lesion. Scale bar 30 µm.
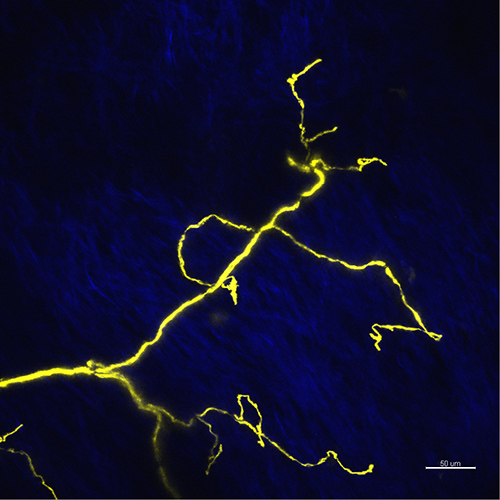
Figure 2. Maximum projection image for nerve endings in flattened skin area. The YFP fluorescence of nerve fibers is shown in yellow, the second harmonic generation of the collagen is shown in blue. Scale bar 50 µm.
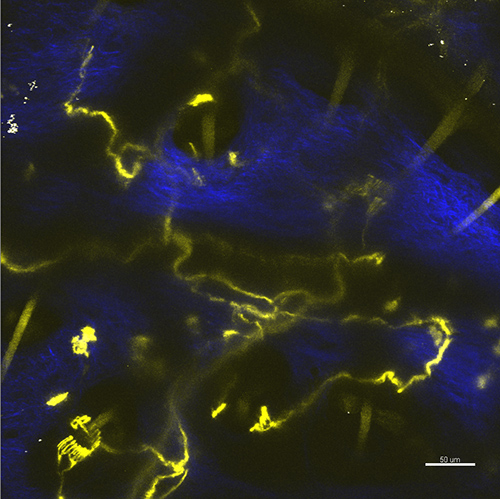
Figure 3. The nerve endings in the curved area of the skin obtained as a maximum z-projection for multiple optical sections. The YFP fluorescence of nerve fibers and autofluorescence of the hair is shown in yellow, the second harmonic generation of the collagen is shown in blue. Scale bar 50 µm.
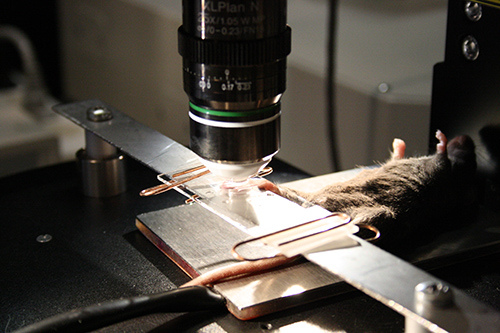
Figure 4. The paw fixation procedure using the microscope glass slide as a cover. The slide is attached to the metal bar using standard paper clips.
Discussion
In this video protocol we demonstrate the method for non-invasive longitudinal two-photon imaging of single nerve endings.
The dynamics of skin innervations is affected in such diseases as psoriasis and peripheral neuropathy2, and in traumatic injuries9. Two-photon imaging allows detailed analysis of the nerve fibers structures in collagen matrix. The use of transgenic reporter mice helps to avoid the problems concerning the staining of the nerve fibers. Thy1-YFP-H strain seems to be sufficiently robust for morphological data analysis10 whereas the Thy1-mitoCFP mice may provide an opportunity of functional studies of mitochondrial dynamics in the nerve fibers11. The YFP-16/ICR line can be used for the observation of Meissner bodies12. The alternative approach may be the viral injection into dorsal root ganglion for the selective tracking of specific neuronal populations13.
It is worth noticing that the production of selective lesion within the collagen matrix is strongly hindered in comparison to the upper layers of skin. That is why sometimes exposure times for the dissection of nerve fibers can differ as much as tenfold while the intensity of the fluorescence for the imaging differs on the range of 10-20%. To maintain good quality of the image the constant immersion coupling has to be kept.
The resolution of the image usually depends on the desirable speed of acquisition. Typical time for production of a 30 frames- stack with resolution 800 x 800 pixels is 1 min, so one can decrease the resolution or thickness of imaged area to detect fast changes or increase resolution for fine morphological features studies.
Fixation of the footpad should be tight enough not to allow the drift of the image, but at the same time it should not affect the blood circulation in the skin. For optimization of the procedure, injections of blood vessels tracers (e.g., TexasRed labeled 70 kDa dextran) are possible, allowing one to adjust the plastic packaging material pressure via monitoring the blood flow. Motion artifacts in the preparation described in this protocol are unlikely because the animal is deeply anaesthetized, the paw itself is firmly attached to the fixation assembly, and because heartbeat and breathing movement are not directly transmitted to the paw. However, slight movements are possible especially if high magnification imaging is performed. In this case we can recommend using ImageJ plugins designed to compensate motion artifacts.
The repetitive imaging of the same area may be achieved by using such reference points as carpal pad14, or in case of injection of some substances in the footpad the spot of injection can serve as a landmark6. The other possibility is tattooing of the skin.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Neurotar Ltd. for technical assistance, CIMO Foundation and FGSN for financial support.
Materials
| Round cover glass | Electron Microscopy Science | 72296-05 | 5 mm diameter #1.5 thickness |
| Eye drops Viscotears | Novartis | 2mg/g | |
| Superglue Loctite 401 | Henkel | 135429 | |
| Ketaminol | Intervet | Ketamine 50 mg/ml, working solution 10 mg/ml (use it 80 µg per gramm of animal weight) | |
| Rompun | Bayer Healthcare | Xylazine 20 mg/ml, working solution 1.25 mg/ml (use it 10 µg per gramm of animal weight) | |
| Working solution is a mixture of Ketamine 10 mg/ml and Xylazine 1.25 mg/ml in PBS | |||
| Ethanol 70% | |||
| Distilled water or Milli-Q water | |||
| Syringes 1ml | BD | 300013 | |
| 30G 1/2" needles | BD | 304000 | |
| Plastic packaging material | Could be purchaized from general hardware store | ||
| FV-1000MPE microscope | Olympus | FV-1000MPE | Microscope with motorized stage and rigid metal bar for fixation |
| 25X XLPlan objective | Olympus | XLPLN 25XWMP | Water imersion objective optimized for multiphoton imaging |
| Mai-Tai DeepSee laser (2W) | SpectraPhysics | ||
| Heating pad | Supertech | TMP-5b | Heating pad with a temperature controller |
| Metal ring fixator | Neurotar Ltd. | ||
| ImageJ | NIH | Open source software for image processing and analysis, http://rsbweb.nih.gov/ij/ | |
| Thy1-YFPH mice strain | JaxLab | 003782 |
References
- Lumpkin, E. A., Caterina, M. J. Mechanisms of sensory transduction in the skin. Nature. 445, 858-865 (2007).
- Kennedy, W. R., Wendelschafer-Crabb, G., Johnson, T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology. 47, 1042-1048 (1996).
- Wu, K. K. Morton’s interdigital neuroma: a clinical review of its etiology, treatment, and results. J. Foot Ankle Surg. 35, 112-119 (1996).
- Lauria, G., Lombardi, R. Skin biopsy: a new tool for diagnosing peripheral neuropathy. BMJ. 334, 1159-1162 (2007).
- Cheng, C., Guo, G. F., Martinez, J. A., Singh, V., Zochodne, D. W. Dynamic plasticity of axons within a cutaneous milieu. J. Neurosci. 30, 14735-14744 (2010).
- Wang, B., Zinselmeyer, B. H., McDole, J. R., Gieselman, P. A., Miller, M. J. Non-invasive Imaging of Leukocyte Homing and Migration in vivo. J Vis Exp. (46), e2062 (2010).
- Sacconi, L., O’Connor, R. P., Jasaitis, A., Masi, A., Buffelli, M., Pavone, F. S. In vivo multiphoton nanosurgery of cortical neurons. J Biomed Opt. 12, 050502 (2007).
- Robinson, L. R. Traumatic injury to peripheral nerves. Muscle Nerve. 23, 863-873 (2000).
- Feng, G., Mellor, R. H., Bernstein, M., Keller-Peck, C., Nguyen, Q. T., Wallace, M., Nerbonne, J. M., Lichtman, J. W., Sanes, J. R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 28, 41-51 (2000).
- Marinkovic, P., Reuter, M. S., Brill, M. S., Godinho, L., Kerschensteiner, M., Misgeld, T. Axonal transport deficits and degeneration can evolve independently in mouse models of amyotrophic lateral sclerosis. Proc Natl Acad Sci U.S.A. 109, 4296-4301 (2012).
- Amit, S., Yaron, A. Novel systems for in vivo monitoring and microenvironmental investigations of diabetic neuropathy in a murine model. J Neural Transm. 119, 1317-1325 (2012).
- Yu, H., Fischer, G., Jia, G., Reiser, J., Park, F., Hogan, Q. H. Lentiviral gene transfer into the dorsal root ganglion of adult rats. Mol Pain. 7, 63 (2011).
- Yuryev, M., Khiroug, L. Dynamic longitudinal investigation of individual nerve endings in the skin of anesthetized mice using in vivo two-photon microscopy. J Biomed Opt. 17, 046007 (2012).

