一种快速探测激光方法有利于叶热性能的非侵入性和无接触测定
Summary
A method was developed to determine the specific heat capacity and thermal conductivity of leaf tissue by non-invasive, contact-free near infrared laser probing, which requires less than 1 min per sample.
Abstract
植物可以产生有价值的物质如次级代谢产物和重组蛋白。从植物生物质后者的纯化可以通过热处理(热烫)精简。一个热烫装置可以更精确地设计,如果叶子的热性质详细地说, 即 ,比热容和热导率是已知的。这些特性的测量是耗时且劳动密集型的,并且通常需要将样品直接接触侵入性的方法。这样可以减少产品的产量和可能是遏制需求, 如不兼容,在良好生产规范的范围内。为了解决这些问题,一种非侵入性,无接触方法的开发,用于确定在一分钟左右的比热容和完整植物叶的热导率。该方法涉及定义的长度和强度的短激光脉冲的到的一个小区域的应用程序叶样品,引起正在使用近红外传感器测量的温度上升。的温度上升与已知的叶性质(厚度和密度)相结合,以确定比热容量。然后热导率是根据随后的温度下降的信息计算出的,以热辐射和对流热传递考虑。相关的计算和样品处理关键方面进行了讨论。
Introduction
生物材料的大规模加工通常需要的热处理步骤,例如巴氏杀菌。对于这样的方法的设备可以更精确地设计,如果生物材料的热性能是公特征在于,包括特定的热容量(C P,S)和热导率(λ)。这些参数可以容易地为液体,悬浮液和匀浆由量热法1来确定。然而,固体样品中测量这种参数可以是劳动密集型的,并且经常需要与样品或者甚至它的破坏2直接接触。例如,光热技术需要在样品和检测器3之间的直接接触。这种限制是食品加工过程中可以接受的,但是是高度调节的过程不相容如在良好生产规范4的上下文中在植物中生产的生物制药蛋白质。一世n个这样的背景下,可在七周的生长期为植物个体作为一种质量控制工具所需热性能的重复( 例如,每周)的监测。如果这样的监测需要和消耗对于每个测量一个叶,就没有生物质留在收获时间来处理。
此外,仅使用叶份代替会导致伤害的植物和增加坏死或病原体感染的风险,又减少了工艺产率。病原体感染的可能性也会增加,如果将用于直接接触到样品的方法,诱导该植物的整个批次可以通过与污染的传感器装置接触被感染的危险。类似方面必须考虑对植物监测强调像干旱, 例如 ,在一个生理生态环境。例如,失水往往是由在鲜生物量的改变,这需要一种侵入特雷监视正在调查5, 如植物,解剖叶的atment。相反,在确定比热容量,这取决于样品的水含量,以非侵入性方式在这里描述,可以用作植物的水合状态的替代参数。在这两种情况下(药品生产和生态生理学),通过破坏或侵入性的测量技术人工诱导应力将是有害的,因为他们可以扭曲的实验数据。因此,先前报道闪光方法6或样品的银板7之间的位置不适合于这样的工艺和实验,因为它们要么需要直接接触到样品或者是破坏性的。所述参数c P,S和λ必须以设计的工艺设备用于预煮步骤,可以简化产物纯化,从而降低制造成本8-10来确定。使用CP,S和λ现在可以通过无接触的非破坏性近红外(NIR)激光以一致和可再现的方式11探测被快速确定,并且这种新的方法将在下面进行详细说明。用这种方法得到的结果进行了成功地用于模拟传热在烟叶12,允许适当的处理设备的设计和相应的参数,如热烫温度的选择。
该方法是容易设置( 图1),并具有两个阶段,测量和分析,其每一个包括两个主要步骤。在测量阶段,叶子样品首先在本地通过短激光脉冲加热和最大采样温度被记录下来。然后将样品的温度分布被记录为50秒的持续时间。在分析阶段,叶属性,如密度(轻松,准确地通过比重瓶measurem确定耳鼻喉科)与最大样本温度相结合来计算中 c p,第在第二步骤中,叶的温度分布被用作输入一个能量平衡方程,以传导,对流和辐射进去,来计算λ。
在协议部分提供了详细的一步一步的指导,扩大对影随行的内容。典型的测量,然后在结果部分中。最后,该方法的优点和限制在讨论部分与潜在的改进和进一步的应用沿着突出。
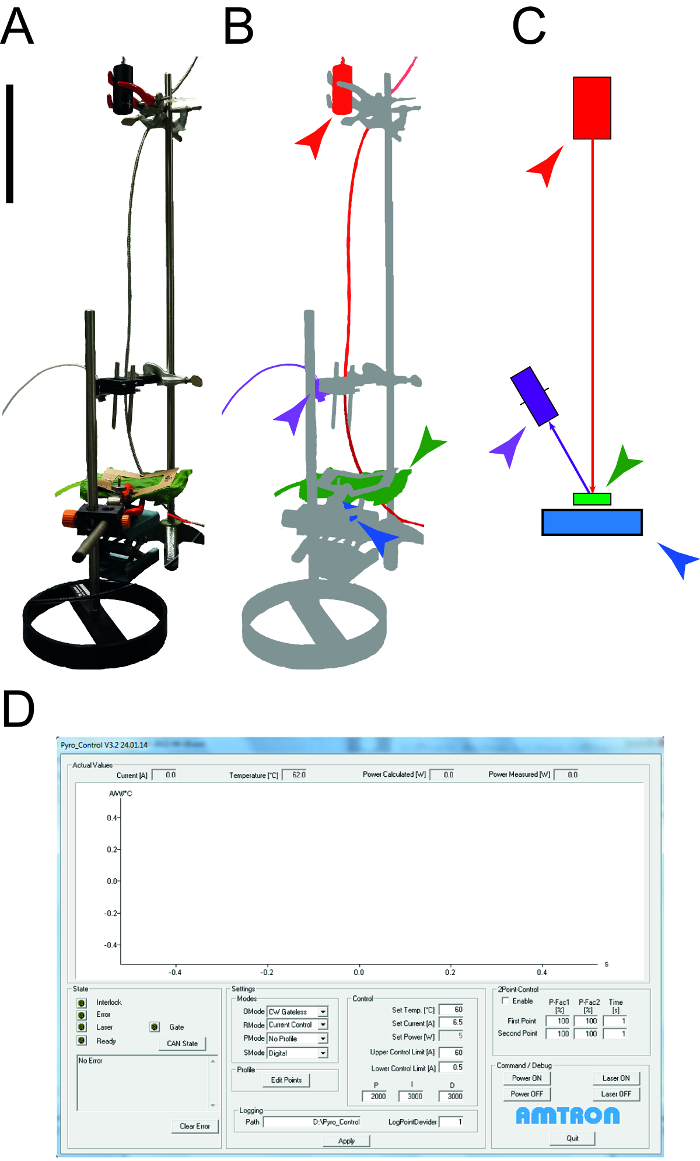
图1:用于确定叶热性能的装置。 一 。所述测量装置的照片来确定比热容量和乐的热导率鸟类。外围设备(计算机,示波器)未示出。 B点 。的测定装置的示意图。激光器和连接的设备以红色突出显示,用于温度测量的近红外检测器示于紫,叶样品是绿色和光电二极管功率传感器是蓝色的。 ℃。绘制测量设置具有相同的颜色代码作为B的元件的尺寸条表示0.1米。 ð。屏幕截图示出激光控制软件的典型元素。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
上述的非接触式的,非破坏性测量方法可用于确定以同时和再现的方式中 c p,S和ʎ。特别ʎ的计算取决于几个参数是错误敏感。尽管如此,这些错误的影响是线性或分比例,并为所有参数变化系数被认为是少于10%。即使该方法可因此被认为是坚固的,一些技术改进可制成以减少错误的剩余源。
安装样品放入组件在技术上是挑战性的,因为扁平叶子表?…
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors are grateful to Dr. Thomas Rademacher and Ibrahim Al Amedi for cultivating the plants used in this study. We would like to thank Dr. Richard M. Twyman for his assistance with editing the manuscript. This work was in part funded by the European Research Council Advanced Grant “Future-Pharma”, proposal number 269110, the Fraunhofer Zukunftsstiftung (Future Foundation), the Fraunhofer-Gesellschaft Internal Programs under Grant No. Attract 125-600164.
Materials
| 1" tube | Thorlabs | SM1L10E | Tube for fiber holder |
| Agarose | Sigma Aldrich | A0701 | Agarose |
| Bi-Convex lense f=25.4 | Thorlabs | LB1761 | Lense |
| Digital Handheld Optical Power and Energy Meter Console | Thorlabs | PM100D | Console for thermal surface absorber sensor |
| Digital Phosphor Oscilloscope | Tektronix | DPO7104 | Oscilloscope |
| DMR light microscope | Leica | n.a. | Light microscope |
| Falcon 50mL Conical Centrifuge Tubes | Fisher Scientific | 14-432-2 | Pycnometer |
| Ferty 2 Mega | Kammlott | 5.220072 | Fertilizer |
| Fiber holder | Thorlabs | Fiber holder | |
| Forma -86C ULT freezer | ThermoFisher | 88400 | Freezer |
| Greenhouse | n.a. | n.a. | For plant cultivation |
| Grodan Rockwool Cubes 10x10cm | Grodan | 102446 | Rockwool block |
| Infrared Detector Optris CT | Optris | OPTCTLT15 | Infrared detector |
| Infrared Detector Software Compact Connect | Optris | n.a. | Control software for infrared detector |
| Lambda 1050 UV/Vis spectrophotometer | PerkinElmer | L1050 | UV/VIS Spectrophotometer |
| Laser 400μm, 1550nm Conduction Cooled Single Bar Fiber Coupled Module | DILAS | M1F-SS2.1 | Laser |
| Laser cover | Amtron | LM200 | Laser Cover |
| Laser Driver | Amtron | CS 408 | Laser Driver |
| Osram cool white 36 W | Osram | 4930440 | Light source |
| Photodiode sensor | Thorlabs | PDA20H-EC | Power sensor for transmission measurements |
| Precision weight Ohaus Analytical Plus | Ohaus | 80251552 | Precision weight |
| Sample frame | Fraunhofer ILT | n.a. | Fixation of the leaf sample |
| Software Pyro Control | Amtron | n.a. | Laser Power Control Software |
| Stainless-steel-holder | n.a. | n.a. | Holder for measurement set-up |
| Teflon plates 2cm | Fraunhofer ILT | n.a. | Teflon attenuation |
| Thermal surface absorber Power sensor | Thorlabs | S314C | Sensor for laser power measurements |
| Vibratome | Leica | 1491200S001 | Vibratome |
| Zoc/Pro 6.51 | EmTec Innovative Software | n.a. | Laser Control Software |
References
- Wilhelm, E. . Heat Capacities: Liquids, Solutions and Vapours. , 516 (2010).
- Costa, J. M., Grant, O. M., Chaves, M. M. Thermography to explore plant-environment interactions. J. Exp. Bot. 64, 3937-3949 (2013).
- Jayalakshmy, M. S., Philip, J. Thermophysical Properties of Plant Leaves and Their Influence on the Environment Temperature. International Journal of Thermophysics. 31, 2295-2304 (2010).
- Buyel, J. F. Process development strategies in plant molecular farming. Curr. Pharm. Biotechnol. 16, 966-982 (2015).
- Schuster, A. C., et al. Effectiveness of cuticular transpiration barriers in a desert plant at controlling water loss at high temperatures. AoB PLANTS. 8, (2016).
- Parker, W. J., Jenkins, R. J., Abbott, G. L., Butler, C. P. Flash Method of Determining Thermal Diffusivity, Heat Capacity, and Thermal Conductivity. J Appl Phys. 32, 1679-1684 (1961).
- Hays, R. L. The thermal conductivity of leaves. Planta. 125, 281-287 (1975).
- Menzel, S., et al. Optimized blanching reduces the host cell protein content and substantially enhances the recovery and stability of two plant derived malaria vaccine candidates. Front. Plant Sci. , (2015).
- Buyel, J. F., Hubbuch, J., Fischer, R. Blanching intact leaves or heat precipitation in an agitated vessel or heat exchanger removes host cell proteins from tobacco extracts. J. Vis. Exp. , (2015).
- Beiss, V., et al. Heat-precipitation allows the efficient purification of a functional plant-derived malaria transmission-blocking vaccine candidate fusion protein. Biotechnol. Bioeng. 112, 1297-1305 (2015).
- Buyel, J. F., Gruchow, H. M., Tödter, N., Wehner, M. Determination of the thermal properties of leaves by non-invasive contact free laser probing. J. Biotechnol. 217, 100-108 (2016).
- Buyel, J. F. Numeric simulation can be used to predict heat transfer during the blanching of leaves and intact. Biochem. Eng. J. , (2015).
- Hedlund, H., Johansson, P. Heat capacity of birch determined by calorimetry: implications for the state of water in plants. Thermochim Acta. 349, 79-88 (2000).
- Chandrakanthi, M., Mehrotra, A. K., Hettiaratchi, J. P. A. Thermal conductivity of leaf compost used in biofilters: An experimental and theoretical investigation. Environ. Pollut. 136, 167-174 (2005).
- Larcher, W. . Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups. , (2003).
- Cowen, R. A gamma-ray burst’s enduring fireball. Science News. 152, 197 (1997).
- Jones, H. G., et al. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 36, 978-989 (2009).
- Defraeye, T., Verboven, P., Ho, Q. T., Nicolai, B. Convective heat and mass exchange predictions at leaf surfaces: Applications, methods and perspectives. Comput. Electron. Agric. 96, 180-201 (2013).
- Arndt, S. K., Irawan, A., Sanders, G. J. Apoplastic water fraction and rehydration techniques introduce significant errors in measurements of relative water content and osmotic potential in plant leaves. Physiol. Plant. 155, 355-368 (2015).
- Jones, H. G., Schofield, P. Thermal and other remote sensing of plant stress. General and Applied Plant Physiology. 34, 19-32 (2008).
- Jones, H. G., Archer, N., Rotenberg, E., Casa, R. Radiation measurement for plant ecophysiology. J. Exp. Bot. 54, 879-889 (2003).
- Dupont, C., Chiriac, R., Gauthier, G., Toche, F. Heat capacity measurements of various biomass types and pyrolysis residues. Fuel. 115, 644-651 (2014).
- Chaerle, L., et al. Multi-sensor plant imaging: Towards the development of a stress-catalogue. Biotechnol. J. 4, 1152-1167 (2009).
- Hackl, H., Baresel, J. P., Mistele, B., Hu, Y., Schmidhalter, U. A Comparison of Plant Temperatures as Measured by Thermal Imaging and Infrared Thermometry. J. Agron. Crop. Sci. , 415-429 (2012).
- Yuan, L., et al. Spectral analysis of winter wheat leaves for detection and differentiation of diseases and insects. Field Crops Res. 156, 199-207 (2014).

