A Hyperandrogenic Mouse Model to Study Polycystic Ovary Syndrome
Summary
We describe the development of a lean PCOS-like mouse model with dihydrotestosterone pellet to study the pathophysiology of PCOS and the offspring from these PCOS-like dams.
Abstract
Hyperandrogenemia plays a critical role in reproductive and metabolic function in females and is the hallmark of polycystic ovary syndrome. Developing a lean PCOS-like mouse model that mimics women with PCOS is clinically meaningful. In this protocol, we describe such a model. By inserting a 4 mm length of DHT (dihydrotestosterone) crystal powder pellet (total length of pellet is 8 mm), and replacing it monthly, we are able to produce a PCOS-like mouse model with serum DHT levels 2 fold higher than mice not implanted with DHT (no-DHT). We observed reproductive and metabolic dysfunction without changing body weight and body composition. While exhibiting a high degree of infertility, a small subset of these PCOS-like female mice can get pregnant and their offspring show delayed puberty and increased testosterone as adults. This PCOS-like lean mouse model is a useful tool to study the pathophysiology of PCOS and the offspring from these PCOS-like dams.
Introduction
Hyperandrogenism is the hallmark of polycystic ovary syndrome (PCOS) according to NIH criteria and of the Androgen Excess and PCOS (AE-PCOS) Society. Women with PCOS have difficulty getting pregnant and have increased risk of pregnancy complications1. Even if they get pregnant, their female offspring have an adverse health outcomes2,3. Animal models have been developed using various strategies4,5,6,7,8,9,10,11,12 and exhibiting many features of PCOS (anovulation, and or impaired glucose and insulin tolerance) with increased body weight and obesity associated with enlarged adipocyte size and increased adipocyte weight. There are two major strategies to produce animal models that are used to study PCOS. One is treatment with high levels of androgens directly (exogenous androgen injection/insertion) or indirectly (such as blocking androgen conversion to estrogen with aromatase inhibitor) after birth13. Another is by fetal hyperexposure of androgens during gestation14,15 to study the offspring. For example, female offspring from rhesus monkey16,17, sheep18, and rodents exposed to male levels of androgen during the intrauterine period develop PCOS-like traits later in life. These models significantly enhanced our understanding of elevated androgen effects, and fetal programing, and uterine environmental effects. However, these models have their own limitations: 1) animals develop obesity and it is therefore difficult to separate the effects of hyperandrogenemia from obesity induced reproductive and metabolic dysfunction; 2) before pregnancy, women with PCOS already exhibit high levels of androgen, thus oocytes have been exposed to androgen excess before fertilization; 3) the pharmacological doses of testosterone (T) or dihydrotestosterone (DHT) used after birth or during gestation may not reflect the androgen environment of PCOS. Testosterone and DHT levels have been measured in ovarian follicular fluid and/or serum, and testosterone and DHT levels are 1.5 to 3.9 fold higher in women with PCOS5,19,20,21,22,23 compared to unaffected women. We created an adult mouse model23,24,25 that develops reproductive and metabolic dysfunction within two weeks of the initiation of chronic DHT exposure from insertion of a pellet with 4mm length of crystal DHT powder (total length of pellet is 8mm). This model produces serum DHT levels that are about 2-fold higher (referred to as 2xDHT) than that of control mice without DHT treatment. The 2xDHT mice do not exhibit alterations of basal serum estradiol, testosterone, LH and do not develop obesity, and show similar ovarian weight, serum levels of cholesterol, free fatty acids, leptin, TNFα and IL-623,24,25 relative to controls even up to 3.5 months after DHT insertion23,24,25. Additionally, by mating females that have already developed features of PCOS, we can study the impact of a hyperandrogenic maternal environment on the metabolic and reproductive health of the offspring15.
This new paradigm (relevant to NIH and AE-PCOS Society criteria) models the disease by producing relatively similar levels of androgens to those of women with PCOS 2- to 3-fold higher testosterone or DHT levels compared to unaffected women. However, this model is maintained by continual exogenous DHT and not from programmed endogenous hyperandrogenism once DHT is withdrawn. The overall goal of this article is to focus on 1) how to make the DHT pellet; 2) how to generate a lean-PCOS like mouse model; 3) strategies to evaluate female offspring from these dams. Other measurements and assessment of phenotypes are not addressed in this manuscript but can be found in5,15,23,24,25,26.
Protocol
Here, we present detailed protocols for DHT pellet preparation and insertion, and for reproductive and metabolic testing. The mice used in this study were a mixed background (C57/B6, CD1, 129Sv) and were maintained with food and water ad libitum in a 14/10 h light/dark cycle at 24 °C in the Broadway Research Building animal facility at the Johns Hopkins University School of Medicine. All procedures were approved by the Johns Hopkins University Animal Care and Use Committee.
1. Create PCOS-like Mouse Model
- DHT pellet preparation
- Autoclave silastic tubing to sterilize. Cut silastic tubes to a length of 15 mm with a razor blade;
- Seal one side by injecting medical adhesive silicone into the tube with a 20 G blunted needle attached to a 3 mL syringe. Plunger should be withdrawn from the syringe and then adhesive inserted into the reservoir. The plunger is reinserted and adhesive pushed down until it begins to emerge from the needle. The blunted needle is made by cutting the sharp tip side of 20 G needle with any strong scissors. The length of silicon in the tube should be more than 2 mm to allow for post-production trimming;
- Dry overnight; check for air bubbles on the sealed side. Those with no air bubbles are used for DHT pellets. Others can be used for no-DHT control pellets.
- Wear gloves, mask, goggles and lab coat before making DHT-pellet in a hood to avoid DHT exposure to skin.
- Pour DHT powder into a plastic weigh boat and press the open side of the adhesive capped tubes (produced in A1.1) into the DHT powder.
- DHT powder can be tamped down with a large paper clip that is straightened. Continue until DHT reaches a height of 4 mm (or desired length). Check the length of DHT with a ruler, and seal the open side with silicon, dry overnight.
- Cut each sealed side to make the length of silicon 2 mm long. The total length of pellet will be 8 mm.
- Seal both side of empty silastic tubing as control (no-DHT) pellet.
- Keep pellets in a 50 mL conical tube wrapped with foil (to prevent light exposure) at room temperature until use. The DHT pellets maintain full efficacy for at least 3 months of storage.
- DHT insertion and replacement
- Up to 20 DHT pellets or control pellets are submerged separately in a 50 mL conical tube with 30 mL sterile 0.9% saline for 24 h at 37 °C for equilibration just before insertion.
- Prior to surgery, the surfaces and gloves are disinfected with clidox and the work surfaces are covered with clean plastic backed absorbent paper (surgical pad). All instruments that come in contact with animals are decontaminated prior to entering the animal facility by autoclaving. Instruments will be allowed to dry and cool prior to use.
- 2-month-old female mice are used (4–5 mice/cage). Mice are injected intraperitoneally with Xylazine (3.5 mg/kg bw) and Ketamine (78.8 mg/kg bw) using an insulin syringe. Calculations for mixing and dosing anesthesia is in Table 1.
- After adequate anesthesia is achieved, as measured by loss of toe reflex and slowed breathing, the mouse will be prepped for surgery.
- Disinfect the skin of the area with betadine using sterile gauze and clean with 70% ethanol. The area will again be painted with betadine.
- Cut a hole around 5 mm length with scissors under the skin near the neck. Using a 10 G trochar, make a small tunnel (15 mm) in the rostral direction. The pellet is inserted dorsally with the trochar.
- The opening is then sealed with surgical adhesive. Manually approximate the wound edges with forceps and gentle brushing strokes to apply a thin film of liquid adhesive to the approximated wound edges. Buildup 3 thin layers of adhesive to ensure the adhesive is evenly distributed over the wound. The adhesive should extend 1 cm on each side of the apposed wound edges. Suture or surgical clips can also be used to close the hole. Put mice back into cage, individually housed, with a heat pad for recovery. Replace pellet every 4 weeks to maintain a constant level of androgen exposure. The original pellet will be removed and new pellet will be inserted as described in A1.15 in the similar position.
- Test estrous cyclicity after 3 days of DHT insertion. Estrous cycle stage is assessed by vaginal cytology. Vaginal cells are collected for 16 consecutive days by using a p10 pipettor to squirt sterile saline (around 10 µL) into the vaginal cavity and then withdrawing the saline with the same sterile pipette tip. Vaginal cells sloughed from vaginal wall mix with saline and are collected.
- Spread saline with cells onto a labeled slide. Each slide can contain six samples. Slides are labeled to note which sample is placed in each of the six locations.
- After the saline has completely dried out on the slide, put slides into a container with 100% ethanol to fix cells. Slides should be fixed for at least 5 min, but can remain in fixative indefinitely until further use.
- Put slides into staining solutions for 1 min each for buffer B and C. Wash slides with tap water and dry at room temperature.
- Examine cell morphology under a 10X objective light microscope. Cell morphology to distinguish proestrus (P), estrus (E), metestrus (M) and diestrus (D) is described in references27,28,29.
- Count the days in each stage and divided by the total number of days to calculate percent time at each stage.
- Test glucose tolerance at 21 days after DHT insertion by fasting the mice overnight (16 h) and injecting 2 g/kg body weight of 20% dextrose intraperitoneally. For example, if a mouse body weight is 25 g, this mouse will be injected with 250 µL of 20% dextrose with a 50 cc (0.5 mL) insulin syringe. Glucose levels are assessed by glucometer and test strips by sampling blood from tail vein at 0, 15, 30, 60, 90, 120 min. This is described in detail elsewhere30.
- Blood collection
- Collect blood at various days after DHT insertion (we limit collection volume to 100 µL for steroid and 30ul for LH/FSH assay) between 9 and 10 am by submandibular vein bleeding with lancet. This is described in11.
- Centrifuge blood at 6,000 x g at 4 ˚C for 10 min, and collect the serum layer into another 1.5 mL tube that can be stored at -80 °C until assay.
2. Assess Reproductive Profiles of Female Offspring from Chronically DHT Inserted Dams
- Mating
- The female mouse to be tested is moved into the cage with a proven fertile male (that has previously had pups with a female mouse) at 15 days following pellet insertion.
- Document dam’s body weight every week to determine if the mouse is pregnant. Dam weight increases of more than 3 g in a week indicates pregnancy.
- Collect blood of dams the second week after we observe increased body weight of dams greater than 3 g from the week before.
- Puberty assessed by vaginal opening and first estrus
- Check vaginal opening by visual inspection every day after pups are weaned at 21 post-natal day (PND), measure the anogenital distance and record the age of vaginal opening.
- Once vagina opens, collect vaginal cells daily, as described in 1.2.8–1.2.11 to check estrous cyclicity.
- Observe cell morphology as described 1.2.12, and the first estrus is defined as the date that all cells are cornified epithelial cells.
- Body weight and ear punch
- Dip the lancet tip into the tattoo paste and mark each pup with tattoo on the toe as described in reference12 at 7 PND, and weigh mice every 7 days until 70days of age.
- Ear punch mice to number them using system in Figure 1 between 12 to 16 PND. To distinguish sex, examine ventral side of body, females will have visible nipples.
- Collect blood at 21, 26, 70 PND after birth as described above in 1.3.1
- Hormonal Assay
- DHT levels in blood serum are measured by both enzyme-linked immunosorbent assay (ELISA) and by liquid chromatography tandem mass spectrometry (LC-MS)23,24,31,32. T is measured by LC-MS, or a rat/mouse ELISA that has been validated by the University of Virginia Ligand Assay Core in the Center for Research in Reproduction23,24.
Representative Results
Serum DHT levels and Glucose tolerance test
DHT levels were measured from collected serum by both ELISA and by LC-MS according to protocol 1.24–1.25, and 2.9, 3.0. The DHT absolute values are different between mass spectrometry and ELISA, however, the relative fold (around 2-fold) of DHT vs no-DHT insertion is similar from both assays and across experiments15,23,24 (Figure 2A). DHT levels are significantly increased from preconception through pregnancy in both DHT and no-DHT mice, however, DHT levels are 2-fold higher in the DHT mice compared to the no-DHT mice at both pregestational (one day before mating) and gestational (around 14 days) time points (Figure 2B). Since absolute levels of DHT differ between ELISA and LC-MS, we calculate relative levels (fold change: DHT level with DHT insertion vs no-DHT insertion) within assays. Female mice with DHT showed impaired glucose tolerance relative to no-DHT (Figure 3) at 2 weeks following DHT insertion according to protocol 1.23.
Female offspring body weight and puberty
While generally infertile, some dams get pregnant and have pups (around 30% pregnancy rate, so testing fertility of 10 dams we will usually get 3 pregnant dams, refer to protocol 2.1). We therefore can evaluate chronic androgen effects in dams on the female offspring. Female offspring from DHT inserted dams are called DHT-daughters, and those from no-DHT inserted dams are called no-DHT daughters. We observed no difference between no-DHT and DHT-daughters for the age of vaginal opening. However, the first estrus of DHT-daughters is significantly delayed (day 35 for no-DHT daughter; day 42 for DHT-daughter). This is associated with reduced body weight at both 35 and 42 PND in DHT-daughters relative to no-DHT daughters (Figure 4; refer to protocol 2.4–2.7).
Hormonal levels and estrous cyclicity in female offspring
Testosterone levels are not altered at 21 PND, but are reduced at 26PND. However, testosterone is increased at 70 PND (Figure 5, refer to protocol 2.9–3.0). Adult DHT daughters showed disrupted estrous cyclicity compared to adult no-DHT daughters, experiencing significantly longer times in M/D and less time in P and E (Figure 6A, B; according to the method in protocol 1.17–1.22).
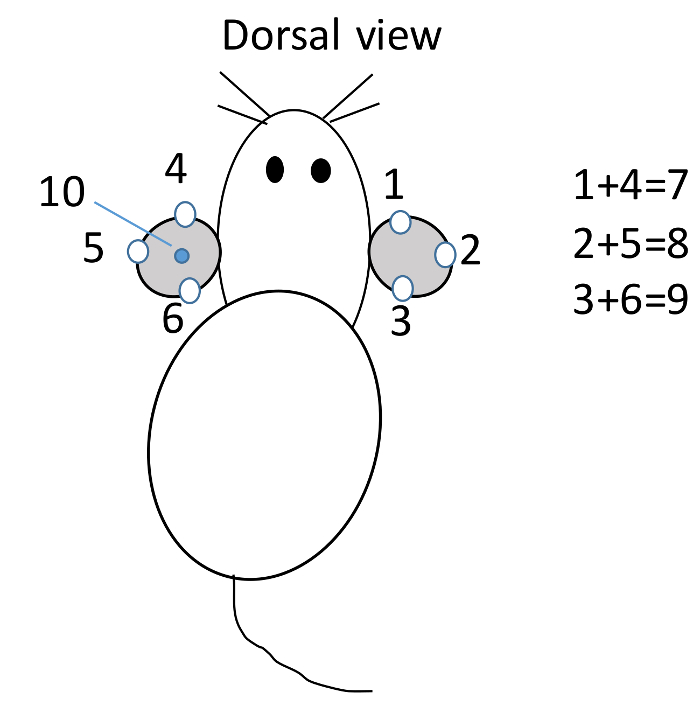
Figure 1: Mouse identification. Within one cage, mouse is ear punched at different position to represent the mouse number. Mouse #1 to 3 is punched on the right ear, and #4 to 6 is punched on the left ear viewed dorsally. If mouse is punched on ears at both #1 and #4 position, it is #7; at 2 and 5, is #8; at 3 and 6 is #9; at the middle of left ear is #10; at 1 and 10 position is #11; etc. Please click here to view a larger version of this figure.
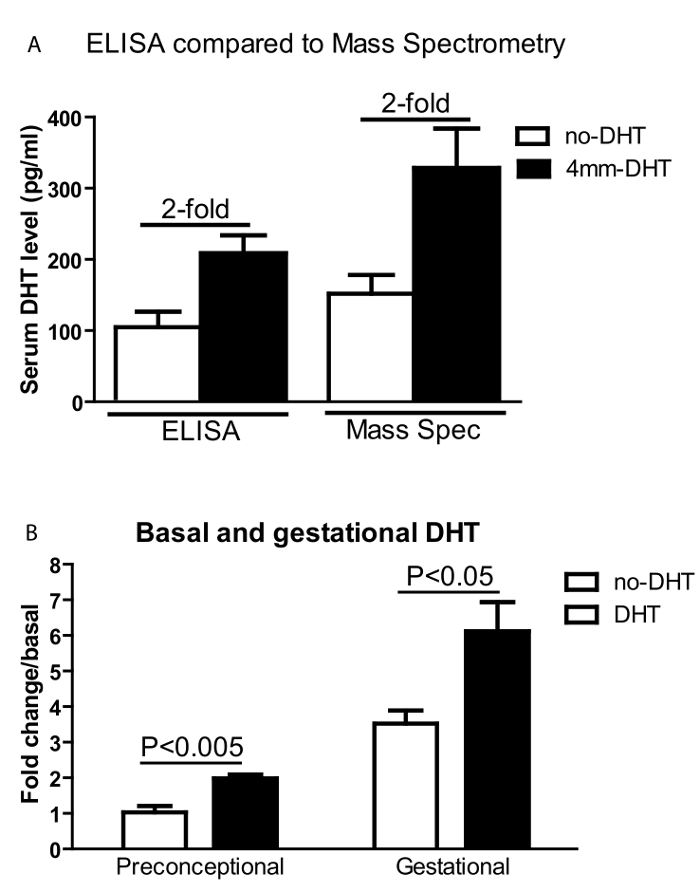
Figure 2: Serum DHT levels. Serum DHT levels. (A) Serum DHT levels are measured by both ELISA and Mass-Spectrometry. Although the absolute values are different, the two assays showed similar fold differences between DHT and no-DHT treated mice. (B) Serum DHT fold change relative to non-DHT levels at preconceptional and gestational time points. No-DHT (open bars) and DHT-implanted (black bars) female mice before (one day before mating) and during pregnancy (around 14 days of gestation). Values are mean ±S.E.M. N = 5-8 per group. Please click here to view a larger version of this figure.
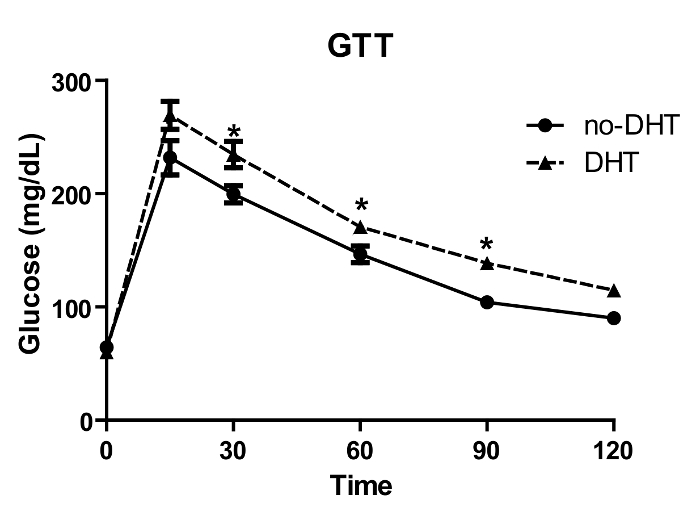
Figure 3: Glucose tolerance test (GTT). No-DHT and DHT implanted mice were fasted overnight and glucose (2 g/kg BW) was injected intraperitoneally, and tail blood glucose was measured at different time points. DHT treated mice showed significantly increased glucose levels between 30 to 120 min compared to no-DHT treated mice. Values are mean ±S.E.M. N = 4-12 per group. * P <0.05 Please click here to view a larger version of this figure.
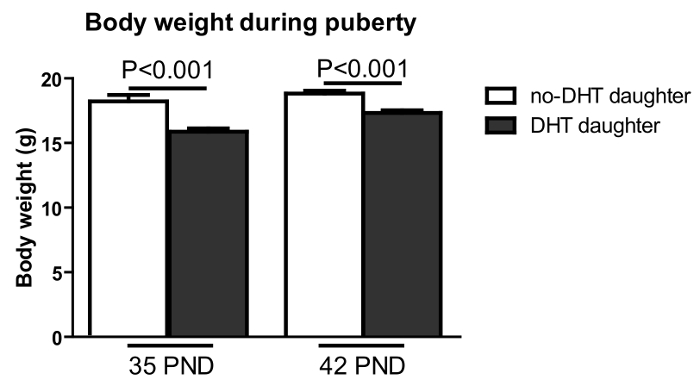
Figure 4: Pre-pregnancy maternal DHT treatment resulted in reduced body weight in female offspring at 35 and 42 PND. Body weight (Y-axis) was measured on post-natal days as shown (X-axis). DHT-exposed offspring: black bar; no –DHT offspring: open bar. Values are mean ±S.E.M. N = 9-14 per group. Please click here to view a larger version of this figure.
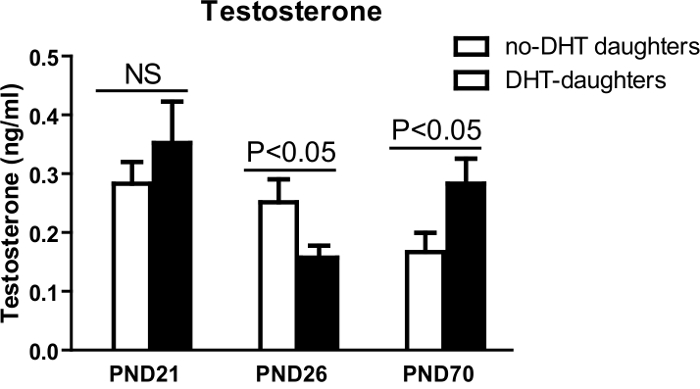
Figure 5: Serum DHT levels. Blood was collected in the morning on PND 21, 26, 70 in the morning. Serum DHT levels (Y-axis) from female offspring of no-DHT daughters (open bars) and DHT-daughters (black bars). Values are mean ±S.E.M. N = 5-11 per group. This figure has been modified from reference2 Please click here to view a larger version of this figure.
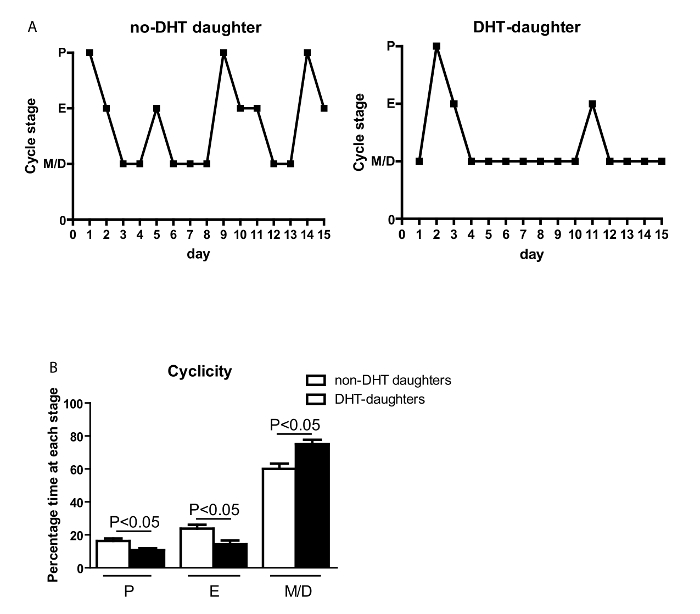
Figure 6: Chronic maternal androgen excess leads to disturbed cyclicity in adult daughters. (A) Representation of estrous cyclicity of female offspring. (B) The percentage of time spent at each estrous stage (Y-axis) during 15 days (X-axis) measured by cytological examination of vaginal cells. Values are mean ±S.E.M. N = 5-9 per group. The estrous cycle stage (Y-axis). M/D: met/diestrus; P: proestrus; E: estrus. Please click here to view a larger version of this figure.
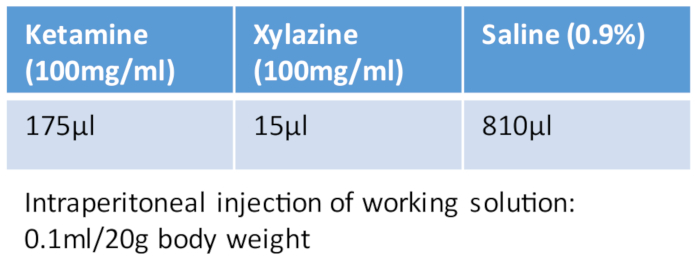
Table 1: Ketamine/xylazine cocktail.
Discussion
Hyperandrogenism is a key feature of PCOS. The serum DHT levels (two fold higher in DHT mice than in no-DHT mice) used in this protocol are lower than those reported by other investigators in previous studies and are calibrated to proportionally mimic women with PCOS5,19,20,21. Unlike other models, this 2-fold DHT model does not alter the body weight and whole body composition compared with no-DHT mice for up to 3.5 months after DHT insertion23,24. These adult DHT implanted mice are maintained by continual exogenous DHT. Although whole body composition is not altered, alteration of the structure and function of the adipocytes has been observed in lean PCOS women20. A careful examination of different depots is warranted. We observed impaired estrous cyclicity within one week after DHT insertion15 and reduced fertility during a 3 month fertility evaluation23.
The small subset of DHT treated female mice that were able to get pregnant and have offspring provided an opportunity to assess the impact of pre-gestational, gestational and nursing hyperandrogenemia on fetal development. In order to get a properly controlled experiment, multiple breeding females are required which necessitates that at least 10 mating pairs be established. DHT-daughters are only exposed to DHT during gestation and before 21 PNA. DHT-daughters showed reduced body weight compared to no-DHT daughters, indicating that obesity or overweight is not a confounding variable in the observed physiological effects15.
In initial studies, we validated the serum DHT levels over time. We found no significant differences in serum DHT at 1, 2, 3, and 4 weeks23,24. DHT levels declined after 4 weeks, therefore, we replace DHT pellet every 4 weeks. We do not observe reduced effects of DHT even they have been stored at room temperature for 3 months. It is important to incubate pellets in saline for 24 h just before insertion. This step is critical for even release of DHT from the pellet (DHT release slowly through the silastic tubing). Estrous cyclicity can be examined after 3 days of DHT insertion, and vaginal smear can be also examined directly in the saline without dry and stain under a light microscope after you familiar with the cell types27.
Metabolic phenotype can be assessed at, or after, 2 weeks (14 days) of DHT insertion. However, the effects of DHT on metabolism at the end of the fourth week (28 days) are slightly attenuated, though no attenuation is observed for reproductive dysfunction. Therefore, we assess metabolism function normally at the end of 2, 3, 5 , 6, 7 weeks (14, 21, 35, 42, and 49 days, 2 times total insertion). The production of slow release steroid pellets in the lab is a mature technique that has been widely adopted by different laboratories33,34. It represents a cost-effective alternative to commercially available products, which can exceed $50/pellet (90 day DHT pellet, 5mg/pellet, IRA, NA-161). The commercial product does have the advantage of not needing to be replaced for up to 90 days, while the pellet described in this report needs to be replaced every 28 days. For large-scale studies, investigators may find it more economical to produce their own pellets even with the added work required to replace pellets every 4 weeks.
As observed by others, models with chronic DHT implantation after birth do not show increased LH pulsatile frequency and this may point out different pathological mechanisms of elevated androgen induced reproductive function between developmental and late-onset acquired hyperandrogenism. With this protocol, we are able to investigate female offspring from chronically hyperandrogenic dams. We can leverage this model to try to fully understand the reproductive (e.g. follicle development, ovulation (corpora lutea), and fertility) and metabolic consequences (e.g. glucose or lipid metabolism, body composition, adipose depot distribution). Our protocol adds new tool to investigate elevated androgen induced reproductive and metabolic dysfunction; and dissociates the pathophysiology caused by elevated androgen from those that could be due to obesity. This model can be used to explore the tissue specific effects of elevated androgens in females. In addition, the methodology reported here for DHT pellet production can be easily applied to the study of other steroid hormones.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institutes of Health (Grants R00-HD068130 to S.W.) and the Baltimore Diabetes Research Center: Pilots and Feasibility Grant (to S.W.).
Materials
| Crystalline 5α-DHT powder | Sigma-Aldrich | A8380-1G | ||
| Dow Corning Silastic tubing | Fisher Scientific | 11-189-15D | 0.04in/1mm inner diameter x0.085in/2.15mm outer diameter | |
| Medical adhesive silicone | Factor II, InC. | A-100 | ||
| Goggles, lab coats, gloves and masks. | ||||
| 10 µL pipette tips without filter | USA Scientific | 11113700 | ||
| Microscope slide for smear | Fisher Scientific | 12-550-003 | ||
| Diff Quik for staining cells | Fisher Scientific | NC9979740 | ||
| Lancet | Fisher Scientific | NC9416572 | ||
| 3 mL Syring | Becton, Dickinson and Company (BD), | 30985 | ||
| attached needle: 20G | BD | 305176 | ||
| Ruler: any length than 10cm with milimeter scale. | ||||
| Xylazine | Vet one AnnSeA LA, MWI, Boise | NDC13985-704-10 | 100mg/ml | |
| Ketamine Hydrochloride | Hospira, Inc | NDC 0409-2051-05 | 100mg/ml | |
| Surgical staple | AutoClip® System, Fine Science Tool | 12020-00 | ||
| Insulin syringe | BD | 329461 | 1/2 CC, low dose U-100 insulin syringe | |
| Trochar | Innovative Research of America | MP-182 | ||
| Microscope | Carl Zeiss Primo Star | 415500-0010-001 | Germany | |
| Ear punch | Fisher Scientific | 13-812-201 | ||
| Testosterone rat/mouse ELISA kit | IBL | B79174 | ||
| DHT ELISA kit | Alpha Diagnostic International | 1940 | ||
| One touch ultra glucometer | Life Scan, Inc. | |||
| One touch ultra test stripes | Life Scan, Inc. | |||
| Eppendorf tube | Fisher Scientific | 05-402-18 | ||
| Razor blade | Fisher Scientific | 12-640 | ||
| Clidox | Fisher Scientific | NC0089321 | ||
| surgical underpad | Fisher Scientific | 50587953 |
|
|
| Betadine Antiseptic Solution | Walgreens | |||
| 3M Vetbond (n-butyl cyanoacrylate) | 3M Science. Applied to Life | |||
| Animal tattoo ink paste | Ketchum manufacturing Inc. | Brockville, Ontario, Canada | ||
| Scale | Ohaus Corporation | HH120D | Pine Brook, NJ | |
| Electronic digital caliper | NEIKO Tools USA | 01407A | available from Amazon |
References
- Palomba, S., de Wilde, M. A., Falbo, A., Koster, M. P., La Sala, G. B., Fauser, B. C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update. 21 (5), 575-592 (2015).
- Doherty, D. A., Newnham, J. P., Bower, C., Hart, R. Implications of polycystic ovary syndrome for pregnancy and for the health of offspring. Obstet. Gynecol. 125 (6), 1397-1406 (2015).
- de Wilde, M. A., et al. Cardiovascular and Metabolic Health of 74 Children From Women Previously Diagnosed With Polycystic Ovary Syndrome in Comparison With a Population-Based Reference Cohort. Reprod. Sci. , (2018).
- Caldwell, A. S., et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 155 (8), 3146-3159 (2014).
- van Houten, E. L., Kramer, P., McLuskey, A., Karels, B., Themmen, A. P., Visser, J. A. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 153 (6), 2861-2869 (2012).
- Cardoso, R. C., Puttabyatappa, M., Padmanabhan, V. Steroidogenic versus Metabolic Programming of Reproductive Neuroendocrine, Ovarian and Metabolic Dysfunctions. Neuroendocrinology. 102 (3), 226-237 (2015).
- Dumesic, D. A., Abbott, D. H., Padmanabhan, V. Polycystic ovary syndrome and its developmental origins. Rev. Endocr. Metab Disord. 8 (2), 127-141 (2007).
- Kauffman, A. S., et al. A Novel Letrozole Model Recapitulates Both the Reproductive and Metabolic Phenotypes of Polycystic Ovary Syndrome in Female Mice. Biol Reprod. 93 (3), 69 (2015).
- Kelley, S. T., Skarra, D. V., Rivera, A. J., Thackray, V. G. The Gut Microbiome Is Altered in a Letrozole-Induced Mouse Model of Polycystic Ovary Syndrome. PLoS One. 11 (1), e0146509 (2016).
- Kafali, H., Iriadam, M., Ozardali, I., Demir, N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch. Med. Res. 35 (2), 103-108 (2004).
- Maliqueo, M., Benrick, A., Stener-Victorin, E. Rodent models of polycystic ovary syndrome: phenotypic presentation, pathophysiology, and the effects of different interventions. Semin. Reprod. Med. 32 (3), 183-193 (2014).
- Yanes, L. L., et al. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend. Med. 8 (2), 103-115 (2011).
- Kauffman, A. S., et al. A Novel Letrozole Model Recapitulates Both the Reproductive and Metabolic Phenotypes of Polycystic Ovary Syndrome in Female Mice. Biol. Reprod. 93 (3), 69 (2015).
- Filippou, P., Homburg, R. Is foetal hyperexposure to androgens a cause of PCOS?. Hum. Reprod. Update. 23 (4), 421-432 (2017).
- Wang, Z., Shen, M., Xue, P., DiVall, S. A., Segars, J., Wu, S. Female Offspring From Chronic Hyperandrogenemic Dams Exhibit Delayed Puberty and Impaired Ovarian Reserve. Endocrinology. 159 (2), 1242-1252 (2018).
- Abbott, D. H., Barnett, D. K., Bruns, C. M., Dumesic, D. A. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome?. Hum. Reprod. Update. 11 (4), 357-374 (2005).
- Abbott, D. H., Dumesic, D. A., Franks, S. Developmental origin of polycystic ovary syndrome – a hypothesis. J. Endocrinol. 174 (1), 1-5 (2002).
- Padmanabhan, V., Veiga-Lopez, A. Sheep models of polycystic ovary syndrome phenotype. Mol. Cell. Endocrinology. 373 (1-2), 8-20 (2013).
- Pierre, A., et al. Dysregulation of the Anti-Mullerian Hormone System by Steroids in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 102 (11), (2017).
- Dumesic, D. A., et al. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J. Clin. Endocrinol. Metab. 101 (11), 4178-4188 (2016).
- Fassnacht, M., Schlenz, N., Schneider, S. B., Wudy, S. A., Allolio, B., Arlt, W. Beyond adrenal and ovarian androgen generation: Increased peripheral 5 alpha-reductase activity in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 88 (6), 2760-2766 (2003).
- Dikensoy, E., Balat, O., Pence, S., Akcali, C., Cicek, H. The risk of hepatotoxicity during long-term and low-dose flutamide treatment in hirsutism. Arch. Gynecol. Obstet. 279 (3), 321-327 (2009).
- Ma, Y., et al. Androgen Receptor in the Ovary Theca Cells Plays a Critical Role in Androgen-Induced Reproductive Dysfunction. Endocrinology. , en20161608 (2016).
- Andrisse, S., et al. Low Dose Dihydrotestosterone Drives Metabolic Dysfunction via Cytosolic and Nuclear Hepatic Androgen Receptor Mechanisms. Endocrinology. , en20161553 (2016).
- Andrisse, S., Billings, K., Xue, P., Wu, S. Insulin signaling displayed a differential tissue-specific response to low-dose dihydrotestosterone in female mice. Am. J. Physiol.Endocrinol. Metab. 314 (4), E353-E365 (2018).
- van Houten, E. L., Visser, J. A. Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function?. Reprod. Biol. 14 (1), 32-43 (2014).
- Caligioni, C. S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. , (2009).
- Wu, S., et al. Conditional knockout of the androgen receptor in gonadotropes reveals crucial roles for androgen in gonadotropin synthesis and surge in female mice. Mol. Endocrinol. 28 (10), 1670-1681 (2014).
- Nelson, J. F., Felicio, L. S., Randall, P. K., Sims, C., Finch, C. E. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol. Reprod. 27 (2), 327-339 (1982).
- Dinger, K., et al. Intraperitoneal Glucose Tolerance Test, Measurement of Lung Function, and Fixation of the Lung to Study the Impact of Obesity and Impaired Metabolism on Pulmonary Outcomes. Journal of Visualized Experiments. (133), (2018).
- Nilsson, M. E., et al. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology. 156 (7), (2015).
- McNamara, K. M., Harwood, D. T., Simanainen, U., Walters, K. A., Jimenez, M., Handelsman, D. J. Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 121 (3-5), 611-618 (2010).
- Klein, S. L., Bird, B. H., Glass, G. E. Sex differences in Seoul virus infection are not related to adult sex steroid concentrations in Norway rats. J. Virol. 74 (17), 8213-8217 (2000).
- Siracusa, M. C., Overstreet, M. G., Housseau, F., Scott, A. L., Klein, S. L. 17beta-estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J. Immunol. 180 (3), 1423-1431 (2008).

