Rabies Necropsy Techniques in Large and Small Animals
Summary
The goal of this protocol is to demonstrate safe necropsy techniques in small and large animals to obtain satisfactory tissue samples for rabies testing.
Abstract
The New York State Department of Health (NYSDOH) Rabies Laboratory receives between 6,000 to 9,000 specimens annually and performs rabies testing for the entire state, with the exception of New York City. The Rabies laboratory necropsies a variety of animals ranging in size from bats to bovids. Most of these specimens are animals exhibiting neurological signs, however, less than 10% actually test positive for rabies; implying trauma, lesions or other infectious agents as the cause of these symptoms. Due to the risk of aerosolizing undiagnosed infectious agents, the Rabies Laboratory does not use power tools or saws. Three necropsy techniques will be presented for animals whose skulls are impenetrable with scissors. The laboratory has implemented these techniques to decrease potential exposure to infectious agents, eliminate unnecessary manipulation of the specimen and reduce processing time. The advantages of a preferred technique opposed to another is subject to the trained individual processing the specimen.
Introduction
Working on the necropsy floor of a rabies laboratory is inherently dangerous. At times, specimens arrive with embedded porcupine quills, foreign objects including arrows/bullets/pellets or exposed bone shards that may penetrate the protective shipping wrap. Improper packaging can result in leakage, endangering individuals who unpack specimens. In addition to physical injury, necropsy technicians risk exposure to unknown zoonotic infectious agents from the CNS and body fluids of the specimens. Additionally, ectoparasites carried by the specimen may transmit other zoonotic diseases, as fleas and ticks are commonly seen on submitted animals. Depending on geographic location and species involved the diseases exposed vary. Arboviruses such as Eastern equine encephalitis virus (EEEV) or West Nile virus (WNV), tick-borne diseases including Lyme disease or tularemia, bacteria causing Q fever or tuberculosis, and infectious prions name a small number of the possible dangers1,2,3.
The purpose of these methods is to demonstrate safe and efficient necropsy techniques using instruments that minimize the potential for aerosolization unlike power tools or saws4,5. Commonly, the necropsy of small animals in the rabies laboratory requires cutting away the cranial muscles and using a hammer and chisel to open the caudal dorsal portion of the calvarium6. Removing this area of calvarium exposes the hind brain, including the entire cerebellum and cranial brain stem. Modified necropsy techniques may be performed on the ventral part of the skull, avoiding the large cranial muscles and thicker regions of the skull. However, these modified necropsy techniques are only possible when the specimen is without cervical vertebrae.
Similarly, brain tissue in large animals can be removed by separating the cranial muscles and opening the caudal dorsal portion of the skull7. Considerable effort is required to expose the cerebellum and brain stem as the skulls of larger animals are generally thicker. To avoid penetrating the skull, the head of a large animal is positioned so the ventro-caudal portion of the skull is facing the technician. Using modified instruments, the cerebellum and brain stem are removed through the foramen magnum. This is similar to the sample acquisition method recommended by the TSE European Union Reference Laboratory for Transmissible Spongiform Encephalopathy (TSE) investigations8. Cranial vertebrae should be removed beforehand to provide access to the foramen magnum.
Application of these techniques are beneficial to suitably trained technicians in rabies laboratories. As the rabies laboratory receives samples of various sizes, from juvenile bats to adult draft horses9, the technician has several methods to choose from based on the individual circumstance. The method demonstrated for a large animal is also appropriate for veterinarians who perform necropsies in the field, since shipping an entire large animal head for rabies testing is cumbersome and costly. Implementing any of these techniques will improve safety by decreasing the potential of aerosol production, reduce the handling of the specimen and save processing time. However, as the field does not have the same advantages as a laboratory set up specific for rabies testing, it is essential that any modifications made to these procedures focus on safety, especially the use of personal protective equipment (PPE).
Protocol
All methods described were approved by the Wadsworth Center Institutional Animal Care and Use Committee (IACUC).
1. Preparation
- Don PPE, at minimum eye protection (glasses or face shield), surgical or N-95 mask, and non-latex gloves.
- Prepare work area, ideally a bio-safety cabinet (BSC), with a disposable work surface covering (e.g., kraft paper or absorbent pads) and clean necropsy instruments (Figure 1).
- Place the specimen on the work surface and use instruments to manipulate it to assess condition of the sample including evidence of decomposition, damage to skull, potential hazards (e.g., porcupine quills, scalpel blades), and the quality of the decapitation.
2. Ventral method
NOTE: When the specimen is properly decapitated at the jaw-line, the foramen magnum and the occipital condyle will be exposed. The ventral method is less complicated for retrieving the cerebellum and brain stem.
- Position the specimen with ventral side up and nose directing distally toward the back of BSC.
- Hold an orthopedic hammer/mallet in right hand (if right-handed) and at same time hold a councilmen chisel in left hand.
- Position the chisel at a 45° angle with the corner point of the chisel directing between the right side of temporal bone and occipital bone making a “V” opening.
- Strike the top of the chisel with the hammer until the two bones separate. Make the cut to adjacent to the basisphenoid bone.
- Repeat on the left side of temporal bone/occipital bone (Figure 2A).
- Bend the “V” area of skull downward with the chisel. Expose the entire rhombencephalon area of the brain (cerebellum and brain stem) (Figure 2B).
- Scoop out the brain stem and cerebellum with scissors and forceps. Remove any remaining pieces from the skull if the brain stem and cerebellum did not come out in a single piece.
3. Dorsal method
NOTE: If the specimen has a poor decapitation (foramen magnum not visible) and the neck cannot be easily removed during necropsy or if damage to the cerebellum is suspected, the dorsal method should be utilized.
- Position the specimen dorsally with the nose directing distally toward the back of BSC.
- Using tumor tenacula, grasp the left temporal muscle with teeth of tenacula and lock by squeezing the handle.
- Cut the temporal muscle down to bone with sharp carving knife.
- Rotate the specimen 180° with tenacula and knife (not hand) and repeat the process on the opposite temporal muscle. Expose the skull.
- Position a chisel at a 45° angle with the corner point of the chisel on the center of the skull at the juncture of the parietal and intraparietal bone.
- Strike the top of the chisel with a hammer until a horizontal opening is made on the top half of the skull at parietal bone.
- Rotate the specimen 180° and repeat the process on the opposite side.
- Insert the point of the chisel into the end of cut 1 (Figure 3A) and at 90° of horizontal opening. Strike with the hammer until the opening reaches occipital bone (approximately 10 cm depending upon size of the specimen).
- Roll the specimen and repeat on the opposite side at the end of cut 2.
NOTE: With specimen dorsal and nose positioned toward the back of BSC, the openings in the skull resemble an upside-down “U”. - Insert the teeth of the tenacula into the skull at the bottom of the “U” and pry towards oneself. Expose the caudal end of the cerebrum and the cerebellum (Figure 3B).
- Use scissors as a scoop and pry out entire cerebellum from within the cavity.
- Use tissue forceps to tease out the brain stem from the foramen.
4. Large animal method
- Position the specimen so that the dorsal part of the skull is in contact with the necropsy surface with the caudal portion of the skull and foramen magnum facing the technician.
- Insert the modified stiletto knife into the foramen magnum in between the spinal cord and spinal meninges as far as possible.
- Score around the spinal cord to separate the cerebellum and brain stem from the spinal meninges. After the knife is inserted through the foramen magnum, gently angle the knife to follow along the skull as much as possible.
- Insert a chemistry spatula or thin, long handled spoon into the space between the neural tissue and spinal meninges.
- Probe around the spinal cord and cerebellum to ensure the connection to the spinal meninges have been severed.
- Hold the brain stem with forceps. With the other hand, advance the spoon rostrally then dorsally to scoop up the cerebellum. Simultaneously pull back on the brain stem with the forceps and scoop out the cerebellum using the spoon.
NOTE: It may take more than one attempt to recover adequate cerebellum for rabies testing.
5. Post necropsy
- Dispose of all disposable materials (gloves, pads, work area coverings) and unused tissues in biohazardous waste.
- Clean and disinfect all instruments with method available (e.g., industrial dishwasher, autoclave, chemical disinfectant, boiling).
- Clean and disinfect all work surfaces with 20% bleach and/or 70% ethanol.
Representative Results
All terrestrial samples submitted with skulls between January 31, 2019 and February 28, 2019 had information regarding the presence of a neck and the method of necropsy collected. During that time, 170 heads were necropsied with 18 species represented. 52% (89/170) were properly decapitated. The remaining had at least one vertebra attached including three whole body specimens. The ventral method was used 75% (128/170) of the time, of those, necks were present on 49. Specimens submitted with a neck will have it removed during necropsy to allow for the ventral method whenever possible. Three large animals (cow, deer, pig) were submitted and in two cases the large animal protocol was used. The large animal protocol was not used on the pig because extra brain tissue samples were required for additional testing. A squirrel was submitted with a crushed skull and simply cutting away the skin exposed brain tissue, thus none of the above methods were used (Table 1).
On fresh intact submissions, all three methods will result with the required tissue for reliable rabies diagnostic test results. Occasionally, the cerebellum and brain stem cannot be removed intact, although after removing all tissue from the hindbrain these tissues can be identified and processed accordingly.
These three valuable methods cannot compensate for poor specimen quality caused prior to receipt at the lab. Trauma, decomposition and poor decapitation methods can affect the outcome regardless of how efficiently the samples are collected.

Figure 1: Instruments used in rabies necropsy. Curved sharp-blunt mayo scissors, smooth-tipped tissue dressing forceps without teeth, councilman orthopedic bone chisel, orthopedic mallet-hammer, locking tumor-tenacula, restaurant-quality carving knife, modified stiletto knife, chemistry spoon, and sharpened tablespoon. Please click here to view a larger version of this figure.
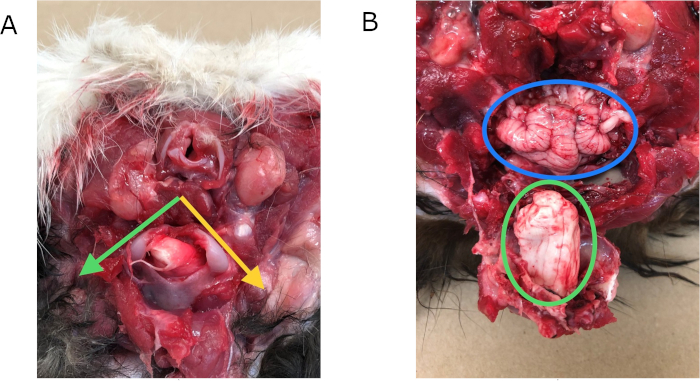
Figure 2: Ventral method of necropsy. (A) Location of cuts: Place point of a chisel at base of arrow, cut in direction of green arrow and repeat following the yellow arrow forming a "V" around the foramen magnum. Pry "V" down to expose the brain stem and cerebellum. (B) Brain stem (green) and cerebellum (blue) when exposed using the ventral method of necropsy. Please click here to view a larger version of this figure.
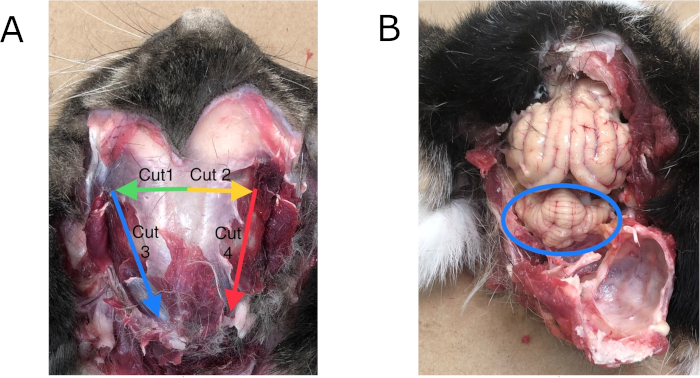
Figure 3: Dorsal method of necropsy. (A) Location of cuts: Place point of a chisel at base of arrow and cut in direction of arrow in the order noted forming a "U". Pry "U" down to expose the cerebellum with the brain stem below it. (B) Cerebellum (circled) when exposed using the dorsal method. Brain stem lies directly beneath and is not visible until cerebellum is removed. Please click here to view a larger version of this figure.
| species | vertebrae attached | method used V=ventral, D=dorsal, LA=large animal | total | comments |
| bear | no | V | 2 | |
| cat | no | V | 32 | |
| cat | no | D | 3 | 2 small enough to open skull with scissors, 1 had abcess that was being investigated exposing top of skull |
| cat | yes | V | 11 | |
| cat | yes | D | 8 | |
| cow | no | LA | 1 | |
| coyote | no | V | 1 | |
| coyote | yes | V | 2 | |
| coyote | yes | D | 3 | |
| deer | no | LA | 1 | |
| dog | no | V | 19 | |
| dog | no | D | 3 | |
| dog | yes | V | 2 | 1 was small dog |
| dog | yes | D | 18 | |
| ferret | no | V | 1 | |
| fisher | no | V | 1 | |
| flying squirrel | yes | D | 1 | whole body |
| grey fox | no | V | 2 | |
| grey fox | yes | V | 4 | |
| pig | yes | V | 1 | |
| porcupine | no | V | 1 | |
| raccoon | no | V | 16 | |
| raccoon | no | D | 1 | frozen |
| raccoon | yes | V | 26 | |
| red fox | no | V | 2 | |
| skunk | no | V | 1 | |
| skunk | yes | V | 3 | |
| squirrel | no | V | 1 | |
| squirrel | yes | D | 1 | full body, crushed skull, used scissors to cut away skin to exposed brain cavity |
| weasle | no | D | 1 | |
| woodchuck | yes | D | 1 | whole body |
| Total | 170 | |||
| Breakdown of total | ||||
| with neck | 81 | |||
| no neck | 89 | |||
| ventral method | 128 | |||
| dorsal method | 39 | |||
| large animal method | 2 | |||
| other method | 1 | |||
| ventral method with neck | 49 |
Table 1: Breakdown of specimens requiring tissue removal from the skull submitted from January 31, 2019 through February 28, 2019 at the New York State Department of Health Rabies Laboratory.
Discussion
Specimens submitted for rabies necropsy often have a history of clinical signs compatible with a neurological illness. The presence of clinical illness may be associated with a variety of disorders, including zoonotic diseases, increasing the risk to staff of a laboratory acquired infection. To reduce these risks, techniques have been implemented that decrease the handling and manipulation of specimens.
The methods demonstrated represent a necropsy event to remove desired tissues from a single animal only. More commonly multiple specimens are processed in a shift and care is needed to ensure no cross contamination between samples. A clean worksurface (disposable kraft paper or pads), a new set of clean, disinfected instruments, and glove changes are mandatory. Once tissues are obtained, the individual laboratory's protocol for processing can be followed, including making slides for microscopy or RNA extraction for molecular methods.
There are several essential prerequisites for successfully implementing these techniques in the laboratory or field. Prior rabies vaccination and PPE are critical for anyone necropsying a rabies suspect animal. Individuals working in rabies laboratories should have their serum tested every six months to ensure an adequate level of anti-rabies antibodies are present10. It is important to remember that other zoonotic diseases, such as EEEV, WNV and Bovine Spongiform Encephalopathy (BSE), present similar signs as rabies and may also occur in rabies suspect animals11,12.
Appropriate well-maintained instruments are essential to safely perform necropsy. Once a specimen has been removed from its biohazard bag, it should only be manipulated with instruments, not hands, to decrease the potential for accidents. Prior to small animal necropsy, the technician should evaluate the condition of the specimen to determine whether a preferred ventral approach through the base of the skull is possible. With large animals it may be too cumbersome to fully evaluate the condition of the specimen as additional vertebrae may need to be removed before retrieving tissue through the foramen magnum.
Limitations present themselves in all necropsy techniques including specimen condition, tissue quality and the amount of remaining cervical vertebrae. The cervical vertebrae will not affect the results of rabies analysis, but severely decomposed tissue may result in unsatisfactory results. More sensitive molecular methods in rabies diagnostics may allow successful testing in certain samples unable to be tested by direct fluorescent antibody assay (DFA), including severely decomposed specimens13. However, no amount of sensitivity can replace the need for proper tissue sampling.
A common problem in the rabies laboratory is receiving inappropriate or inadequate brain tissue for testing when large animal necropsies are performed in the field. Without the required tissue, and if additional tissues are unavailable for resubmission, our rabies laboratory will perform testing on the tissue available but is unable to verify the specimen negative, instead it is unsatisfactory for testing. There are other published methods for field tissue collections such as the straw method or retro orbital route14. Both methods collect brain tissue without the need to open the skull. A straw or disposable pipette is inserted either through the foramen magnum or a hole created in the eye socket and pushed through the brain, essentially taking a core sample and not necessarily sampling the full cross section of the brain stem. As these field methods do not collect samples in a manner to be considered satisfactory for testing in our laboratory, these processes are not demonstrated or explored in this paper.
In the field large animal necropsy can be challenging for individuals who are not trained to remove the correct tissue for rabies testing. Instead the entire head of the animal, which can weigh between 20-45 kg, is submitted creating cumbersome transport for both the field veterinarian and rabies laboratory technicians. Frequent requests for training on large animal necropsy technique have been made to our laboratory. The objective of this manuscript is to distribute this information to individuals and groups whose work can benefit from these techniques.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We are grateful to the New York State Department of Health Wadsworth Center for supporting this project. We also would like to acknowledge the support from Amy Willsey and Frank Blaisdell of Department of Health Wadsworth Center, and LL Ranch, Altamont, NY.
Materials
| Chemistry spoon | Any | ||
| Curved, sharp-blunt mayo scissors | Sklar | 14-2055 | Sklar Operating Scissors 5-1/2 Inch Premium OR Grade Stainless Steel Finger Ring Handle Curved Sharp/Blunt |
| Large sharp restaurant-quality carving knife | Dexter | P94848 | 8" Scalloped Utility Knife, white handle |
| Locking tumor-tenacula | Diamond Scientific and Surgicals | N/A | Czerny Tenaculum Forcep |
| Modified stiletto knife (6.5 inch long blade carving knife ground to 0.5 inch wide) | Dexter | P94848 | Modified 8" Scalloped Utility Knife, white handle |
| Orthopedic mallet-hammer | Mortech | N/A | Postmortem hammer with hook |
| Sharp councilman orthopedic bone chisel | Shandon | 60-5 | Councilman's Chisel Blade: 2 in x 2.25 in standard 7 in |
| Sharpened tablespoon or other long handled spoon | Any | ||
| Smooth-tipped tissue dressing forceps without teeth | Shandon | 63-03 | Shandon Broad Point Dressing Thumb Forceps |
| Powder-free non-latex gloves | Any | ||
| Safety glasses, goggles, or faceshield | Any | ||
| Surgery or N-95 mask | Any | ||
| Kraft paper, butcher paper, absorbent pad, etc | Any |
References
- Centers for Disease Control and Prevention (CDC). West Nile virus activity – United States, 2009. MMWR Morbidity and Mortality Weekly Report. 59 (25), 769-772 (2010).
- McDaniel, C. J., Cardwell, D. M., Moeller, R. B., Gray, G. C. Humans and cattle: A review of bovine zoonoses. Vector Borne and Zoonotic Diseases. 14 (1), 1-19 (2014).
- Zoonotic diseases. Merck Veterinary Manual Available from: https://www.merckvetmanual.com/public-health/zoonoses/zoonotic-diseases (2019)
- Wenner, L., Pauli, U., Summermatter, K., Gantenbein, H., Vidondo, B., Posthaus, H. Aerosol generation during bone-sawing procedures in veterinary autopsies. Veterinary Pathology. 54 (3), 425-436 (2017).
- Green, F. H. Y., Yoshida, K. Characteristics of aerosols generated during autopsy procedures and their potential role as carriers of infectious agents. Applied Occupational and Environmental Hygiene. 5 (12), 853-858 (1990).
- Barrat, J., Meslin, F. X., Kaplan, M. M., Koprowski, H. Simple technique for the collection and shipment of brain specimens for rabies diagnosis. Laboratory techniques in Rabies 4th Edition. , 425-427 (1996).
- Ness, S. L., Bain, F. T. How to perform an equine field necropsy. American Association of Equine Practitioners. 55, 313-316 (2009).
- . Sample requirements for TSE testing and confirmation – EURL guidance Available from: https://science.vla.gov.uk/tse-lab-net/documents/tse-oie-rl-samp.pdf (2019)
- . Rabies reports Available from: https://www.wadsworth.org/programs/id/rabies/reports (2019)
- . Protocol for postmortem diagnosis of rabies in animals by direct fluorescent antibody testing: A minimum standard for rabies diagnosis in the United States Available from: https://www.cdc.gov/rabies/pdf/rabiesdfaspv2.pdf (2019)
- Miller, L. D., Davis, A. J., Jenny, A. L., Fekadu, M., Whitfield, S. G. Surveillance for lesions of bovine spongiform encephalopathy in U.S. cattle. Developments in Biological Standardizations. 80, 119-121 (1993).
- Andrews, C., Gerdin, J., Patterson, J., Buckles, E. L., Fitzgerald, S. D. Eastern equine encephalitis in puppies in Michigan and New York states. Journal of Veterinary Diagnostic Investigation. 30 (4), 633-636 (2018).
- Appler, K., Brunt, S., Jarvis, J. A., Davis, A. D. Clarifying indeterminate results on the rabies direct fluorescent antibody test using real-time reverse transcriptase polymerase chain reaction. Public Health Reports. 134 (1), 57-62 (2019).
- Rupprecht, C. E., Fooks, A. R., Abela-Ridder, B. Chapter 7. Brain removal. Laboratory techniques in Rabies 5th Edition. , 67-72 (2018).

