通过可见光光解 核 黄素-5′-磷酸灭活病原体
Summary
在这里,我们提出了一种方案,在低强度的蓝光和紫光照射下,用黄素单核苷酸(FMN)光解过程中产生的活性氧灭活致病菌。FMN光解被证明是一种简单而安全的卫生过程方法。
Abstract
核黄素-5′-磷酸(或黄素单核苷酸;FMN)对可见光敏感。各种化合物,包括活性氧(ROS),可以在用可见光照射时通过FMN光解产生。FMN光解产生的ROS对微生物有害,包括金黄色葡萄球菌(S. aureus)等致病菌。本文提出了一种在可见光照射下通过涉及FMN的光化学反应使金黄色葡萄球菌失活的方案。FMN光解过程中产生的超氧自由基阴离子()通过硝基蓝四唑(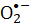 NBT)还原进行评估。归因于反应
NBT)还原进行评估。归因于反应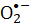 性物种的金黄色葡萄球菌的微生物活力用于确定该过程的有效性。细菌灭活率与FMN浓度成正比。紫光比蓝光照射更有效地灭活金黄色葡萄球菌,而红光或绿光不驱动FMN光解。本文展示了FMN光解作为一种简单安全的卫生过程方法。
性物种的金黄色葡萄球菌的微生物活力用于确定该过程的有效性。细菌灭活率与FMN浓度成正比。紫光比蓝光照射更有效地灭活金黄色葡萄球菌,而红光或绿光不驱动FMN光解。本文展示了FMN光解作为一种简单安全的卫生过程方法。
Introduction
核黄素-5′-磷酸(FMN)是通过核黄素侧链的核黄素5′-位置磷酸化形成的,并且是许多细胞过程产生能量所必需的所有黄素蛋白。它还对人体的某些功能起着维生素的作用1。FMN在水中的溶解度大约是核黄素2的200倍。
细菌的抗菌光动力灭活(aPDI)是控制对细菌3,4耐药性的有效方法,因为它不依赖于细菌耐药性的模式。临床上,aPDI用于治疗软组织感染,以减少由于多重耐药细菌5,6,7,8,9引起的院内皮肤感染。aPDI还通过产生活性氧(ROS)产生细胞死亡。ROS,如超氧自由基()、单线态氧、羟基自由基(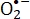 •OH)和过氧自由基,是自由基或含有活性氧10,11,12的分子,通常具有活性13。与ROS引起的DNA损伤类似,膜过氧化和内皮细胞破坏也是细胞中ROS引起的不良生化反应12。
•OH)和过氧自由基,是自由基或含有活性氧10,11,12的分子,通常具有活性13。与ROS引起的DNA损伤类似,膜过氧化和内皮细胞破坏也是细胞中ROS引起的不良生化反应12。
aPDI用于病原菌涉及在化合物存在下使用可见光或紫外光源灭活微生物,例如甲基氯化亚砜14、PEI-c e6共轭物15、卟啉16、二氧化钛17、甲苯胺蓝O 18和氧化锌纳米颗粒19。甲苯胺蓝O和亚甲蓝是吩噻嗪染料,亚甲蓝具有毒性。氧化锌纳米颗粒和紫外线照射与不利的健康和环境影响有关。因此,在可见光照射下通过光解开发可靠、安全、简单的光敏剂值得进一步研究。
微量营养素核黄素或FMN无毒,确实用于食品制造或喂养20。FMN和核黄素都对光照射高度敏感2。在紫外1,2,21,22,23和蓝光照射10,24下,这两种化合物达到激发态。通过光解产生的活化核黄素或FMN被提升到其三重态,同时产生ROS2,25。Kumar等人报告说,被紫外光激活的核黄素选择性地导致病原微生物中DNA的鸟嘌呤部分损伤增加26。在紫外光照射下,光动力激活的核黄素被证明可以促进双链DNA中8-OH-dG的产生,8-OH-dG是氧化应激的生物标志物27。据报道,金黄色葡萄球菌和大肠杆菌在核黄素或FMN光解过程中被ROS灭活10,24,28。作者先前的一项研究表明,涉及核黄素和FMN的光解反应减少了结晶紫,一种三芳基甲烷染料和产生 抗菌剂,并消除了结晶紫28的大部分抗菌能力。当黄素腺嘌呤二核苷酸或FMN被蓝光照射时,产生的ROS在HeLa细胞中产生细胞凋亡,以便在体外中毒29。在核黄素存在下使用光化学处理,Cui等人通过抑制淋巴细胞的增殖和细胞因子产生来灭活淋巴细胞30。
抗菌剂,并消除了结晶紫28的大部分抗菌能力。当黄素腺嘌呤二核苷酸或FMN被蓝光照射时,产生的ROS在HeLa细胞中产生细胞凋亡,以便在体外中毒29。在核黄素存在下使用光化学处理,Cui等人通过抑制淋巴细胞的增殖和细胞因子产生来灭活淋巴细胞30。
核黄素的光解用于紫外线10,24灭活血液病原体,但在紫外线照射下血液成分会受损30。另据报道,暴露于紫外线的血小板逐渐增强其膜上激活标志物P-选择素和LIMP-CD63的性能。需要研究紫外线和高强度照射的细胞毒性,在涉及可见光的FMN光反应期间简单且安全的光敏剂将非常有用。
波长较短的光具有更多的能量,并且更有可能对细胞造成严重损害。然而,在合适的光敏剂存在下,用低强度紫光照射可以抑制病原微生物。因此,当用紫光照射时,FMN的光敏化和产生对于研究非常重要,以确定FMN光 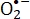 解的ROS增加细菌灭活的途径。
解的ROS增加细菌灭活的途径。
抗菌控制是一个常见问题,新抗生素的开发通常需要数十年时间。用紫光照射后,以FMN介导的光灭活可以消灭环境致病菌。本研究提出了一种有效的体 外 抗菌方案,使用紫光驱动FMN光解,从而产生 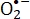 aPDI。 金黄色葡萄球菌 的微生物活力用于确定FMN诱导的aPDI的可行性。
aPDI。 金黄色葡萄球菌 的微生物活力用于确定FMN诱导的aPDI的可行性。
Protocol
Representative Results
Discussion
光敏剂增加化合物的光化学反应以产生ROS。病原微生物可以在光敏剂存在下通过光照射灭活。本研究确定了由于外源性光敏剂 FMN 的紫光照射产生的 ROS 引起的 金黄色葡萄球菌 的 aPDI。
如图3所示,对于FMN,使用紫光或蓝光照射5分钟后,444nm处的吸光度显着降低,并且在绿光或红光照射下FMN吸光度没有显着变化。NBT还原用于确定通过电荷转移过程<s…
Divulgations
The authors have nothing to disclose.
Acknowledgements
作者感谢黄德华博士和谢宗哲先生对实验的支持。
Materials
| Blue, green and red LED lights | Vita LED Technologies Co., Tainan, Taiwan | DC 12 V 5050 | |
| Dimethyl Sulfoxide | Sigma-Aldrich, St. Louis, MO | 190186 | |
| Infrared thermometer | Raytek Co. Santa Cruz, CA | MT4 | |
| LB broth | Difco Co., NJ | ||
| L-Methionine | Sigma-Aldrich, St. Louis, MO | 1.05707 | |
| NBT | Bio Basic, Inc. Markham, Ontario, Canada | ||
| Power supply | China tech Co., New Taipei City, Taiwan | YP30-3-2 | |
| Riboflavin 5′-phosphate | Sigma-Aldrich, St. Louis, MO | R7774 | |
| RNase | New England BioLabs, Inc. Ipswich, MA | ||
| Solar power meter | Tenmars Electronics Co., Taipei, Taiwan | TM-207 | |
| Staphylococcus aureus subsp. aureus | Bioresource Collection and Research Center (BCRC), Hsinchu, Taiwan | 10451 | |
| UV-Vis optical spectrometer | Ocean Optics, Dunedin, FL | USB4000 | |
| UV-Vis spectrophotometer | Hitachi High-Tech Science Corporation,Tokyo, Japan | U-2900 | |
| Violet LED | Long-hui Electronic Co., LTD, Dongguan, China |
References
- Jian, H. L., Cheng, C. W., Chen, L. Y., Liang, J. Y. The photochemistry of riboflavin. MC-Transaction on Biotechnology. 3, 1-11 (2011).
- Lin, Y., Eitenmiller, R. R., Landen, W. O. Riboflavin. Vitamin analysis for the health and food sciences. , 329-360 (2008).
- Xie, L. J., Wang, R. L., Wang, D., Liu, L., Cheng, L. Visible-light-mediated oxidative demethylation of N(6)-methyl adenines. Chemical Communications. 53 (77), 10734-10737 (2017).
- Tim, M. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. Journal of Photochemistry and Photobiology B: Biology. 150, 2-10 (2015).
- Maisch, T., et al. Photodynamic inactivation of multi-resistant bacteria (PIB) – a new approach to treat superficial infections in the 21st century. Journal of the German Society of Dermatology. 9 (5), 360-366 (2011).
- Hamblin, M. R. Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes. Current Opinion in Microbiology. 33, 67-73 (2016).
- Del Rosso, J. Q. Oral Doxycycline in the management of acne vulgaris: Current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. The Journal of Clinical and Aesthetic Dermatology. 8 (5), 19-26 (2015).
- Wong, T. W., et al. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by indocyanine green and near infrared light. Dematologica Sinica. 36 (1), 8-15 (2018).
- Yuann, J. M. P., et al. Effects of free radicals from doxycycline hyclate and minocycline hydrochloride under blue light irradiation on the deactivation of Staphylococcus aureus, including a methicillin-resistant strain. Journal of Photochemistry and Photobiology B: Biology. 226, 112370 (2022).
- Liang, J. Y., Cheng, C. W., Yu, C. H., Chen, L. Y. Investigations of blue light-induced reactive oxygen species from flavin mononucleotide on inactivation of E. coli. Journal of Photochemistry and Photobiology B: Biology. 143, 82-88 (2015).
- Yang, M. J., et al. Effects of blue-light-induced free radical formation from catechin hydrate on the inactivation of Acinetobacter baumannii, Including a carbapenem-resistant strain. Molecules. 23 (7), 1631 (2018).
- Yuann, J. M. P., et al. A study of catechin photostability using photolytic processing. Processes. 9 (2), 293 (2021).
- Yuann, J. M. P., Wang, J. S., Jian, H. L., Lin, C. C., Liang, J. Y. Effects of Clinacanthus nutans (Burm. f) Lindau leaf extracts on protection of plasmid DNA from riboflavin photoreaction. MC-Transaction on Biotechnology. 4 (1), 45-59 (2012).
- Rineh, A., et al. Attaching NorA efflux pump inhibitors to methylene blue enhances antimicrobial photodynamic inactivation of Escherichia coli and Acinetobacter baumannii in vitro and in vivo. Bioorganic and Medicinal Chemistry Letters. 28 (16), 2736-2740 (2018).
- Dai, T., et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrobial Agents and Chemotherapy. 53 (9), 3929-3934 (2009).
- Nitzan, Y., Ashkenazi, H. Photoinactivation of Acinetobacter baumannii and Escherichia coli B by a cationic hydrophilic porphyrin at various light wavelengths. Current Microbiology. 42 (6), 408-414 (2001).
- Tseng, C. C., et al. Altered susceptibility to the bactericidal effect of photocatalytic oxidation by TiO2 is related to colistin resistance development in Acinetobacter baumannii. Applied Microbiology and Biotechnology. 100 (19), 8549-8561 (2016).
- Boluki, E., et al. Antimicrobial activity of photodynamic therapy in combination with colistin against a pan-drug resistant Acinetobacter baumannii isolated from burn patient. Photodiagnosis and Photodynamic Therapy. 18, 1-5 (2017).
- Yang, M. Y., et al. Blue light irradiation triggers the antimicrobial potential of ZnO nanoparticles on drug-resistant Acinetobacter baumannii. Journal of Photochemistry and Photobiology B, Biology. 180, 235-242 (2018).
- . Code of Federal Regulations Available from: https://www.gpo.gov/fdsys/pkg/CFR-2009-title21-vol6/pdf/CFR-2009-title21-vol6-sec582-5695.pdf (2022)
- Lu, C. Y., et al. Generation and photosensitization properties of the oxidized radical of riboflavin: a laser flash photolysis study. Journal of Photochemistry and Photobiology B, Biology. 52 (1-3), 111-116 (1999).
- Sato, K., et al. The primary cytotoxicity in ultraviolet-a-irradiated riboflavin solution is derived from hydrogen peroxide. The Journal of Investigative Dermatology. 105 (4), 608-612 (1995).
- Tripathi, A. K., et al. Attenuated neuroprotective effect of riboflavin under UV-B irradiation via miR-203/c-Jun signaling pathway in vivo and in vitro. Journal of Biomedical Science. 21 (1), 39 (2014).
- Liang, J. Y., et al. Blue light induced free radicals from riboflavin on E. coli DNA damage. Journal of Photochemistry and Photobiology B, Biology. 119, 60-64 (2013).
- Ottaway, P. B. . The Technology of Vitamins in Food. , 233-244 (1993).
- Kumar, V., et al. Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level. Photochemistry and Photobiology. 80 (1), 15-21 (2004).
- Ito, K., Inoue, S., Yamamoto, K., Kawanishi, S. 8-Hydroxydeoxyguanosine formation at the 5’site of 5′-GG-3’sequences in double-stranded DNA by UV radiation with riboflavin. Journal of Biological Chemistry. 268 (18), 13221-13227 (1993).
- Liang, J. Y., Yuann, J. M. P., Hsie, Z. J., Huang, S. T., Chen, C. C. Blue light induced free radicals from riboflavin in degradation of crystal violet by microbial viability evaluation. Journal of Photochemistry and Photobiology B, Biology. 174, 355-363 (2017).
- Yang, M. Y., Chang, C. J., Chen, L. Y. Blue light induced reactive oxygen species from flavin mononucleotide and flavin adenine dinucleotide on lethality of HeLa cells. Journal of Photochemistry and Photobiology B, Biology. 173, 325-332 (2017).
- Cui, Z., Huang, Y., Mo, Q., Wang, X., Qian, K. Inactivation of lymphocytes in blood products using riboflavin photochemical treatment with visible light. Photochemistry and Photobiology. 84 (5), 1195-1200 (2008).
- Wong, T. W., Cheng, C. W., Hsieh, Z. J., Liang, J. Y. Effects of blue or violet light on the inactivation of Staphylococcus aureus by riboflavin-5′-phosphate photolysis. Journal of Photochemistry and Photobiology B: Biology. 173, 672-680 (2017).
- Russell, L. F., Vanderslice, J. T. Comprehensive review of vitamin B2 analytical methodology. Journal of Micronutrient Analysis. 8, 257-310 (1990).
- Cheng, C. W., Chen, L. Y., Chou, C. W., Liang, J. Y. Investigations of riboflavin photolysis via coloured light in the nitro blue tetrazolium assay for superoxide dismutase activity. Journal of Photochemistry and Photobiology B: Biology. 148, 262-267 (2015).
- Huang, S. T., et al. Effects of 462 nm light-emitting diode on the inactivation of Escherichia coli and a multidrug-resistant by tetracycline photoreaction. Journal of Clinical Medicine. 7 (9), 278 (2018).
- Barua, M. G., Escalada, J. P., Bregliani, M., Pajares, A., Criado, S. Antioxidant capacity of (+)-catechin visible-light photoirradiated in the presence of vitamin B2. Redox Report: Communications in Free Radical Research. 22 (6), 282-289 (2017).
- Castillo, C., Criado, S., Díaz, M., García, N. A. Riboflavin as a sensitiser in the photodegradation of tetracyclines. Kinetics, mechanism and microbiological implications. Dyes and Pigments. 72 (2), 178-184 (2007).
- Huvaere, K., Sinnaeve, B., Van Bocxlaer, J., Skibsted, L. H. Flavonoid deactivation of excited state flavins: reaction monitoring by mass spectrometry. Journal of Agricultural and Food Chemistry. 60 (36), 9261-9272 (2012).
- Massad, W. A., Bertolotti, S., Garcia, N. A. Kinetics and mechanism of the vitamin B2-sensitized photooxidation of isoproterenol. Photochemistry and Photobiology. 79 (5), 428-433 (2004).
- Maclean, M., MacGregor, S. J., Anderson, J. G., Woolsey, G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiology Letters. 285 (2), 227-232 (2008).
- Huang, S. T., et al. The influence of the degradation of tetracycline by free radicals from riboflavin-5′-phosphate photolysis on microbial viability. Microorganisms. 7 (11), 500 (2019).
- Maisch, T., et al. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PLoS One. 9 (12), 111792 (2014).
- Grijzenhout, M., et al. Ultraviolet-B irradiation of platelets induces a dose-dependent increase in the expression of platelet activation markers with storage. British Journal of Haematology. 83 (4), 627-632 (1993).

