Inactivation of Pathogens via Visible-Light Photolysis of Riboflavin-5′-Phosphate
Summary
Here, we present a protocol to inactivate pathogenic bacteria with reactive oxygen species produced during photolysis of flavin mononucleotide (FMN) under blue and violet light irradiation of low intensity. FMN photolysis is demonstrated to be a simple and safe method for sanitary processes.
Abstract
Riboflavin-5'-phosphate (or flavin mononucleotide; FMN) is sensitive to visible light. Various compounds, including reactive oxygen species (ROS), can be generated from FMN photolysis upon irradiation with visible light. The ROS generated from FMN photolysis are harmful to microorganisms, including pathogenic bacteria such as Staphylococcus aureus (S. aureus). This article presents a protocol for deactivating S. aureus, as an example, via photochemical reactions involving FMN under visible light irradiation. The superoxide radical anion (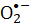 ) generated during the FMN photolysis is evaluated via nitro blue tetrazolium (NBT) reduction. The microbial viability of S. aureus that is attributed to reactive
) generated during the FMN photolysis is evaluated via nitro blue tetrazolium (NBT) reduction. The microbial viability of S. aureus that is attributed to reactive 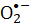 species was used to determine the effectiveness of the process. The bacterial inactivation rate is proportional to FMN concentration. Violet light is more efficient in inactivating S. aureus than blue light irradiation, while the red or green light does not drive FMN photolysis. The present article demonstrates FMN photolysis as a simple and safe method for sanitary processes.
species was used to determine the effectiveness of the process. The bacterial inactivation rate is proportional to FMN concentration. Violet light is more efficient in inactivating S. aureus than blue light irradiation, while the red or green light does not drive FMN photolysis. The present article demonstrates FMN photolysis as a simple and safe method for sanitary processes.
Introduction
Riboflavin-5′-phosphate (FMN) is formed by phosphorylation at the riboflavin 5′-position of the ribityl side-chain and is required by all flavoproteins for numerous cellular processes to generate energy. It also plays the role of vitamin for some functions in the human body1. FMN is approximately 200 times more soluble in water than riboflavin2.
The antibacterial photodynamic inactivation (aPDI) of bacteria is an efficient way to control resistance to bacteria3,4 because it does not depend on the mode of bacterial resistance. Clinically, aPDI is used to treat soft tissue infections in order to decrease infection of nosocomial skin due to multi-resistant bacteria5,6,7,8,9. aPDI also produces cell death by generating reactive oxygen species (ROS). ROS, such as superoxide radicals (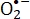 ), singlet oxygen, hydroxyl radicals (•OH), and peroxyl radicals, are free radicals or molecules that contain reactive oxygen10,11,12 and are normally reactive13. Similar to DNA damage that is caused by ROS, membrane peroxidation and destruction of endothelial cells are also adverse biochemical reactions that are attributed to ROS in cells12.
), singlet oxygen, hydroxyl radicals (•OH), and peroxyl radicals, are free radicals or molecules that contain reactive oxygen10,11,12 and are normally reactive13. Similar to DNA damage that is caused by ROS, membrane peroxidation and destruction of endothelial cells are also adverse biochemical reactions that are attributed to ROS in cells12.
The use of aPDI for pathogenic bacteria involves a visible or UV light source to inactivate microorganisms in the presence of chemical compounds, such as methylthioninium chloride14, PEI-ce6 conjugate15, porphyrin16, titanium dioxide17, toluidine blue O18, and zinc oxide nanoparticles19. Toluidine blue O and methylene blue are phenothiazinium dyes and methylene blue has toxic properties. Zinc oxide nanoparticles and UV irradiation are linked to adverse health and environmental effects. As such, the exploitation of a reliable, secure, and simple photosensitizer via photolysis under visible irradiation deserves further study.
The micronutrient, riboflavin or FMN, is not toxic and is indeed used for food manufacturing or feeding20. Both FMN and riboflavin are highly sensitive to light irradiation2. Under UV1,2,21,22,23 and blue light irradiation10,24, these two compounds achieve an excited state. The activated riboflavin or FMN that is produced by photolysis is promoted to its triplet state and ROS are generated simultaneously2,25. Kumar et al. reported that riboflavin activated by UV light selectively causes increased injury to the guanine moiety of DNA in pathogenic microorganisms26. Under irradiation by UV light, photodynamically activated riboflavin is demonstrated to promote the generation of 8-OH-dG, which is a biomarker for oxidative stress, in double-stranded DNA27. It is reported that S. aureus and E. coli are deactivated by ROS during riboflavin or FMN photolysis10,24,28. A previous study by the authors showed that the photolytic reactions involving riboflavin and FMN reduce crystal violet, a triarylmethane dye and an antibacterial agent that generates 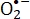 , and eliminate most of the antimicrobial capability of crystal violet28. When flavin adenine dinucleotide or FMN is irradiated by blue light, the resulting ROS produce apoptosis in HeLa cells for their poisoning in vitro29. Using photochemical treatment in the presence of riboflavin, Cui et al. inactivated lymphocytes by inhibiting their proliferation and cytokine production30.
, and eliminate most of the antimicrobial capability of crystal violet28. When flavin adenine dinucleotide or FMN is irradiated by blue light, the resulting ROS produce apoptosis in HeLa cells for their poisoning in vitro29. Using photochemical treatment in the presence of riboflavin, Cui et al. inactivated lymphocytes by inhibiting their proliferation and cytokine production30.
The photolysis of riboflavin is used for the inactivation of blood pathogen by UV10,24, but blood components can be impaired under UV light irradiation30. It is also reported that platelets exposed to UV progressively enhance the performance of the activation markers P-selectin and LIMP-CD63 on their membranes. The cytotoxicity of UV and high-intensity irradiation needs to be investigated and a photosensitizer that is uncomplicated and safe during an FMN photoreaction involving visible light would be of great use.
Light of shorter wavelengths has more energy and is much more likely to cause severe damage to cells. However, in the presence of a suitable photosensitizer, irradiation with low-intensity violet light can inhibit pathogenic microorganisms. The photosensitization and the generation of 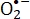 by FMN when irradiated with violet light is thus important to study, in order to determine the pathway by which ROS from FMN photolysis increases the inactivation of bacteria.
by FMN when irradiated with violet light is thus important to study, in order to determine the pathway by which ROS from FMN photolysis increases the inactivation of bacteria.
Antimicrobial control is a common issue and the development of new antibiotics frequently takes decades. After irradiation with violet light, photoinactivation that is intermediated by FMN can annihilate environmental pathogenic bacteria. This study presents an effective antimicrobial protocol in vitro using violet light for driving FMN photolysis and thus generating 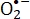 for aPDI. The microbial viability of S. aureus is used to determine the feasibility of FMN-induced aPDI.
for aPDI. The microbial viability of S. aureus is used to determine the feasibility of FMN-induced aPDI.
Protocol
Representative Results
Discussion
A photosensitizer increases the photochemical reaction of chemical compounds to generate ROS. Pathogenic microorganisms can be inactivated by light irradiation in the presence of photosensitizers. This study determines the aPDI of S. aureus due to ROS generated by violet light irradiation of an exogenous photosensitizer, FMN.
As shown in Figure 3, for FMN, the absorbance at 444 nm is reduced significantly after 5 min of irradiation using violet or blue li…
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors are grateful to Dr. Tak-Wah Wong and Mr. Zong-Jhe Hsieh for their support with experiments.
Materials
| Blue, green and red LED lights | Vita LED Technologies Co., Tainan, Taiwan | DC 12 V 5050 | |
| Dimethyl Sulfoxide | Sigma-Aldrich, St. Louis, MO | 190186 | |
| Infrared thermometer | Raytek Co. Santa Cruz, CA | MT4 | |
| LB broth | Difco Co., NJ | ||
| L-Methionine | Sigma-Aldrich, St. Louis, MO | 1.05707 | |
| NBT | Bio Basic, Inc. Markham, Ontario, Canada | ||
| Power supply | China tech Co., New Taipei City, Taiwan | YP30-3-2 | |
| Riboflavin 5′-phosphate | Sigma-Aldrich, St. Louis, MO | R7774 | |
| RNase | New England BioLabs, Inc. Ipswich, MA | ||
| Solar power meter | Tenmars Electronics Co., Taipei, Taiwan | TM-207 | |
| Staphylococcus aureus subsp. aureus | Bioresource Collection and Research Center (BCRC), Hsinchu, Taiwan | 10451 | |
| UV-Vis optical spectrometer | Ocean Optics, Dunedin, FL | USB4000 | |
| UV-Vis spectrophotometer | Hitachi High-Tech Science Corporation,Tokyo, Japan | U-2900 | |
| Violet LED | Long-hui Electronic Co., LTD, Dongguan, China |
References
- Jian, H. L., Cheng, C. W., Chen, L. Y., Liang, J. Y. The photochemistry of riboflavin. MC-Transaction on Biotechnology. 3, 1-11 (2011).
- Lin, Y., Eitenmiller, R. R., Landen, W. O. Riboflavin. Vitamin analysis for the health and food sciences. , 329-360 (2008).
- Xie, L. J., Wang, R. L., Wang, D., Liu, L., Cheng, L. Visible-light-mediated oxidative demethylation of N(6)-methyl adenines. Chemical Communications. 53 (77), 10734-10737 (2017).
- Tim, M. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. Journal of Photochemistry and Photobiology B: Biology. 150, 2-10 (2015).
- Maisch, T., et al. Photodynamic inactivation of multi-resistant bacteria (PIB) – a new approach to treat superficial infections in the 21st century. Journal of the German Society of Dermatology. 9 (5), 360-366 (2011).
- Hamblin, M. R. Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes. Current Opinion in Microbiology. 33, 67-73 (2016).
- Del Rosso, J. Q. Oral Doxycycline in the management of acne vulgaris: Current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. The Journal of Clinical and Aesthetic Dermatology. 8 (5), 19-26 (2015).
- Wong, T. W., et al. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by indocyanine green and near infrared light. Dematologica Sinica. 36 (1), 8-15 (2018).
- Yuann, J. M. P., et al. Effects of free radicals from doxycycline hyclate and minocycline hydrochloride under blue light irradiation on the deactivation of Staphylococcus aureus, including a methicillin-resistant strain. Journal of Photochemistry and Photobiology B: Biology. 226, 112370 (2022).
- Liang, J. Y., Cheng, C. W., Yu, C. H., Chen, L. Y. Investigations of blue light-induced reactive oxygen species from flavin mononucleotide on inactivation of E. coli. Journal of Photochemistry and Photobiology B: Biology. 143, 82-88 (2015).
- Yang, M. J., et al. Effects of blue-light-induced free radical formation from catechin hydrate on the inactivation of Acinetobacter baumannii, Including a carbapenem-resistant strain. Molecules. 23 (7), 1631 (2018).
- Yuann, J. M. P., et al. A study of catechin photostability using photolytic processing. Processes. 9 (2), 293 (2021).
- Yuann, J. M. P., Wang, J. S., Jian, H. L., Lin, C. C., Liang, J. Y. Effects of Clinacanthus nutans (Burm. f) Lindau leaf extracts on protection of plasmid DNA from riboflavin photoreaction. MC-Transaction on Biotechnology. 4 (1), 45-59 (2012).
- Rineh, A., et al. Attaching NorA efflux pump inhibitors to methylene blue enhances antimicrobial photodynamic inactivation of Escherichia coli and Acinetobacter baumannii in vitro and in vivo. Bioorganic and Medicinal Chemistry Letters. 28 (16), 2736-2740 (2018).
- Dai, T., et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrobial Agents and Chemotherapy. 53 (9), 3929-3934 (2009).
- Nitzan, Y., Ashkenazi, H. Photoinactivation of Acinetobacter baumannii and Escherichia coli B by a cationic hydrophilic porphyrin at various light wavelengths. Current Microbiology. 42 (6), 408-414 (2001).
- Tseng, C. C., et al. Altered susceptibility to the bactericidal effect of photocatalytic oxidation by TiO2 is related to colistin resistance development in Acinetobacter baumannii. Applied Microbiology and Biotechnology. 100 (19), 8549-8561 (2016).
- Boluki, E., et al. Antimicrobial activity of photodynamic therapy in combination with colistin against a pan-drug resistant Acinetobacter baumannii isolated from burn patient. Photodiagnosis and Photodynamic Therapy. 18, 1-5 (2017).
- Yang, M. Y., et al. Blue light irradiation triggers the antimicrobial potential of ZnO nanoparticles on drug-resistant Acinetobacter baumannii. Journal of Photochemistry and Photobiology B, Biology. 180, 235-242 (2018).
- . Code of Federal Regulations Available from: https://www.gpo.gov/fdsys/pkg/CFR-2009-title21-vol6/pdf/CFR-2009-title21-vol6-sec582-5695.pdf (2022)
- Lu, C. Y., et al. Generation and photosensitization properties of the oxidized radical of riboflavin: a laser flash photolysis study. Journal of Photochemistry and Photobiology B, Biology. 52 (1-3), 111-116 (1999).
- Sato, K., et al. The primary cytotoxicity in ultraviolet-a-irradiated riboflavin solution is derived from hydrogen peroxide. The Journal of Investigative Dermatology. 105 (4), 608-612 (1995).
- Tripathi, A. K., et al. Attenuated neuroprotective effect of riboflavin under UV-B irradiation via miR-203/c-Jun signaling pathway in vivo and in vitro. Journal of Biomedical Science. 21 (1), 39 (2014).
- Liang, J. Y., et al. Blue light induced free radicals from riboflavin on E. coli DNA damage. Journal of Photochemistry and Photobiology B, Biology. 119, 60-64 (2013).
- Ottaway, P. B. . The Technology of Vitamins in Food. , 233-244 (1993).
- Kumar, V., et al. Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level. Photochemistry and Photobiology. 80 (1), 15-21 (2004).
- Ito, K., Inoue, S., Yamamoto, K., Kawanishi, S. 8-Hydroxydeoxyguanosine formation at the 5’site of 5′-GG-3’sequences in double-stranded DNA by UV radiation with riboflavin. Journal of Biological Chemistry. 268 (18), 13221-13227 (1993).
- Liang, J. Y., Yuann, J. M. P., Hsie, Z. J., Huang, S. T., Chen, C. C. Blue light induced free radicals from riboflavin in degradation of crystal violet by microbial viability evaluation. Journal of Photochemistry and Photobiology B, Biology. 174, 355-363 (2017).
- Yang, M. Y., Chang, C. J., Chen, L. Y. Blue light induced reactive oxygen species from flavin mononucleotide and flavin adenine dinucleotide on lethality of HeLa cells. Journal of Photochemistry and Photobiology B, Biology. 173, 325-332 (2017).
- Cui, Z., Huang, Y., Mo, Q., Wang, X., Qian, K. Inactivation of lymphocytes in blood products using riboflavin photochemical treatment with visible light. Photochemistry and Photobiology. 84 (5), 1195-1200 (2008).
- Wong, T. W., Cheng, C. W., Hsieh, Z. J., Liang, J. Y. Effects of blue or violet light on the inactivation of Staphylococcus aureus by riboflavin-5′-phosphate photolysis. Journal of Photochemistry and Photobiology B: Biology. 173, 672-680 (2017).
- Russell, L. F., Vanderslice, J. T. Comprehensive review of vitamin B2 analytical methodology. Journal of Micronutrient Analysis. 8, 257-310 (1990).
- Cheng, C. W., Chen, L. Y., Chou, C. W., Liang, J. Y. Investigations of riboflavin photolysis via coloured light in the nitro blue tetrazolium assay for superoxide dismutase activity. Journal of Photochemistry and Photobiology B: Biology. 148, 262-267 (2015).
- Huang, S. T., et al. Effects of 462 nm light-emitting diode on the inactivation of Escherichia coli and a multidrug-resistant by tetracycline photoreaction. Journal of Clinical Medicine. 7 (9), 278 (2018).
- Barua, M. G., Escalada, J. P., Bregliani, M., Pajares, A., Criado, S. Antioxidant capacity of (+)-catechin visible-light photoirradiated in the presence of vitamin B2. Redox Report: Communications in Free Radical Research. 22 (6), 282-289 (2017).
- Castillo, C., Criado, S., Díaz, M., García, N. A. Riboflavin as a sensitiser in the photodegradation of tetracyclines. Kinetics, mechanism and microbiological implications. Dyes and Pigments. 72 (2), 178-184 (2007).
- Huvaere, K., Sinnaeve, B., Van Bocxlaer, J., Skibsted, L. H. Flavonoid deactivation of excited state flavins: reaction monitoring by mass spectrometry. Journal of Agricultural and Food Chemistry. 60 (36), 9261-9272 (2012).
- Massad, W. A., Bertolotti, S., Garcia, N. A. Kinetics and mechanism of the vitamin B2-sensitized photooxidation of isoproterenol. Photochemistry and Photobiology. 79 (5), 428-433 (2004).
- Maclean, M., MacGregor, S. J., Anderson, J. G., Woolsey, G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiology Letters. 285 (2), 227-232 (2008).
- Huang, S. T., et al. The influence of the degradation of tetracycline by free radicals from riboflavin-5′-phosphate photolysis on microbial viability. Microorganisms. 7 (11), 500 (2019).
- Maisch, T., et al. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PLoS One. 9 (12), 111792 (2014).
- Grijzenhout, M., et al. Ultraviolet-B irradiation of platelets induces a dose-dependent increase in the expression of platelet activation markers with storage. British Journal of Haematology. 83 (4), 627-632 (1993).

