Nuclei Isolation from Mouse Cardiac Progenitor Cells for Epigenome and Gene Expression Profiling at Single-Cell Resolution
Summary
Here, we present a protocol describing cell nuclei preparation. After microdissection and enzymatic dissociation of cardiac tissue into single cells, the progenitor cells were frozen, followed by isolation of pure viable cells, which were used for single-nucleus RNA sequencing and the single-nucleus assay for transposase-accessible chromatin with high-throughput sequencing analyses.
Abstract
The developing heart is a complex structure containing various progenitor cells controlled by complex regulatory mechanisms. The examination of the gene expression and chromatin state of individual cells allows the identification of the cell type and state. Single-cell sequencing approaches have revealed a number of important characteristics of cardiac progenitor cell heterogeneity. However, these methods are generally restricted to fresh tissue, which limits studies with diverse experimental conditions, as the fresh tissue must be processed at once in the same run to reduce the technical variability. Therefore, easy and flexible procedures to produce data from methods such as single-nucleus RNA sequencing (snRNA-seq) and the single-nucleus assay for transposase-accessible chromatin with high-throughput sequencing (snATAC-seq) are needed in this area. Here, we present a protocol to rapidly isolate nuclei for subsequent single-nuclei dual-omics (combined snRNA-seq and snATAC-seq). This method allows the isolation of nuclei from frozen samples of cardiac progenitor cells and can be combined with platforms that use microfluidic chambers.
Introduction
Among birth defects, congenital heart defects (CHDs) are the most common, occurring in about 1% of live births each year1,2. Genetic mutations are identified in only a minority of cases, implying that other causes, such as abnormalities in gene regulation, are involved in the etiology of CHD2,3. Cardiac development is a complex process of diverse and interacting cell types, making the identification of causal noncoding mutations and their effects on gene regulation challenging. Organogenesis of the heart begins with cellular progenitors that give rise to different subtypes of cardiac cells, including myocardial, fibroblast, epicardial, and endocardial cells4,5. Single-cell genomics is emerging as a key method for studying heart development and assessing the impact of cellular heterogeneity in health and disease6. The development of multi-omics methods for the simultaneous measurement of different parameters and the expansion of computational pipelines has facilitated the discovery of cell types and subtypes in the normal and diseased heart6. This article describes a reliable single-nucleus isolation protocol for frozen cardiac progenitor cells obtained from mouse embryos that is compatible with downstream snRNA-seq and snATAC-seq (as well as snRNA-seq and snATAC-seq combined)7,8,9.
ATAC-seq is a robust method that allows the identification of regulatory open chromatin regions and the positioning of nucleosomes10,11. This information is used to draw conclusions about the location, identity, and activity of transcription factors. The activity of chromatin factors, including remodelers, as well as the transcriptional activity of RNA polymerase, can, thus, be analyzed since the method is highly sensitive for measuring quantitative changes in chromatin structure1,2. Thus, ATAC-seq provides a robust and impartial approach to uncovering the mechanisms controlling transcriptional regulation in a specific cell type. ATAC-seq protocols have also been validated to measure chromatin accessibility in single cells, revealing variability in the chromatin architecture within cell populations10,12,13.
Although there have been notable advances in the field of single cells in recent years, the main difficulty is the processing of the fresh samples needed to perform these experiments14. To circumvent this difficulty, various tests have been carried out with the aim of conducting analyses such as snRNA-seq and snATAC-seq with frozen cardiac tissue or cells15,16.
Several platforms have been used to analyze single-cell genomics data17. The widely used platforms for single-cell gene expression and ATAC profiling are platforms for multiple microfluidic droplet encapsulation17. As these platforms use microfluidic chambers, debris or aggregates can clog the system, resulting in non-usable data. Thus, the success of single-cell studies depends on the accurate isolation of individual cells/nuclei.
The protocol presented here uses a similar approach to recent studies using snRNA-seq and snATAC-seq to understand congenital heart defects18,19,20,21,22,23. This procedure utilizes the enzymatic dissociation of freshly microdissected cardiac tissue followed by the cryopreservation of mouse cardiac progenitor cells. After thawing, the viable cells are purified and processed for nuclear isolation. In this work, this protocol was successfully used to obtain snRNA-seq and snATAC-seq data from the same nuclear preparation of mouse cardiac progenitor cells.
Protocol
The animal procedure adopted in this study was approved by the animal ethics committees of the Aix-Marseille University (C2EA-14) and was carried out according to protocols approved by the appointed national ethical committee for animal experimentation (Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche; Authorization Apafis N°33927-2021111715507212).
1. Setting up the timed mating prior to dissection
- To generate mouse embryos, perform a timed mating between adult mice 9.5 days prior to heart region isolation. In this procedure, wild-type C57BL/6J mice aged 2-6 months were used, and timed mating was performed overnight.
- The next morning, check if the female mice have a vaginal plug. Consider the day the plug is identified as embryonic day (E) 0.5.
2. Tissue preparation and cell isolation
- Euthanize 2-6 months old pregnant female C57BL/6J at day 9.5 post-conception via CO2 with an increasing concentration followed by cervical dislocation. Clean the lower part of the abdomen with 70% ethanol.
NOTE: The use of anesthetics before cervical dislocation is not recommended as this would expose the embryos to environmental changes that could impact subsequent transcriptomic and epigenomic studies. - Open the abdomen and peritoneum with scissors. Visualize the uterine horns, and use forceps to excise both horns.
- Place the horns in a Petri dish containing cold complete medium with 1% FBS. While no specific volume is required, care must be taken to ensure that the embryos are completely immersed in the medium. An approximate volume of 10 mL per 10 cm Petri dish is appropriate.
- Under the stereomicroscope set at 5x magnification and using forceps, remove the endometrial tissue, placenta, and yolk sac from each embryo.
- Place the embryos in a Petri dish with cold complete medium with 1% FBS. Dissect the embryonic heart region, as described on the schematic embryo illustrated in Figure 1, in cold complete medium.
- Pool together the heart regions dissected from five mouse embryos in a 1.5 mL microcentrifuge tube. Prechill swinging bucket centrifuges to 4 °C.
- Centrifuge at 300x g for 1 min at RT in a swinging bucket centrifuge. Remove the supernatant, and wash the tissues by adding 1 mL of tissue culture-grade PBS.
- Centrifuge at 300 x g for 1 min at RT. Remove the supernatant, and repeat the washing steps for a total of two washes. Repeat the wash twice, replacing the PBS with 0.05% trypsin/EDTA (1x).
- For tissue digestion, add 50 µL of 0.25% trypsin/EDTA for 10 min at 37 °C. Apply gentle mechanical dissociation after 5 min by up and down gestures using a wide-orifice filter tip.
- Inactivate trypsin digestion activity by adding 350 µL of complete medium to the dissociated cells. Pass the resuspended cells through a 40 µm cell strainer.
3. Cell counting and viability assessment
NOTE: The cell count and viability are critical parameters for the success of single-cell experiments. The snATAC-seq approach is sensitive to minor variations in cell number. Too few cells lead to over-digestion of the chromatin, which results in a higher number of reads and the mapping of inaccessible chromatin regions (noise). Similarly, having too many cells generates high-molecular-weight fragments that are hard to sequence. For the accurate determination of the cell and nuclei concentrations, the cell count and viability were assessed by two different methods.
- Perform a trypan blue assay for cell counting, as described below.
- Vortex 0.4% trypan blue staining solution, spin for 10 s at room temperature at 2,000 x g, and transfer 10 µL into a microtube.
- Using a pipette, mix the cell suspension, and add 10 µL of suspension to the already aliquoted 10 µL of trypan blue solution. Mix carefully 10 times with a pipette.
- Transfer 10 µL of the stained cells into a counting slide. Place the slide into the counter to automatically calculate the cell concentration and viability.
- Perform a fluorescence-based assessment of mortality as described below.
NOTE: This assay is based on the use of fluorescent dyes (ethidium homodimer-1, EthD-1, and calcein AM) to evaluate the cell viability. A commercially available kit was used, and the provider's instructions were followed for the appropriate preparation and usage.- Prepare a 10 µM EthD-1 solution by adding 5 µL of the supplied 2 mM EthD-1 stock solution to 1 mL of sterile tissue-culture grade D-PBS, and vortex for 5 s to ensure complete mixing.
- Transfer 2.5 µL of the supplied 4 mM calcein AM stock solution to the already prepared EthD-1 solution. Vortex for 5 s to ensure complete mixing.
- Add 10 µL of the resulting 10 µM EthD-1 and 10 µM calcein AM working solution to 10 µL of cell suspension.
NOTE: Calcein is highly susceptible to hydrolysis in aqueous solutions, so use this working solution within 1 day. - Transfer 10 µL of the stained cells onto a counting slide. Insert the slide into the automated fluorescent cell counter. Count the live cells using a GFP filter and the dead cells with an RFP filter.
NOTE: The two counting methods gave similar cell counts and viability, and the total number of freshly dissociated cells was estimated to average around 20,000 cells per embryonic heart region (0.06 mm3).
4. Cell freezing
- Centrifuge the cell suspension at 500 x g for 5 min at 4 °C. Carefully remove the supernatant without touching the bottom of the tube, and resuspend the pellet in 1 mL of chilled freezing medium.
- Transfer the cell suspension into a pre-cooled cryovial, and place the cryovial in a pre-cooled freezing container.
- Place the container at −80 ◦C for 4 h to 8 h. Transfer the cryovial to liquid nitrogen for downstream single-nucleus RNA and ATAC sequencing experiments.
NOTE: The cryovial should be transported on dry ice prior to usage. In our experience, the cells may be stored in liquid nitrogen for at least 6 months with no loss of cell viability. The storage temperature should be kept below −150 °C all times to prevent the formation of ice crystals.
5. Cell thawing and removal of dead cells
- Place the cryovials in a 37 °C water bath for 1-2 min. Remove it when a tiny crystal remains in the cryovial.
- Add 1 mL of pre-warmed (37 °C) complete medium. Mix with a pipette three times. Transfer the suspension to a 15 mL conical tube containing 10 mL of warm complete medium.
- Pass the entire cell suspension over a 30 µm strainer. Rinse the strainer with 1-2 mL of warm complete medium, and collect the flowthrough in the same conical tube.
- Centrifuge at 300 x g for 5 min, and remove supernatant. Wash one time with PBS, and transfer the cell suspension into a 1.5 mL microtube.
- Centrifuge at 300 x g for 5 min, and remove the supernatant without disrupting the cell pellet.
- Add 100 µL of the provided magnetic microbeads to the pelleted cells, and homogenize five times using a wide-bore pipette tip. Incubate for 15 min at RT.
- In the meantime, rinse the magnetic separation column (capacity: 1 x 107 to 2 x 108 cells) with 500 µL of 1x binding buffer.
- Once the incubation is completed, dilute the cell suspension containing the microbeads with 500 µL of 1x binding buffer.
- Apply the cell suspension (0.6 µL) to the prepared magnetic separation column. The flow-through is the negatively selected live cell fraction, and the magnetically retained fraction is the positively selected dead cells.
- Collect the live cell effluent in a 15 mL centrifuge tube. Rinse the 1.5 mL microtube that contained the cell suspension and the column with 2 mL of 1x binding buffer, and collect the effluent in the same 15 mL tube.
- Centrifuge at 300 x g for 5 min. Remove the supernatant without disrupting the cell pellet.
- Add 1 mL of the PBS-BSA solution, and mix gently by pipetting five times using a wide-orifice pipette tip. Transfer the cell suspension into a 1.5 mL microtube.
- Centrifuge at 300 x g for 5 min. Remove the supernatant without disrupting the cell pellet.
- Add 1 mL of PBS-BSA to resuspend the pellet, and repeat the washing step for a total of two washes.
- Resuspend the cells in 100 µL of PBS-BSA solution. Mix gently by pipetting 10 times.
- Determine the concentration and viability of the cells using the previously described methods (step 3.1 and step 3.2). To proceed to the next step, ensure a cell count of ≥100,000 cells. See Figure 2 for representative results.
NOTE: Cryopreservation results in a loss of approximately 40% of the initial starting material of live cells. Depending on the starting number of cells, bead separation on a magnetic column results in high cell viability but divides the total number of cells by two-fold to five-fold. The utilization of a column-based magnetic kit to remove the dead cells is advised only when sufficient starting material is available.
6. Nuclei isolation
NOTE: snATAC and snRNA-seq combined are performed with a suspension of clean and intact nuclei. The optimization of the lysis conditions (lysis time and NP40 concentration) for the used cell type is recommended. The optimal lysis timepoint and detergent concentration are those that result in the maximum number of cells being lysed without disrupting the nuclear morphology. The loss of cells/nuclei can be reduced by using a swinging bucket centrifuge instead of a fixed-angle centrifuge. To minimize nuclei retention on plastics, it is recommended to use low-retention pipette tips and centrifuge tubes. This can increase the nuclei recovery. The coating of the pipette tips and centrifuge tubes with 5% BSA is a less expensive but more time-consuming alternative.
- Clean the working place and materials with 70% ethanol and RNase removing solution.
- Prechill swinging bucket centrifuges to 4 °C. Freshly prepare lysis buffer, lysis dilution buffer, 0.1x lysis buffer, and wash buffer (see Table 1). Maintain the buffers on ice.
- Centrifuge the cell suspension at 500 x g for 5min at 4 °C in a swinging bucket centrifuge. Gently remove all the supernatant without touching the bottom of the tube to avoid dislodging the cell pellet.
- Add 100 µL of chilled 0.1x lysis buffer. Gently mix by pipetting 10 times. Incubate for 5 min on ice.
- Add 1 mL of chilled wash buffer, and gently mix by pipetting five times. Centrifuge at 500 x g for 5 min at 4 °C. Remove the supernatant without disrupting the nuclei pellet.
- Repeat the washes with chilled wash buffer for a total of three washes. Prepare the diluted nuclei buffer. After resuspension, keep the nuclei stock solution on ice.
7. Quality and quantity assessments of the isolated nuclei
NOTE: Based on the initial cell count and estimating approximately 50% nuclei loss during cell lysis, resuspend the appropriate number of cells in cold diluted nuclei buffer. The calculation of the resuspension volume with chilled diluted nuclei buffer is based on the targeted nuclei recovery and the corresponding nuclei stock concentrations recommended by the manufacturer's user guide. See an example of this calculation in Supplementary Protocol 1.
- Based on the initial cell count and estimating approximately 50% nuclei loss during cell lysis, resuspend the appropriate number of cells in cold diluted nuclei buffer.
- Determine the actual nuclei concentration. Mix 2 µL of nuclei stock solution with 8 µL of diluted nuclei buffer and add the mix to the pre-aliquoted 10 µL of trypan blue or EthD-1/calceinAM working solution. Count the nuclei using an automated cell counter. Less than 5% of the cells should be viable. See Figure 3 for representative results.
- Calculate the volume of nuclei stock and the volume of diluted nuclei buffer to obtain a total volume of 5 µL at the recommended concentration for the transposition reaction (refer to the manufacturer's user guide). Proceed immediately to the transposition reaction according to the user guide17.
NOTE: All the steps from the transposition reaction to the construction of the ATAC and GEX libraries were performed according to the user guide of the kit used for snRNA-seq and snATAC-seq combined17.
8. Quality analysis of the snRNA-seq and snATAC-seq libraries
- Before proceeding to next-generation sequencing, validate the quality and size distribution of the final snATAC-seq and gene expression GEX libraries. Assess the quality and quantity of sequences identified in the libraries using fragment analysis systems. See Figure 4 for representative quality assessment results.
NOTE: The final trace of the snATAC-seq libraries should show the periodicity of nucleosome winding, and the sizes should range from 200 bp to several Kbp, whereas the sizes in the gene expression library should range from 300 bp to 600 bp.
Representative Results
Compared to the preparation of single-cell suspensions for single-cell approaches, the preparation of single-nuclei suspensions is much more challenging and requires a higher degree of resolution and processing. The key factor for successful combined snRNA-seq and snATAC-seq is a clean and intact nuclei suspension. The protocol for efficient nuclei isolation must be adapted to each tissue type and condition (fresh or frozen). Here, an optimized protocol is described for the isolation of nuclei from frozen mouse embryonic cardiac cells. All the steps from embryonic cardiac region dissection and cardiac cell dissociation to nuclei isolation are summarized in Figure 1. For the starting material, a cell count of 1 x 105-1 x 106 cells and a cell viability >70% are recommended for efficient nuclei isolation. As shown in Figure 2, the additional step performed in this protocol to sort and remove the dead cells after thawing allowed for obtaining a cleaner cell suspension with a significantly higher cell viability and for improving the quality of the starting sample. In addition to nuclei isolation, this protocol provides detailed information on how to assess the quality and quantity of the isolated nuclei (Figure 3). The quality of the isolated nuclei greatly impacts the quality of the snRNA-seq and snATAC-seq combined data; here, the quality of the isolated nuclei was assessed by the evaluation of the nuclear membrane morphology. The isolated nuclei appeared smooth and uniformly round, as shown in Figure 3C, under brightfield microscopy, indicating an effective isolation process. For more accuracy, two different counting methods were used, including trypan blue and a kit for the fluorescence-based assessment of viability, to quantify the cells and nuclei. The fluorescence assay was used to determine the cell viability based on cellular integrity and intracellular esterase activity. This dye solution allows for distinguishing live cells from dead cells by simultaneously visualizing green fluorescent calcein AM, which indicates the intracellular esterase activity, and red fluorescent ethidium homodimer 1, which reflects the loss of cellular integrity. Using a fluorescent dye for counting helps distinguish nuclei from cell clumps and debris. Using this protocol, the cell viability passed from 80%-90% before nuclei isolation to <5% after nuclei isolation, as shown in Figure 3A,B.
Our procedure was compared to the existing method, which involves freezing fresh tissue directly in liquid nitrogen and performing the lysing step without prior enzymatic dissociation (Supplementary Protocol 2 and Supplementary Figure 1). Both approaches were tested to determine which method gives a better-quality nuclei suspension. Snap-freezing whole embryonic cardiac tissue gave rise to a high percentage of non-lysed cells detected as live, indicating inappropriate cell lysis (Supplementary Figure 1A). Aggregates and non-individual cells were observed despite filtration through filters (Supplementary Figure 1A,B).
The isolated nuclei were used to generate libraries for joint snATAC-seq and snRNA-seq. Figure 4 illustrates the quality control results of the cDNA used for the construction of the gene expression (GEX) library, and the ATAC library. The quality control was performed using automated electrophoresis (Figure 5A). The mRNA-derived cDNAs were quantified, and the yield was sufficient for subsequent use in GEX library construction. A high-quality GEX library ranging from ~300 bp to 600 bp and a high-quality ATAC library with periodic nucleosome winding ranging from 200 bp to 7 kbp were obtained. These libraries were sequenced by high-throughput sequencing and successfully generated single-nucleus gene expression and chromatin accessibility (Figure 5A). These raw data were ready to be demultiplexed and bioinformatically analyzed to generate transcriptional and epigenetic profiling of mouse E9.5 cardiac progenitor cells. SnRNA-seq and snATAC-seq were clustered, identified, and labeled based on known marker genes using unsupervised clustering with the Seurat toolkit for single genomics24 . This initial analysis confirmed the presence of all the expected cardiac cell types at E9.5 (Figure 5B).
All of the above results support the success of this protocol in isolating nuclei that are suitable for downstream single nuclei approaches from frozen embryonic cardiac cells.
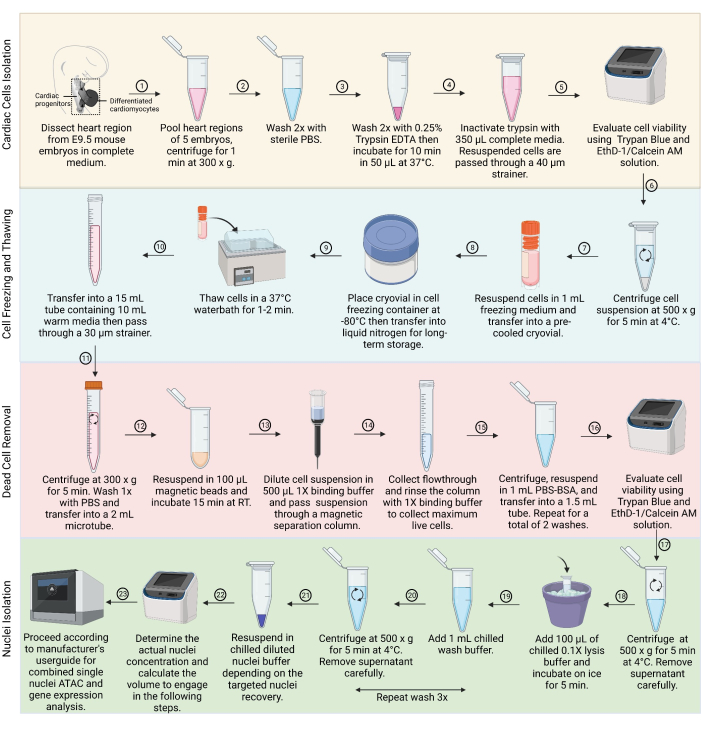
Figure 1: Representation of the main steps in the sample preparation for combined snRNA-seq and snATAC-seq profiling. This flowchart recapitulates all the steps, including cardiac tissue dissection and cardiac cell dissociation, cell freezing and thawing, live cell purification by removing dead cells, and nuclei isolation, that are carried out for the simultaneous detection of mRNA and chromatin accessibility from the same cell. Abbreviations: PBS = phosphate-buffered saline; BSA = bovine serum albumin; RT = room temperature. Created with BioRender.com Please click here to view a larger version of this figure.
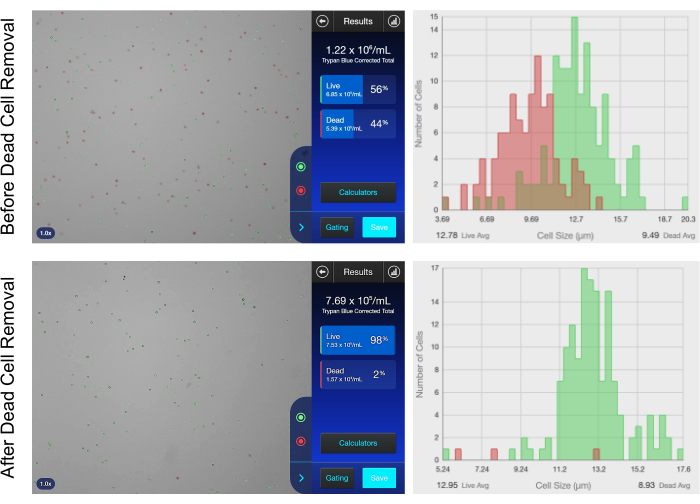
Figure 2: Viability of the optimized thawed cells after dead cell sorting. Images showing the cell count and viability using an automated cell counter before (top) and after (bottom) dead cell removal using the trypan blue exclusion dye. Please click here to view a larger version of this figure.
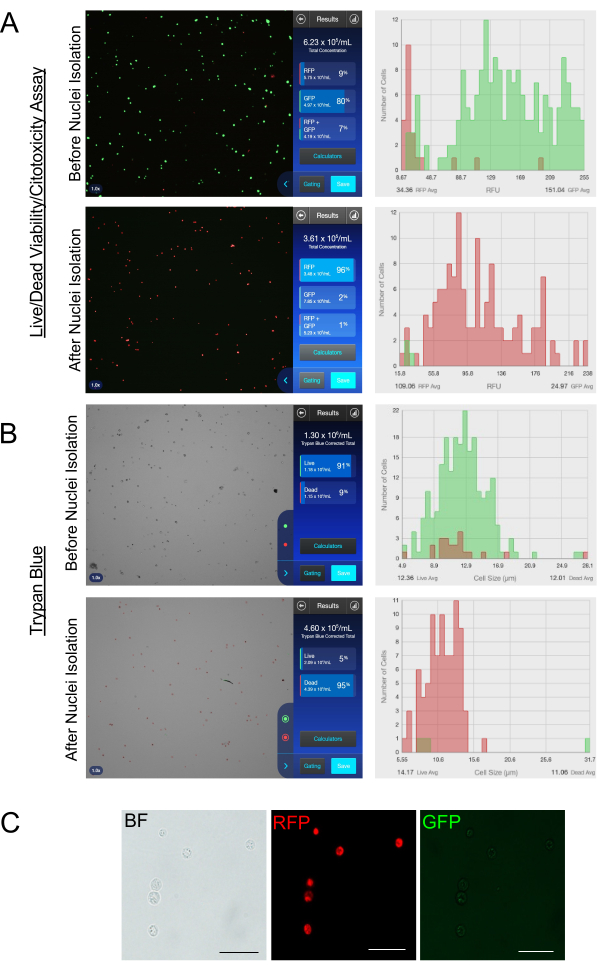
Figure 3: Quality control of the nuclei preparation. (A,B) Images showing the cell count and viability using an automated cell counter before (top) and after (bottom) nuclei isolation. Two counting methods were used: (A) the fluorescent mortality assay and (B)the trypan blue assay. (C) Images showing the quality of the nuclei isolation. Images were taken at 20x (scale bar = 50 µm) using brightfield and fluorescence microscopy. The brightfield image (left) shows nuclear morphology; the RFP (middle) shows cells that lost their membrane integrity, in other words, isolated nuclei; and the GFP (right) that normally indicates the esterase activity of live cells here confirms the absence of live cells in the nuclei suspension. Please click here to view a larger version of this figure.
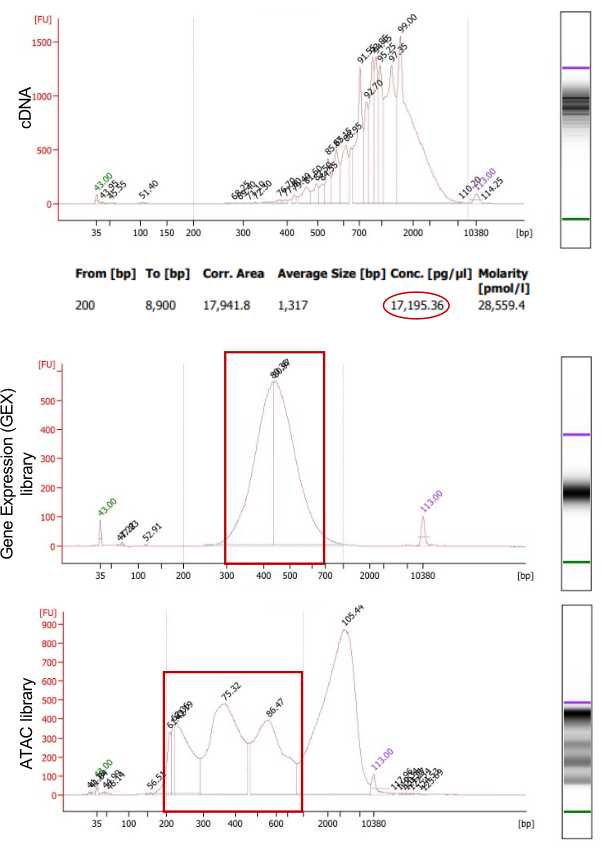
Figure 4: Quality control of the single-cell gene expression and ATAC libraries. DNA analysis with automated electrophoresis showing the quality and size distribution of the cDNA (top), GEX library (middle), and ATAC library (bottom). For the cDNA quantification, the zone ranging from 200 bp to 8,900 bp was selected, and the bioanalyzer estimated the cDNA concentration (in pg/µL; red circle). A sufficient cDNA yield was obtained to proceed with GEX library construction. Please click here to view a larger version of this figure.
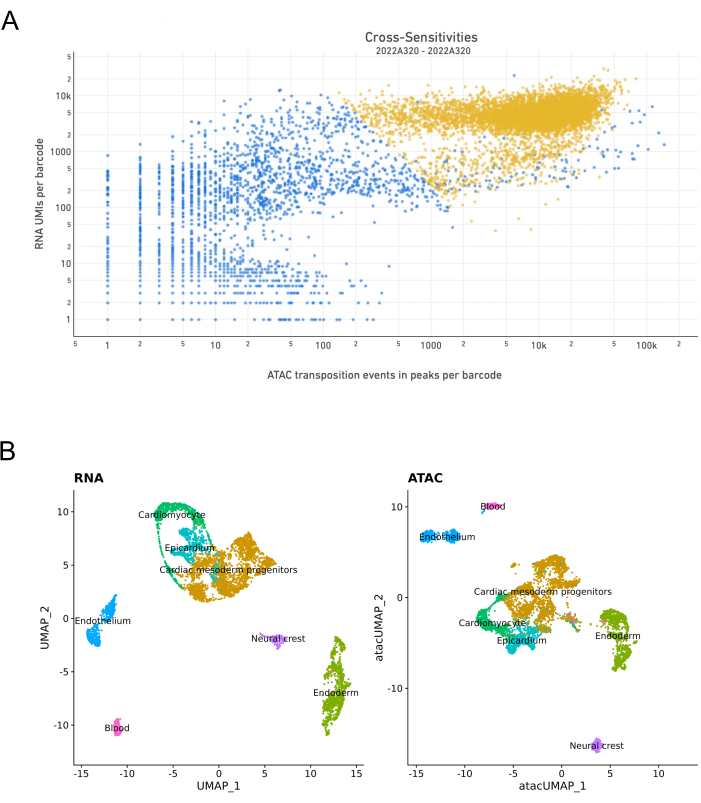
Figure 5: Sample performance assessed with the Cell Ranger software25. (A) Cross-sensitivity plot showing the transposition events in peaks per barcode on the x-axis and RNA unique molecular identifiers (UMIs) per barcode on the y-axis. As expected, the cells are mainly located in the upper-right-hand corner, with high RNA and ATAC data. A clear separation between the cells and non-cells (empty GEMs, background signal) was observed. (B) Representative uniform manifold approximation and projection (UMAP) plot of all the captured cells in scRNA-seq (left) and scATAC-seq (right) colored by cluster identity and grouped based on cell types. A good separation of clusters was observed. Joint GEX and ATAC raw data were obtained from sequencing 7,000 nuclei from the dissected E9.5 mouse embryonic heart region. Please click here to view a larger version of this figure.
Supplementary Figure 1: Quality control of nuclei isolation from the existing method involving freezing fresh tissue directly in liquid nitrogen. (A) Images showing the isolated nuclei count using an automated cell counter and trypan blue as the exclusion dye before (top) and after (bottom) filtration through a 40 µm strainer. A high percentage of non-lysed cells were detected as live. The detection of 20% live cells indicates inappropriate cell lysis. The black arrows indicate aggregates. (B) Images showing the quality of the isolated nuclei. The images were taken at 20x (top and middle with a scale bar of 50 µm) and 40x (bottom with a scale bar of 25 µm) using brightfield and fluorescence microscopy. The brightfield image (left) shows the nuclear morphology; the RFP (right) shows cells that lost their membrane integrity, in other words, isolated nuclei. The black arrows indicate multiplets of nuclei attached by debris. Please click here to download this File.
Table 1: Table of buffers and solutions used in the procedure. Please click here to download this Table.
Supplementary Protocol 1: Assessment of the quantity of the isolated nuclei. Please click here to download this File.
Supplementary Protocol 2: Isolation of nuclei from flash-frozen tissue. Please click here to download this File.
Discussion
The analysis of the cellular composition of the developing heart by combined snRNA-seq and snATAC-seq studies provides a deeper understanding of the origin of congenital heart disease26. Several research laboratories have studied the effects of cardiac tissue cryopreservation on snRNA-seq27. Conducting snRNA-seq and snATAC-seq using fresh micro-dissected tissue from mouse models of human disease can be logistically challenging when comparing different experimental conditions. For a given stage of development, one difficulty is that the control and experimental groups must be processed in the same run to reduce the technical variability introduced by sample processing. When processing fresh tissue is not possible, it is recommended, based on our results, to dissociate and then cryopreserve rather than snap-freeze the whole embryonic cardiac tissue. The choice of one method over the other is based on the fact that if the cardiac tissue is frozen whole, isolating viable single cells after thawing is much more challenging. However, if the tissue dissociation protocol is not optimized, then it may be better to snap-freeze the tissue whole. Dissociation followed by cryopreservation is preferred given that this tissue dissociation protocol yields >90% fresh viable cells with no cell-type bias.
This approach has several benefits, such as the use of frozen cells, which eliminates the requirement for fresh tissue, and the use of a single-cell dissociation step, which allows for the isolation of intact single nuclei; indeed, this is critical for downstream single-cell analysis. However, the enzymatic dissociation may induce transcriptional bias in cell populations. Enzymatic cell dissociation interferes with the cellular microenvironment and, thus, induces transcriptional changes in most cell types28. The primary determinant of these changes is the dissociation time, not the cell type29. To moderate dissociation-induced artifacts it is important to follow the recommended digestion time. The published literature in diverse tissues indicates that a digestion time of 30 min or less induces minor transcriptional changes28,30. In addition, bioinformatics interventions could be used to minimize data distortions31,32.
Obtaining pure and intact single nuclei is the most important factor in single-nucleus genomics. The clogging of the microfluidic chambers of single-cell platforms due to the presence of cell clusters or organic debris leads to poor data quality or, more problematically, to experimental failure. In this procedure, existing protocols were modified by combining enzymatic dissociation, cryopreservation, and purification of viable cardiac progenitor cells. Our procedure allows the isolation of pure nuclei without the need for FACS sorting. Using this procedure, we obtained a high-quality nuclear suspension without clumping, almost no debris, and intact single nuclei. The cell isolation step is crucial to avoid potential nuclei clumping; indeed, this can be avoided by gentle pipetting using large pipette tips or by filtering the nuclei. The use of filters at different steps and subsequent observation under a light microscope can prevent such issues.
The protocol described here was successfully used for snRNA-seq and snATAC-seq profiling from the same nuclei preparation obtained from E9.5 cardiac progenitor cells.This method can be used in pregnant wild-type mice versus mouse models for congenital heart defects. Congenital heart defects likely occur due to the disruption of pathways that control the differentiation, multiplication, migration, and survival of cardiac progenitor cells33,34. To better understand the origin of these defects, this study focused specifically on embryonic stage 9.5, in which the progenitors of the major areas affected in congenital heart defects are present, in particular the cardiac progenitors of the second heart field11 and the neural crest35. The application of this procedure to cells of the adult mouse heart and human heart requires the optimization of the protocol, particularly in terms of the cell dissociation and lysis steps.
The main considerations between manual and automated cell counting are the costs, labor, and accuracy. Automated counters are faster than most skilled scientists performing a manual count. Especially when dealing with a large number of samples, it is of great benefit to be able to simply press count and view the result. This is an important point because once the nuclei are isolated, they must be processed quickly to avoid the loss of their integrity. The primary accuracy benefit of automated systems is that these systems eliminate variability between users or labs. Another advantage is that automated systems typically have a larger field of view than hemocytometers. When the number of cells/nuclei or the concentration of the cells/nuclei are lower, this larger field of view is certainly an advantage over the traditional manual method.
A high percentage of mitochondrial DNA contamination can be observed when the concentration of the non-ionic detergent is too high. By decreasing its concentration in the lysis buffer, this contamination can be reduced without decreasing the yield of the nuclei. The most satisfactory results were obtained in our experiments with a lysis buffer containing 0.1% detergent. Spinning the nuclei in a swing bucket can also reduce the number of mitochondria in the pellet.
Having an appropriate ratio of Tn5 transposase to nuclei is crucial for successful snATAC-seq13,14. For example, having too much Tn5 may lead to a high background due to closed chromatin and low complexity of the sequencing libraries, whereas sub-lysis may not result in a complete PCR amplified library13. To determine the number of nuclei accurately, we used two complementary methods.
Multimodal omics datasets provide the opportunity to investigate multiple levels of genomic organization simultaneously9,36. The joint RNA and ATAC multimodal approach enables the study of upstream regulators and downstream metabolic genes and provides a comprehensive approach for the study of transcriptional networks and chromatin architecture in the context of heart development at the single-cell resolution. This protocol for single-nuclei isolation is compatible with both the individual and joint evaluation of expression and chromatin datasets.Chromatin accessibility is an important epigenetic level of regulation because it enables the activity of transcriptional regulators at specific genomic sites8,9,10,11,12,13. A multitude of information on the identity and target sites of dozens of possible transcription factors are, thus, provided by the DNA sequence in the chromatin profile. The comparison of the information obtained by snATAC-seq with snRNA expression profiling can help to identify the most relevant transcription factors, which can then be further analyzed by Cut&Run37. We hope that this procedure will help interested researchers and encourage them to use this powerful method for their research.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was supported by ERA-CVD-2019 and ANR-JCJC-2020 to SS. We thank the Genomics and Bioinformatics facility (GBiM) from the U 1251/Marseille Medical Genetics lab and the anonymous reviewers for providing valuable comments.
Materials
| 2100 Bioanalyzer Instrument | Agilent | No catalog number | |
| 5M Sodium chloride (NaCl) | Sigma | 59222C-500ML | |
| BSA 10% | Sigma | A1595-50ML | |
| Chromium Next GEM Chip J Single Cell Kit, 16 rxns | 10X Genomics | 1000230 | |
| Chromium Next GEM Single Cell Multiome ATAC + Gene Expression Reagent Bundle, 4 rxns (including Nuclei Buffer 20X) | 10X Genomics | 1000285 | |
| Countess cell counting chamber slides | Invitrogen | C10283 | |
| Countess III FL | Thermofisher | No catalog number | |
| Digitonin (5%) | Thermofisher | BN2006 | |
| DMSO | Sigma | D2650-5x5ML | |
| DNA LoBind Tubes | Eppendorf | 22431021 | |
| D-PBS | Thermofisher | 14190094 | Sterile and RNase-free |
| Dual Index Kit TT Set A 96 rxns | 10X Genomics | 1000215 | |
| Falcon 15 mL Conical Centrifuge Tubes | Fisher Scientific | 352096 | |
| Falcon 50 mL Conical Centrifuge Tubes | Fisher Scientific | 10788561 | |
| HI-FBS | Thermofisher | A3840001 | Heat inactivated |
| High sensitivity DNA kit | Agilent | 5067-4626 | |
| Igepal CA-630 | Sigma | I8896-50ML | |
| LIVE/DEAD Viability/Cytotoxicity Kit | Thermofisher | L3224 | |
| MACS Dead Cell Removal kit: Dead Cell Romoval MicroBeads, Binding Buffer 20X | Miltenyi Biotec | 130-090-101 | |
| MACS SmartStrainers (30 µm) | Miltenyi Biotec | 130-098-458 | |
| Magnesium chloride (MgCl2) | Sigma | M1028-100ML | |
| Milieu McCoy 5A | Thermofisher | 16600082 | |
| MS Columns | Miltenyi Biotec | 130-042-201 | |
| NovaSeq 6000 S2 | Illumina | No catalog number | |
| Penicillin Streptomycin (Pen/Strep) | Thermofisher | 15070063 | |
| PluriStrainer Mini 40µm | PluriSelect | V-PM15-2021-12 | |
| Rock inhibitor | Enzo Life Sciences | ALX-270-333-M005 | |
| Single Index Kit N Set A, 96 rxn | 10X Genomics | 1000212 | |
| Standard 90mm Petri dish Sterilin | Thermofisher | 101R20 | |
| Sterile double-distilled water | Thermofisher | R0582 | |
| Trizma Hydrochloride solution (HCl) | Sigma | T2194-100ML | |
| Trypan Blue stain (0.4%) | Invitrogen | T10282 | |
| Trypsin 0.05% – EDTA 1X | Thermofisher | 25300054 | |
| Tween20 | Sigma | P9416-50ML | |
| Wide orifice filtered pipette tips 200 μl | Labcon | 1152-965-008-9 | |
| ZEISS SteREO Discovery.V8 | ZEISS | No catalog number |
References
- vander Bom, T., et al. The changing epidemiology of congenital heart disease. Nature Reviews. Cardiology. 8 (1), 50-60 (2011).
- Bouma, B. J., Mulder, B. J. Changing landscape of congenital heart disease. Circulation Research. 120 (6), 908-922 (2017).
- Postma, A. V., Bezzina, C. R., Christoffels, V. M. Genetics of congenital heart disease: The contribution of the noncoding regulatory genome. Journal of Human Genetics. 61 (1), 13-19 (2016).
- Buijtendijk, M. F. J., Barnett, P., vanden Hoff, M. J. B. Development of the human heart. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 184 (1), 7-22 (2020).
- Sylva, M., vanden Hoff, M. J., Moorman, A. F. Development of the human heart. American Journal of Medical Genetics. Part A. 164 (6), 1347-1371 (2014).
- Gromova, T., Gehred, N. D., Vondriska, T. M. Single-cell transcriptomes in the heart: When every epigenome counts. Cardiovascular Research. 119 (1), 64-78 (2022).
- Tanay, A., Regev, A. Scaling single-cell genomics from phenomenology to mechanism. Nature. 541 (7637), 331-338 (2017).
- Schwartzman, O., Tanay, A. Single-cell epigenomics: Techniques and emerging applications. Nature Reviews. Genetics. 16 (12), 716-726 (2015).
- Macaulay, I. C., Ponting, C. P., Voet, T. Single-cell multiomics: Multiple measurements from single cells. Trends in Genetics. 33 (2), 155-168 (2017).
- Buenrostro, J. D., Wu, B., Chang, H. Y., Greenleaf, W. J. ATAC-seq: A method for assaying chromatin accessibility genome-wide. Current Protocols in Molecular Biology. 109, 1-9 (2015).
- Stefanovic, S., et al. Hox-dependent coordination of mouse cardiac progenitor cell patterning and differentiation. Elife. 9, e55124 (2020).
- Xu, W., et al. A plate-based single-cell ATAC-seq workflow for fast and robust profiling of chromatin accessibility. Nature Protocols. 16 (8), 4084-4107 (2021).
- Buenrostro, J. D., et al. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 523 (7561), 486-490 (2015).
- Denisenko, E., et al. Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome Biology. 21 (1), 130 (2020).
- Safabakhsh, S., et al. Isolating nuclei from frozen human heart tissue for single-nucleus RNA sequencing. Current Protocols. 2 (7), e480 (2022).
- Pimpalwar, N., et al. Methods for isolation and transcriptional profiling of individual cells from the human heart. Heliyon. 6 (12), 05810 (2020).
- 10x Genomics. Chromium Next GEM Single Cell Multiome ATAC + Gene Expression Reagent Kits User Guide. 10x Genomics. , (2022).
- Jia, G., et al. Single cell RNA-seq and ATAC-seq analysis of cardiac progenitor cell transition states and lineage settlement. Nature Communications. 9 (1), 4877 (2018).
- Wang, L., et al. Single-cell dual-omics reveals the transcriptomic and epigenomic diversity of cardiac non-myocytes. Cardiovascular Research. 118 (6), 1548-1563 (2022).
- Wang, Z., et al. Cell-type-specific gene regulatory networks underlying murine neonatal heart regeneration at single-cell resolution. Cell Reports. 35 (8), 109211 (2021).
- Ameen, M., et al. Integrative single-cell analysis of cardiogenesis identifies developmental trajectories and non-coding mutations in congenital heart disease. Cell. 185 (26), 4937-4953 (2022).
- Gao, R., et al. Pioneering function of Isl1 in the epigenetic control of cardiomyocyte cell fate. Cell Research. 29 (6), 486-501 (2019).
- Manivannan, S., et al. Single-cell transcriptomic profiling unveils dysregulation of cardiac progenitor cells and cardiomyocytes in a mouse model of maternal hyperglycemia. Communications Biology. 5 (1), 820 (2022).
- Cao, Y., et al. Integrated analysis of multimodal single-cell data with structural similarity. Nucleic Acids Research. 50 (21), e121 (2022).
- What is CellRanger. 10x Genomics Available from: https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger (2023)
- Hill, M. C. Integrated multi-omic characterization of congenital heart disease. Nature. 608 (7921), 181-191 (2022).
- Chen, Z., Wei, L., Duru, F., Chen, L. Single-cell RNA sequencing: In-depth decoding of heart biology and cardiovascular diseases. Current Genomics. 21 (8), 585-601 (2020).
- Machado, L., Relaix, F., Mourikis, P. Stress relief: Emerging methods to mitigate dissociation-induced artefacts. Trends in Cell Biology. 31 (11), 888-897 (2021).
- Tabula Muris, C., et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 562 (7727), 367-372 (2018).
- Machado, L., et al. Tissue damage induces a conserved stress response that initiates quiescent muscle stem cell activation. Cell Stem Cell. 28 (6), 1125-1135 (2021).
- Haghverdi, L., Lun, A. T. L., Morgan, M. D., Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nature Biotechnology. 36 (5), 421-427 (2018).
- Yang, Y., et al. SMNN: Batch effect correction for single-cell RNA-seq data via supervised mutual nearest neighbor detection. Briefings in Bioinformatics. 22 (3), 097 (2021).
- Stefanovic, S., Christoffels, V. M. GATA-dependent transcriptional and epigenetic control of cardiac lineage specification and differentiation. Cellular and Molecular Life Sciences. 72 (20), 3871-3881 (2015).
- Gunthel, M., Barnett, P., Christoffels, V. M. Development, proliferation, and growth of the mammalian heart. Molecular Therapy. 26 (7), 1599-1609 (2018).
- Stefanovic, S., Etchevers, H. C., Zaffran, S. Outflow tract formation-embryonic origins of conotruncal congenital heart disease. Journal of Cardiovascular Development and Disease. 8 (4), 42 (2021).
- Hao, Y., et al. Integrated analysis of multimodal single-cell data. Cell. 184 (13), 3573-3587 (2021).
- Bai, H., Lin, M., Meng, Y., Bai, H., Cai, S. An improved CUT&RUN method for regulation network reconstruction of low abundance transcription factor. Cellular Signalling. 96, 110361 (2022).

