Axoplasm Isolation from Rat Sciatic Nerve
Summary
We demonstrate a protocol for axoplasm isolation from adult rat sciatic nerve based on dissection of nerve fascicles and incubation in hypotonic medium to release myelin and lyse non-axonal structures, followed by extraction of the remaining axon-enriched material.
Abstract
Isolation of pure axonal cytoplasm (axoplasm) from peripheral nerve is crucial for biochemical studies of many biological processes. In this article, we demonstrate and describe a protocol for axoplasm isolation from adult rat sciatic nerve based on the following steps: (1) dissection of nerve fascicles and separation of connective tissue; (2) incubation of short segments of nerve fascicles in hypotonic medium to release myelin and lyse non-axonal structures; and (3) extraction of the remaining axon-enriched material. Proteomic and biochemical characterization of this preparation has confirmed a high degree of enrichment for axonal components.
Protocol
This protocol allows axoplasm isolation with minimization of glial and vascular tissue contamination. The method yields approximately 70-100 μg total axoplasm protein per one 8-10 week old Wistar rat.
1. Dissect Sciatic Nerves
- Euthanize two rats by CO2 inhalation followed by cervical dislocation. Confirm death by palpating for lack of heartbeat prior to beginning dissection.
- Swab the dissection area with 70% ethanol.
- Cut the skin, separate muscles and isolate sciatic nerve using scissors and forceps carefully without damaging blood vessels. Dissect both sciatic nerves (about 1.5 cm each) from each animal and collect all four nerves in an eppendorf with 500 μL PBS 0.2X + inhibitors, kept on ice.
- Transfer the nerve segments to plastic dishes containing at least 2 mL of PBS 0.2X + protease inhibitors.
- Remove the epineurium from the nerves using fine forceps under the binocular scope. Separate the fascicles very carefully until they become cloudy and start to float on the surface of solution. This step should be practiced until it can be performed quickly and accurately (not taking more than 10 min per nerve).
2. Incubation, Washing and Elution
- Transfer separated fascicles to a fresh eppendorf tube with 500 μL of PBS 0.2X containing protease inhibitors for incubation for 2 hr at room temperature.
- Wash at least 3 times with 1 mL of the same buffer by transferring the fascicles from eppendorf tube to eppendorf tube and shaking for 5 min after transfer.
- To remove excess of fluid put the fascicles into a new empty eppendorf tube and then transfer the fascicles to a new eppendorf tube with 300 μL of PBS 1X containing protease inhibitor for incubation for 20-30 min at RT.
- Centrifuge 10 min at 10,000 x g at 4°C.
- Take the supernatant and measure protein concentration. This is your axoplasm preparation.
3. Representative Results
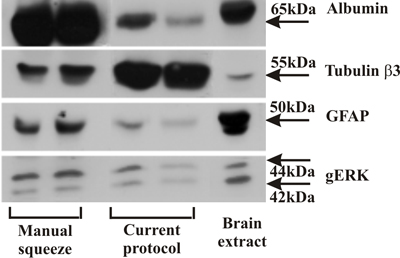
Figure 1. Western blot comparing axoplasm isolated by the manual squeeze method with axoplasm samples isolated as shown in this protocol. Note the reduced levels of albumin and GFAP, representing blood protein and glial cell contaminations, respectively. In contrast, axonal tubulin b3 is enriched in this preparation, indicating a high level of axonal proteins.
Discussion
Biochemical and proteomic analyses have confirmed that this procedure reduces serum and glial cell contamination 3 as compared to previously described methods for axoplasm isolation by mechanical squeeze 1. We have used this axoplasm isolation protocol to explore dynein-based retrograde signaling after sciatic nerve injury2, and expect it to have utility in the exploration of many other processes in adult peripheral nerve, including axon-glia interactions 4.
Disclosures
The authors have nothing to disclose.
Materials
- Male Wistar rats (8-10 weeks old)
- PBS 0.2X and PBS 1X solutions containing protease inhibitors (Roshe) 2 tablets per 50 ml
- Eppendorfs for 1.5 ml
- Sterilized plastic dishes 35 mm
- Dissecting tools including: scissors and fine forceps
- Binocular and table light
Antibodies
Mouse anti-GFAP clone G-A-5 was from Sigma (G6171). Rabbit anti-Albumin was from Cedarlane (CLAG5140). Mouse anti-Tubulin β3 and rabbit anti-gERK were from Sigma (T2200 and M5670 respectively).
References
- Hanz, S., Perlson, E., Willis, D., Zheng, J. Q., Massarwa, R., Huerta, J. J., Koltzenburg, M., Kohler, M., van-Minnen, J., Twiss, J. L. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 40, 1095-1104 (2003).
- Michaelevski, I., Medzihradszky, K. F., Lynn, A., Burlingame, A. L., Fainzilber, M. Axonal transport proteomics reveals mobilization of translation machinery to the lesion site in injured sciatic nerve. Mol Cell Proteomics. 9 (5), 976-987 (2010).
- Rishal, I., Michaelevski, I., Rozenbaum, M., Shinder, V., Medzihradszky, K. F., Burlingame, A. L., Fainzilber, M. Axoplasm isolation from peripheral nerve. Dev Neurobiol. 70, 126-133 (2010).
- Twiss, J. L., Fainzilber, M. Ribosomes in axons–scrounging from the neighbors. Trends Cell Biol. 19, 236-243 (2009).

