Nasal Wipes for Influenza A Virus Detection and Isolation from Swine
Summary
The authors present a protocol to collect swine nasal wipes to detect and isolate influenza A viruses.
Abstract
Surveillance for influenza A viruses in swine is critical to human and animal health because influenza A virus rapidly evolves in swine populations and new strains are continually emerging. Swine are able to be infected by diverse lineages of influenza A virus making them important hosts for the emergence and maintenance of novel influenza A virus strains. Sampling pigs in diverse settings such as commercial swine farms, agricultural fairs, and live animal markets is important to provide a comprehensive view of currently circulating IAV strains. The current gold-standard ante-mortem sampling technique (i.e. collection of nasal swabs) is labor intensive because it requires physical restraint of the pigs. Nasal wipes involve rubbing a piece of fabric across the snout of the pig with minimal to no restraint of the animal. The nasal wipe procedure is simple to perform and does not require personnel with professional veterinary or animal handling training. While slightly less sensitive than nasal swabs, virus detection and isolation rates are adequate to make nasal wipes a viable alternative for sampling individual pigs when low stress sampling methods are required. The proceeding protocol outlines the steps needed to collect a viable nasal wipe from an individual pig.
Introduction
Influenza A viruses (IAV) cause respiratory disease in many species, including domestic birds, swine, and humans. Due to reassortment of the segmented IAV genome rapid viral evolution can occur and new IAV strains frequently emerge. Swine are a species that can serve as a mixing vessel for reassortment of IAVs from multiple host species.1 There are currently three major subtypes of IAV commonly circulating among North American swine (H1N1, H1N2, H3N2), but multiple IAV introductions from humans have led to extensive IAV diversity within those subtypes.2 Rapid evolution of IAVs infecting swine has been evident since 1998 when a triple reassortant IAV containing gene segments from human, avian and swine viruses3 became widespread among pigs in the United States.4 The internal gene segments from that triple reassortant IAV remain highly prevalent among IAVs currently infecting swine.5
Worldwide, IAV is a significant cause of respiratory disease in swine in which typical clinical signs include fever, anorexia, lethargy, coughing, labored breathing, sneezing, nasal discharge and poor weight gain. IAV can be particularly costly to sow farms where reproductive failure due to IAV induced fever and weak-born piglets have been documented.6,7 Within the United States, IAV is commonly detected in commercial swine herds and the extensive antigenic and genomic diversity and continuing evolution among IAV infecting swine has hampered control of this virus.8-11
Public health concerns about the emergence of a pandemic IAV strain resulting from reassortment in swine were realized in 2009 when a swine-lineage IAV containing gene segments from the triple-reassortant North American swine lineage and the Eurasian avian-like swine lineage caused a worldwide pandemic in humans.12 The pandemic virus (A (H1N1)pdm09) has since reassorted with endemic swine IAV strains13,14 and some of these newly reassorted strains have been transmitted back to humans.15 The frequency of reassortment events and emergence of new IAV strains with pandemic potential makes active surveillance of circulating IAV viruses in swine imperative, especially at the swine-human interface.
The swine-human interface is important for bi-directional interspecies transmission of IAV. Human-to-swine transmission occurring in commercial swine production is responsible for a large amount of IAV diversity currently present in the swine population. Agricultural fairs are the largest settings for the comingling of people and swine in the United States and are known sites for the zoonotic transmission of IAV.15-21 In 2012, during the outbreak of a variant H3N2 IAV, 93% of cases reported attendance at an agricultural fair in the days prior to onset of disease.15 Genomic analysis of viral isolates from exhibition pigs compared with human isolates confirmed zoonotic transmission.21 Exhibition swine infected with IAV often do not show clinical signs of illness,21-23 indicating the need for direct diagnostic tests.
Sampling of visibly ill pigs alone will not successfully identify IAV prevalence in swine and cannot be relied on to identify new strains of IAV emerging among swine. Active surveillance is absolutely necessary for detection of emerging strains of IAV in swine and to assess their threat for both swine and public health. Most IAV surveillance activities are voluntary and therefore minimally disruptive methods are needed. The three major ante-mortem sample collection procedures for IAV infecting swine are: nasal swabs, oral fluids, and nasal wipes. Current recommendations for sampling individual swine to detect IAV list the insertion of synthetic-fiber tipped swabs into the nostrils as the preferred method to gather nasal secretions and epithelial cells.24,25 Because pigs may try to avoid this procedure, a team of trained personnel must restrain pigs either manually or with a snare depending on the size of the animal.26 The restraint process is laborious for the personnel, and stressful for the pigs. Additionally, exhibition swine are often involved in multiple competitions at a fair so the perception of added stress on a competition animal can make owners resistant to the surveillance efforts.
With probabilities of IAV detection ranging from 80-100% in IAV infected herds, oral fluids have become a popular alternative to nasal swabs for the molecular detection of IAV in populations of swine.27,28 Additionally, oral fluids may provide a wider window of IAV detection than nasal swabs following initial infection. However, IAV isolation from oral fluids has been problematic with only 50% of virus isolation attempts resulting in IAV recovery.29
Using nasal wipes in lieu of nasal swabs during IAV surveillance in swine overcomes the limitations described above. Nasal wipes do not require the use of a restraining snare and can be performed without stressing the animals or witnesses of the procedure. Minimal technical training is needed to collect nasal wipes, which allows non-veterinary professionals, including swine owners, to collect the surveillance samples. Nasal wipes were previously compared to nasal swabs for the detection and isolation of influenza A virus30 and the detailed protocol for this non-invasive method of sampling is described below.
Protocol
All swine used in the collection of the following data were protected under The Ohio State University Institutional Animal Care and Use Committee (animal use protocol number 2009A0134-R1).
1. Preparation of Viral Transport Medium and Sample Collection Vials
- Add 37 g of Brain Heart Infusion to 900 ml of purified water and mix thoroughly with a stir bar and magnetic stirrer while heating to 70 °C to completely dissolve the powder.
- Autoclave the broth at 121 °C for 15 min. Cool on the bench top until the broth reaches room temperature. Refrigerate at 4 °C overnight if needed.
- Dissolve, by mixing with a magnetic stir bar, 6.02 g of Penicillin G sodium salt and 10 g of streptomycin sulfate into 50 ml of purified water at room temperature. Add additional purified water to bring the total volume to 100 ml. Filter the solution through a 0.22 µm polyethersulfone membrane into a sterile bottle.
- Aseptically add the filtered penicillin and streptomycin solution into the cooled Brain Heart Infusion Broth and mix thoroughly (stir bar or swirl flask by hand) to make the viral transport medium.
- Aseptically test the pH of the viral transport medium; if needed, adjust the pH to 7.4 ± 0.2 using HCl or NaOH.
- Perform quality assurance testing on the viral transport medium by testing for influenza A virus matrix protein by rRT-PCR, as described by Zhang and Harmon.22
- Dispense 5 ml of the viral transport medium into sterile high-density polyethylene bottles with 8 ml capacity. Label the vials for use in the field.
- Store the ready-to-use vials in standard cryo-boxes at -20 °C until sample collection.
2. Collection of Nasal Wipes from Swine
- Thaw the appropriate number of vials immediately prior to sample collection; one vial will be needed per pig sampled. Thawing at room temperature takes approximately 30-45 min. Keep the thawed vials cool on ice packs until use.
- Don appropriate personal protective equipment as dictated by the situation (e.g. coveralls, boot covers, respirator, ear plugs, etc.).
- Enter the animal area. If needed, confine the pig to a small area but do no restrain. Do not rouse resting animals.
- Put on a pair of disposable exam gloves. Take care not to contaminate the gloves by touching pigs, people, or inanimate objects.
- Use a gloved hand to remove a sterile 5.08 cm × 5.08 cm (2 in. × 2 in.) cotton gauze pad from its wrapper. Sterile gauze pads should be used whenever possible. Individually wrapped gauze pads are more convenient to use than those wrapped with two gauze pads per package.
- Hold the cotton gauze pad with the fingertips of one hand exposing as much of the pad as possible for sampling.
- Wipe the gauze pad across the pig’s snout, taking extra care to enter the external nares if possible. Collect visible nasal secretions (approximately 1 ml) with the same gauze pad.
- Using the same hand, fold the gauze pad upon itself to facilitate placement in the vial containing viral transport medium.
- Using the other hand, remove the cap from the vial and place the folded gauze pad into the vial. Recap the vial and shake the vial to ensure the mixing of the gauze pad and viral transport medium.
- Remove the gloves and place in an appropriate receptacle. Change gloves between each pig.
- Verify the vial identifier. Record pig identification number, age, sex, clinical signs, and other notes deemed necessary by investigator.
- Chill the samples immediately after collection. Consider making aliquots of the sample before freezing to prevent multiple freeze-thaw cycles. As soon as possible after collection, place the samples on dry ice for transportation to the laboratory. Store the vials at -80 °C until testing is initiated.
3. Detection of Influenza A Virus Nucleic Acid
- Thaw the samples in a 37 °C dry bead bath for 5 min and then finish thawing the samples on the bench top at room temperature for 20-30 min. The simultaneous addition of several vials to the beads will cause the bath to heat; caution should be exercised as to not overheat the vials.
- Remove 100 µl of viral transport medium for RNA extraction.
- Use a high-throughput (96 well plate format) magnetic bead platform for extracting RNA from a large number of samples.31
- Use real-time reverse transcriptase PCR assays for the rapid detection influenza A virus.31
4. Isolation of Influenza A Virus from Nasal Wipes
- Thaw the samples as described above in Section 3.1
- Treat thawed samples with gentamicin sulfate (1,000 μg/ml), amphotericin B (22.5 μg/ml), and kanamycin sulfate (325 μg/ml).
- Inoculate into Madin-Darby Canine Kidney (MDCK) epithelial cells as described previously.32
Representative Results
Successful use of this method yields rRT-PCR results that, accompanied with the use of an internal control during RNA extraction and rRT-PCR, show samples did not contain PCR inhibitors from any environmental debris picked up during sampling. After sample inoculation, virus isolation wells should be free of visible environmental debris from the sample. There is reasonable agreement between rRT-PCR results and virus isolation results with the understanding that PCR often yields a higher IAV positive rate than virus isolation because PCR detects viral nucleic acid, not necessarily viable virus.
Results show that nasal wipes are a useful alternative to nasal swabs, which are the current gold standard sampling technique for IAV. Edwards et al. performed a comparison of snout wipes and nasal swabs that were collected from pigs at agricultural fairs in the United States during 2013. In that study, pigs were sampled with both nasal swabs and nasal wipes and the samples were test in parallel with rRT-PCR and virus isolation. Edwards et al. reported the concordance for both the detection and isolation of IAV by rRT-PCR and virus isolation in cultured cells was demonstrated by the comparison of the 553 paired nasal swab and nasal wipe samples.30 Of these tested samples, 93.5% (517/553) of rRT-PCR test results were in agreement (Table 1) and 92.4% (511/553) were in agreement using virus isolation in MDCK cells (Table 2).21 The estimated rRT-PCR sensitivity for nasal wipes compared to nasal swabs was 92.9% and the estimated IAV isolation sensitivity for nasal wipes compared to nasal swabs was 82.9%. Edwards et al., previously noted that nasal wipes that were positive both by rRT-PCR and virus isolation averaged a lower Ct value (24.32) than nasal wipes that were positive by rRT-PCR but negative for virus isolation (Ct = 31.96).21
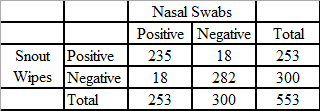
Table 1: rRT-PCR detection of influenza A virus from nasal swabs and snout wipes collected from swine at 29 county fairs (from Edwards et al. (2014) Utility of snout wipe samples for influenza A virus surveillance in exhibition swine populations. Influenza and Other Respiratory Viruses 8(5), 574–579.
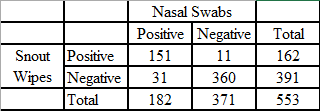
Table 2: Isolation of influenza A virus from nasal swabs and snout wipes collected from swine at 29 county fairs (from Edwards et al. (2014) Utility of snout wipe samples for influenza A virus surveillance in exhibition swine populations. Influenza and Other Respiratory Viruses 8(5), 574–579.)
Discussion
Collecting samples from pigs using polyester-tipped nasal swabs has proven useful in conducting IAV surveillance; however, the use of the nasal swab procedure hinders surveillance efforts due to the required use of a snare for restraint. Nasal wipes represent a refinement of current swine sampling techniques to minimize stress on people and pigs during sample collection. While the method has been developed for and validated in exhibition swine settings, it can be easily applied to other situations where ante-mortem detection of IAV in swine is necessary (i.e. commercial swine, live animal markets, biomedical research, etc.)
As noted above, the major driver of this sampling technique has been the exhibition swine population. During agricultural fairs, swine owners compete to earn financial awards and notoriety based on the overall composition of the pig. Anecdotal evidence indicates that if a pig does not show well, owners may attribute it to the pig’s stress level during restraint and sample collection. Nasal wipes are useful for surveillance efforts that involve sampling individual animals in settings that require low animal stress. The ability to avoid snaring and visibly distressing pigs while performing the nasal wipe technique has improved public acceptance of IAV surveillance efforts in exhibition swine.33 The low stress nature of the nasal wipe method also makes it well suited for commercial swine farms. Furthermore, the nasal wipe method can help reduce costs because it requires fewer personnel and does not mandate specialized training.
In addition to nasal swabs, another commonly used form of pathogen detection in commercial swine involves allowing pigs that are housed together to chew on a cotton rope that has been placed in their pen. They deposit oral fluids which can be collected from the rope.27,34,35 Oral fluid collection produces results that are based on samples collected from a group of animals and has limitations for pathogen prevalence or transmission studies. Additionally, IAV isolation from oral fluids has been poor.29 Oral fluids have been used for individual swine but the time required to hang the ropes and for the pigs to saturate the ropes can be inefficient for personnel resources.36 Using nasal wipes allows for the rapid evaluation of individual animals. The ability to both detect and isolate IAV from nasal wipes provides a distinct advantage over oral fluids. Like other sampling methods, nasal wipes may be most effective during peak viral shedding.
There are some limitations associated with the nasal wipe method that should be considered before implementing the procedure. Pigs are curious animals that root with their snouts. Often there is more debris collected on a nasal wipe, as compared to nasal swabs, because of the moist, dirty environment the wipe contacts on the exterior of the pig snout. The contact between the nasal wipe substrate and the pig is restricted to a more external surface of the respiratory tract than nasal swabs, a situation that often results in unintentional deposition of environmental debris (i.e. bedding, feed, manure, etc.) on the nasal wipes. These environmental contaminants can inhibit PCR or cause cellular toxicity during viral isolation attempts. The cleanliness of sampling location and levels of environmental debris may need to be evaluated before proceeding with nasal wipe protocols. The occurrence of environmental contamination of nasal wipes also means that IAV detected with this method may not have been shed by the tested animal, but rather was present in the environment. While the ability to attribute an IAV isolate to a particular animal may not be a concern for surveillance activities or herd based diagnostics, this may be a limitation to consider for laboratory based transmission studies.
Another issue that needs to be addressed before choosing this method is the increased storage and transportation space that large numbers of nasal wipe samples will require. The vials used for wipes are much larger than the standard 2 ml cryogenic storage vials used for nasal swab storage and will take approximately three to four times more freezer space for long term storage and cooler space for transportation back to the lab. Storing samples for extended periods of time at room temperature, under refrigeration, or freeze-thawing the samples will all result in a decrease of virus viability. Extra care needs to be taken when thawing these samples. Because the gauze absorbs much of the viral transport medium, samples will overheat quickly in a dry bead bath. Inadvertently overheating the samples during the thawing process can also result in a decrease of virus viability. Also, due to decreased IAV recovery in vitro from wipes compared to swabs, it is recommended that nasal wipes not be used in situations requiring high sensitivity for viable virus recovery.30
Successful use of IAV surveillance in pigs by the nasal wipe method can result in greater ease of sampling, increased acceptance of surveillance efforts, and less stress on the animals. In the future, it may be useful for sampling pigs upon entry to a fair or for low-stress testing of individual pigs in commercial swine herds. This method could be investigated for use detecting other respiratory pathogens in swine. Because swine play a critical role in the emergence of novel IAV strains, continued active surveillance for IAV in pigs is critical. As such, this method can aid in the detection and epidemiologic investigation of emergent strains of novel IAVs.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work has been funded in part with federal funds from the Centers of Excellence for Influenza Research and Surveillance (CEIRS), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201400006C.
Materials
| BBL Brain Heart Infusion | Becton, Dickinson and Company | 211059 | |
| Penicillin G Sodium Salt | MP Biomedicals, LLC | 021194537 | 1500 u/mg |
| Streptomycin Sulfate | AMRESCO LLC | 0382 | |
| Gentamicin Solution | Mediatech, Inc. | 30-005-CR | 50 mg/ml |
| Amphotericin B Solution | Fisher | S 24 25 | 250 ug/ml |
| Kanamycin Sulfate | Teknova | K2105 | 5000 ug/ml |
| TPP Rapid Filtermax System | TPP Techno Plastic Products AG | 99150 | |
| Nalgen Diagnostic Bottles | Thermo Scientific | 342002-9025 | HDPE with white PP closure |
| Dermacea Gauze Sponge, 8 ply | Covidien | 441211 | 5.08 cm × 5.08 cm (2 in. × 2 in) |
| Nitrile Exam Gloves | Saftey Choice | 19-170-010 (A-D) |
Riferimenti
- Ma, W., Kahn, R. E., Richt, J. A. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. J. Mol. Genet. Med. 3 (1), 158-166 (2008).
- Nelson, M. I., Vincent, A. L. Reverse zoonosis of influenza to swine: new perspectives on the human-animal interface. Trends Microbiol. 23, (2015).
- Zhou, N. N., et al. Genetic reassortment of avian, swine, and human influenza A viruses in American pigs. J. Virol. 73 (10), 8851-8856 (1999).
- Webby, R. J., et al. Evolution of swine H3N2 influenza viruses in the United States. J. Virol. 74 (18), 8243-8251 (2000).
- Vincent, A., et al. Review of influenza A virus in swine worldwide: a call for increased surveillance and research. Zoonoses Public Health. 61 (1), 4-17 (2014).
- Karasin, A. I., Olsen, C. W., Anderson, G. A. Genetic characterization of an H1N2 influenza virus isolated from a pig in Indiana. J. Clin. Microbiol. 38 (6), 2453-2456 (2000).
- Zhou, N. N., et al. Emergence of H3N2 reassortant influenza A viruses in North American pigs. Vet. Microbiol. 74 (1-2), 47-58 (2000).
- Corzo, C. A., et al. Active Surveillance for Influenza A Virus among Swine, Midwestern United States, 2009-2011. Emerg. Infect. Dis. 19 (6), 954-960 (2013).
- Lorusso, A., et al. Genetic and antigenic characterization of H1 influenza viruses from United States swine from 2008. J. Gen. Virol. 92 (Pt 4), 919-930 (2011).
- Loving, C. L., et al. Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011-2012 H3N2v. J. Virol. 87 (17), 9895-9903 (2013).
- Vincent, A. L., et al. Efficacy of inactivated swine influenza virus vaccines against the 2009 A/H1N1 influenza virus in pigs. Vaccine. 28 (15), 2782-2787 (2010).
- Smith, G. J., et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 459 (7250), 1122-1125 (2009).
- Vijaykrishna, D., et al. Reassortment of Pandemic H1N1/2009 Influenza A Virus in Swine. Science. 328 (5985), 1529 (2010).
- Ducatez, M. F., et al. Multiple Reassortment between Pandemic (H1N1) 2009 and Endemic Influenza Viruses in Pigs, United States. Emerg. Infect. Dis. 17 (9), 1624-1629 (2011).
- Jhung, M. A., et al. Outbreak of variant influenza A(H3N2) virus in the United States. Clin. Infect. Dis. 57 (12), 1703-1712 (2013).
- Vincent, A. L., et al. Characterization of an influenza A virus isolated from pigs during an outbreak of respiratory disease in swine and people during a county fair in the United States. Vet. Microbiol. 137 (1-2), 51-59 (2009).
- Killian, M. L., et al. Simultaneous Infection of Pigs and People with Triple-Reassortant Swine Influenza Virus H1N1 at a U.S. County Fair. Zoonoses Public Health. 60 (3), 196-201 (2013).
- Wong, K. K., et al. Outbreak of influenza A (H3N2) variant virus infection among attendees of an agricultural fair, Pennsylvania, USA, 2011. Emerg. Infect. Dis. 18 (12), 1937-1944 (2011).
- Bowman, A. S., et al. Molecular evidence for interspecies transmission of H3N2pM/H3N2v influenza A viruses at an Ohio agricultural fair, July 2012. Emerg. Microbes. Infect. 1 (10), e33 (2012).
- Wells, D. L., et al. Swine influenza virus infections. Transmission from ill pigs to humans at a Wisconsin agricultural fair and subsequent probable person-to-person transmission. JAMA. 265 (4), 478-481 (1991).
- Bowman, A. S., et al. Swine-to-human transmission of influenza A(H3N2) virus at agricultural fairs, Ohio, USA, 2012. Emerg. Infect. Dis. 20 (9), 1472-1480 (2012).
- Bowman, A. S., Nolting, J. M., Nelson, S. W., Slemons, R. D. Subclinical influenza virus A infections in pigs exhibited at agricultural fairs, Ohio, USA, 2009-2011. Emerg. Infect. Dis. 18 (12), 1945-1950 (2012).
- Gray, G. C., et al. Influenza A(H1N1)pdm09 Virus among Healthy Show Pigs, United States. Emerg. Infect. Dis. 18 (9), 1519-1521 (2012).
- Van Reeth, K., Brown, I. H., Olsen, C. W., Zimmerman, J. J. Ch. 40, Influenza virus. Diseases of Swine. , 557-571 (2012).
- Culhane, M. R., Detmer, S. E., Spackman, E. Ch. 21, Sample types, collection, and transport for influenza A viruses of swine. Methods in Molecular Biology. Animal Influenza Virus, 259-263 (2014).
- Sheldon, C. C., Sonsthagen, T., Topel, J. A. . Animal Restraint for Veterinary Professionals. , (2006).
- Detmer, S. E., Patnayak, D. P., Jiang, Y., Gramer, M. R., Goyal, S. M. Detection of Influenza A virus in porcine oral fluid samples. J. Vet. Diagn. Invest. 23 (2), 241-247 (2011).
- Goodell, C. K., et al. Probability of detecting influenza A virus subtypes H1N1 and H3N2 in individual pig nasal swabs and pen-based oral fluid specimens over time. Vet. Microbiol. 166 (3-4), 3-4 (2013).
- Romagosa, A., Gramer, M., Joo, H. S., Torremorell, M. Sensitivity of oral fluids for detecting influenza A virus in populations of vaccinated and non-vaccinated pigs. Influenza Other Respir. Viruses. 6 (2), 110-118 (2012).
- Edwards, J. L., et al. Utility of snout wipe samples for influenza A virus surveillance in exhibition swine populations. Influenza Other Respir. Viruses. 8 (5), 574-579 (2014).
- Zhang, J., Harmon, K. M., Spackman, E. Ch. 23, RNA extraction from swine samples and detection of influenza A virus in swine by real-time RT-PCR. Animal Influenza Virus. , 277-293 (2014).
- Zhang, J., Gauger, P. C., Spackman, E. Ch. 22, Isolation of swine influenza virus in cell cultures and embryonated chicken eggs. Animal Influenza Virus. , 265-276 (2014).
- Bowman, A. S., et al. The Inability to Screen Exhibition Swine for Influenza A Virus Using Body Temperature. Zoonoses Public Health. , (2015).
- Prickett, J. R., Kim, W., Simer, R., Yoon, K. J., Zimmerman, J. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. J. Swine Health Prod. 16 (2), 86-91 (2008).
- Prickett, J., et al. Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J. Vet. Diagn. Invest. 20 (2), 156-163 (2008).
- Pepin, B., Liu, F. F., Main, R., Ramirez, A., Zimmerman, J. Collection of oral fluid from individually housed sows. J. Swine Health Prod. 23 (1), 35-37 (2015).

