Multiplexing Focused Ultrasound Stimulation with Fluorescence Microscopy
Summary
Low-Intensity Pulsed Ultrasound Stimulation (LIPUS) is a modality for non-invasive mechanical stimulation of endogenous or engineered cells with high spatial and temporal resolution. This article describes how to implement LIPUS to an epi-fluorescence microscope and how to minimize acoustic impedance mismatch along the ultrasound path to prevent unwanted mechanical artefacts.
Abstract
By focusing low-intensity ultrasound pulses that penetrate soft tissues, LIPUS represents a promising biomedical technology to remotely and safely manipulate neural firing, hormonal secretion and genetically-reprogrammed cells. However, the translation of this technology for medical applications is currently hampered by a lack of biophysical mechanisms by which targeted tissues sense and respond to LIPUS. A suitable approach to identify these mechanisms would be to use optical biosensors in combination with LIPUS to determine underlying signaling pathways. However, implementing LIPUS to a fluorescence microscope may introduce undesired mechanical artefacts due to the presence of physical interfaces that reflect, absorb and refract acoustic waves. This article presents a step-by-step procedure to incorporate LIPUS to commercially-available upright epi-fluorescence microscopes while minimizing the influence of physical interfaces along the acoustic path. A simple procedure is described to operate a single-element ultrasound transducer and to bring the focal zone of the transducer into the objective focal point. The use of LIPUS is illustrated with an example of LIPUS-induced calcium transients in cultured human glioblastoma cells measured using calcium imaging.
Introduction
Many diseases require some form of invasive medical intervention. These procedures are often expensive, risky, require recovery periods and thus add a burden to health care systems. Non-invasive therapeutic modalities have the potential to provide safer and cheaper alternatives to conventional surgical procedures. However, current non-invasive approaches such as pharmacotherapy or transcranial magnetic stimulation are often limited by trade-offs between tissue penetration, spatiotemporal resolution and unwanted off-target effects. In this context, a focused ultrasound constitutes a promising non-invasive technology with the potential to manipulate biological functions deep inside tissues with high spatiotemporal accuracy and limited off-target effects.
Focused ultrasound stimulation consists of delivering acoustic energy at precise locations deep inside living organisms. Depending on acoustic pulse parameters, this energy can have a variety of medical uses. For instance, the Food and Drug Administration has approved the use of High-intensity Focused Ultrasound (HiFU) for thermal ablation of prostate tumors, tremor-causing brain regions, uterine fibroids and pain-causing nerve endings in bone metastases1. HiFu-mediated microbubble cavitation is also used to transiently open the blood-brain barrier for the targeted delivery of systemically-administered therapeutics2. The spatial-peak pulse-average intensity (Isppa) and spatial-peak temporal-average intensity (Ispta) used for HiFU applications are typically above several kW cm-2 and produce pulse pressure of several tens of MPa. These intensity values are far above the FDA-approved Isppa and Ispta limits for diagnostic ultrasound, 190 W cm-2 and 720 mW cm-2, respectively3. In contrast, recent studies have shown that non-destructive pulsed ultrasound stimulation that are within or near the range of diagnostic ultrasound intensity limits (LIPUS) can be effective to remotely and safely manipulate neural firing4,5,6,7,8, hormonal secretion9,10 and bioengineered cells11. Yet, the cellular and molecular mechanisms by which cells sense and respond to ultrasound remain unclear, precluding clinical translation of LIPUS. Hence, in the past few years, studies of artificial membranes, cultured cells and animals stimulated with ultrasound have gained momentum to reveal biophysical and physiological processes modulated by LIPUS12,13,14,15.
Sound consists of a vibration propagating through a physical medium. An ultrasound is a sound with a frequency above the human audible range (i.e., above 20 kHz). In a laboratory setting, ultrasound waves are generally produced by piezoelectric transducers that contain a material that vibrates in response to an electrical field oscillating in a specific high-frequency bandwidth. Two types of transducers exist: single element transducers and transducer arrays. Single element piezoelectric transducers possess a curved surface which acts as a focusing lens and hence concentrates acoustic energy into a defined region called the focal zone. Single element transducers are much cheaper and easier to operate than transducer arrays. This article will focus on single element transducers.
The size of the focal zone of a focused single element transducer depends on the geometric properties of the acoustic lens and on its acoustic frequency. To achieve a millimeter-size focal zone with a single element transducer, ultrasound frequencies in the MHz range are generally required. Unfortunately, acoustic waves at such frequency are very rapidly attenuated when propagated in a tenuous medium such as air. Thus, MHz ultrasound waves need to be generated and propagated to the sample in a denser material such as water. This constitutes the first challenge in integrating LIPUS modality to a microscope.
A second challenge is to minimize physical interfaces between materials with different acoustic impedances (which is a product of material density and the acoustic velocity) along the acoustic path. These interfaces can reflect, refract, scatter and absorb acoustic waves, making it difficult to quantify the amount of acoustic energy effectively delivered to a sample. They may also create unwanted mechanical artefacts. For instance, reflections produced perpendicular to acoustic mismatch impedance interfaces create backpropagating waves that interfere with forward-propagating ones. Along the interference path, the waves cancel each other at fixed regions of spaces called nodes and sum up at alternating regions called anti-nodes, creating so-called standing waves (Figure 1). It is important for the experimentalist to be able to control or eliminate these experimental interfaces in vitro as they may not exist in vivo.
Fluorescence measurement of optical reporters is a well-known method to interrogate transparent biological samples in real-time and with no physical disturbance. This approach is thus ideal for LIPUS studies as any physical probes present in the sonicated area will introduce mechanical artefacts. This protocol describes the implementation and operation of LIPUS to a commercial epi-fluorescence microscope.
Protocol
1. Growing Cells on Acoustically-Transparent Polyester Film
- Drill a 12 mm hole size at the bottom of a standard 35 mm culture dish using a vertical press-drill. Move the drill slowly and wear eye protection. Remove pieces of plastic attached to the bottom of the dish using a blade to create a smooth surface on the external side (Figure 2).
- Apply a thin layer of marine-grade epoxy or glue at the external bottom surface of the dish.
- Place a film of polyester (2.5 µm thickness) against the external bottom surface of the dish and press firmly to make sure the epoxy/glue spreads evenly between the film and the thick plastic surface. Gently pull the film in a centrifugal manner with fingers to create a flat surface (Figure 2).
- When the epoxy/glue has dried, briefly rinse-dry the polyester-bottom dish with 95% ethanol and sterilize by placing the dish and the inside surface of its lid under a strong 254 nm UV excitation source. Adjust duration and intensity to deliver a UV dose of approximately 330 mJ cm-2 for complete destruction of most types of micro-organisms. This energy approximately corresponds to a duration of 5 min using a 1,000 µW cm-2 UV illumination.
- Aliquot commercially available extracellular matrix protein mixtures (EMPM) in small tubes (50-100 µL) and store them at -20 °C or less in sterile conditions.
- In a sterile environment (e.g., inside a biosafety cabinet), dilute a frozen stock of EMPM with a desired culture medium to 1:100. Work on ice to prevent EMPM polymerization at room temperature. Quickly apply 100 µL of the medium mixture onto the polyester film. Place the lid back on the dish to maintain sterility.
- Incubate EMPM-coated polyester bottom dishes in a cell culture CO2 incubator at 37 °C for 6-12 h.
- After incubation, aspirate the excess medium and directly seed the surface with cells at the desired density. Work under sterile condition to maintain sterility.
2. LIPUS Implementation
- Place a water tank underneath the objective of an upright microscope with large working volume and without illumination hardware in the transmission path.
- Using commercially-available optomechanical components, place a sample holder below the objective and a transducer holder underneath the sample holder. For subsequent sample search and ultrasound alignment, mount these two holders on translation stages.
- Place the moving parts and actuators of translation stages either outside the tank or above the water line to avoid water damage. Only use non-corrosive materials such as anodized aluminum or stainless steel for immersed optomechanical components.
- Fill the tank with deionized and degassed water before utilizing the immersion transducer. The water line should coincide with the horizontal plane of the sample holder (Figure 3).
NOTE: Deionized water prevents electrical coupling in presence of high electric fields. Degassing will also prevent scattering and alterations of acoustic waves. Drain water after each experiment using a pump or valve so that the water line falls below the position of the transducer. Also, replace or filter water frequently and clean-up the water tank as needed to avoid growth of microorganisms.
3. Oblique Acoustic Excitation
- Using commercially available optomechanical components, orient the transducer in an oblique position with respect to the optical path. This will ensure that any reflected waves will be directed away from the sample (Figure 3 and Figure 4).
4. Driving the Transducer
NOTE: Ultrasound transducers convert oscillating electrical energy into mechanical expansion/contraction of a piezoelectric material. This conversion produces energy loss in the form of heat energy. Hence, while transducers do possess a peak input voltage limit, they also possess an electrical power limit to avoid thermal damage to the piezoelectric element:
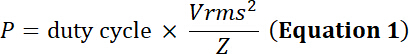
with the duty cycle the relative fraction of time of electrical simulation, P the electrical power (in Watts), Vrms the input root-mean-square voltage (in Volts) of the alternative voltage source and Z the electrical impedance (in Ohms).
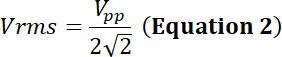
with Vpp the peak-to-peak input voltage applied to the transducer.
- Create a sinusoidal wave form containing the desired frequency, number of cycles per pulse, and pulse repetition frequency using a commercial function generator. However, the relatively high Vpp needed to effectively drive standard ultrasound transducers often requires the addition of a power amplifier to amplify the output (i.e., increase the amplitude of Vpp) of the function generator.
NOTE: For example, a transducer’s manufacturer indicates the power limit for a given transducer is 35 W. Will a sinusoidal peak-to-peak input voltage (Vdi) of 500 mV at a duty cycle of 50% and amplified through a 50 dB/100 W amplifier be within the power limit of this transducer?- To answer this question, calculate the voltage after amplification. For a radio-frequency (RF) power amplifier, the amplification factor (dB) is defined by:
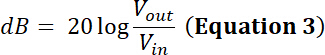
Thus, the amplified voltage has an amplitude output Vpp (Vpp = Vout) of: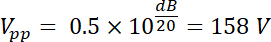
Using Equations 1 and 2, and using 50 Ω as electrical impedance, the corresponding power generated by this voltage is:
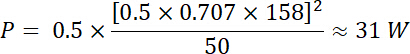
This stimulation is therefore within the power limit of the transducer. - Using the example above, calculate the waveform parameters (Vpp, frequency, pulse duration and pulse repetition frequency) that correspond to the power and voltage limits provided by the transducer’s manufacturer. Make sure to respect these limits to avoid damaging the transducer and other connected instruments.
- To answer this question, calculate the voltage after amplification. For a radio-frequency (RF) power amplifier, the amplification factor (dB) is defined by:
- Choose a function generator that operates within a frequency range compatible with the ultrasound transducer. Adjust the frequency of the function generator to the nominal peak frequency of the transducer.
- Create a sinusoidal voltage pulse of the desired duration and repetition frequency using the burst mode of the function generator. Adjust peak-to-peak voltage to a desired value. Make sure that the pulse duration is shorter than the elapsed time between two consecutive pulses.
- Check that the waveform corresponds to the desired signal by connecting the output of the function generator to the input of an oscilloscope.
- Connect the output of the function generator to the input of a power RF amplifier (Figure 4). Make sure that the stimulation parameters are within the limits of the transducer’s manufacturer.
5. Beam Alignment
- Choose a hydrophone that operates with a frequency range and acoustic intensity compatible with the frequency and intensity of the ultrasound transducer.
- Carefully bring the tip of a hydrophone probe into focus within the objective field of view at the position corresponding to the position of the sample (Figure 4).
- Make sure that both probe and transducer are immersed in deionized and degassed water. Do not bump the tip of the hydrophone with any physical object other than water as this will alter its coating and affect the measurement.
- Perform a gross pre-alignment of the transducer by visually positioning its acoustic axis toward the hydrophone probe. Makes sure that the distance between the transducer’s surface and the hydrophone tip correspond approximately to the transducer’s focal length.
- Connect the hydrophone output to one of the oscilloscope’s signal input. Connect the synchronization trigger from the function generator to another oscilloscope input. Visualize both signals simultaneously on the oscilloscope.
- Drive the transducer with few ultrasound cycles at a low duty cycle and low amplitude to avoid damaging the probe. Check with the hydrophone’s manufacturer safe operation conditions to avoid damaging the hydrophone tip.
- Adjust the s/division knob according to the travel time of ultrasound from the transducer’s surface to the hydrophone. Look for a hydrophone signal on the oscilloscope after the synchronization trigger.
- Slowly actuate the transducer using a motorized or manual XYZ stage. Leave the transducer into the position that correlates with the maximal hydrophone signal (Figure 4).
NOTE: If no signal is detected it is possible that the intensity of the acoustic pulses is too low or that the beam is mis-aligned or scattered by an object. Check regularly that the hydrophone and transducer are visually pre-aligned and that no bubbles or physical object are present in the path except the polyester film. If no signal is still detected, increase the input voltage by a small amount to increase the amplitude of hydrophone signal.
6. Determination of Ultrasound Pulse Pressure and Intensity
- With the beam aligned, measure the peak-to-peak amplitude of the hydrophone output at the oscilloscope for various voltages driving the transducer. Make sure not to exceed the pressure limit recommended by the hydrophone’s manufacturer.
- Convert these measurements into pressure and/or acoustic intensity values using the calibration method provided by the hydrophone’s manufacturer.
NOTE: The acoustic intensity can be determined from the pressure and vice versa using the formula:
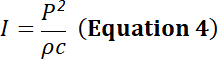
with I the acoustic pressure (in W m-2), P the acoustic pressure (in Pa), ρ the density of propagating material (1,000 kg m-3 for water) and c the speed of sound in propagating medium (for water, c = 1,500 m s-1). - Create calibration curves using these measurements.
NOTE: The pressure vs. voltage and intensity vs. voltage curves have a linear and parabolic shape, respectively. - Determine the pressure and/or intensity value of a desired driving voltage by using the corresponding calibration curve.
7. Calcium-Sensitive/LIPUS Live-Cell Fluorescence Imaging
- Replace the cell’s culture medium with a desired imaging buffer containing 5 µM of a cell-permeant calcium-sensitive dye (e.g., Fluo-4 AM). Incubate the culture dish in a CO2 incubator at 37 °C for 1 h.
- Carefully wash cells with the same buffer free of dye.
- Place the dish in the sample holder. Excite the cells using blue light illumination (490 nm) and adjust excitation intensity and camera exposure to avoid excessive bleaching or pixel saturation.
- Perform time-lapse imaging using desired image acquisition settings. Use an immersion objective for better image quality and with long working distance to reduce undesired reflections (see Figure 4).
Representative Results
Figure 5 is an example of LIPUS experiment multiplexed with calcium imaging. Glioblastoma cells (A-172) were grown on EMPM coated polyester film in standard culture medium (supplemented with 10% serum and 1% antibiotics) and incubated with the calcium-sensitive fluorescent reporter Fluo-4 AM. Cells were imaged using a 10X immersion lens and illuminated with a white LED light source and fluorescence light was collected using a standard GFP filter set. LIPUS was applied by manually by driving a 4 MHz transducer with a pulse waveform of 158 V peak-to-peak amplitude, 0.1 ms pulse duration and 10 ms pulse repetition frequency (i.e., 1% duty cycle). These parameters correspond to Isppa = 88 W cm-2 (well below the diagnostic limit of 190 W cm-2)and Ispta = 877 mW cm-2 (slightly above the diagnostic limit of 720 mW cm-2), respectively. The results show this stimulation produced robust calcium elevations (Figure 5B, 5C, 5D).
For short pulse durations (i.e., with no significant heat dissipation during the pulse) and assuming the heat capacity of the sample (i.e., cells grown on polyester film and immersed in aqueous solution) is similar to that of water, the change of temperature (∆Tmax) produced during each pulse can be estimated by16
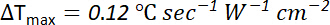
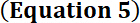
with a pulse duration of 0.1 ms and pulse intensity of 88 W cm-2
∆Tmax = 0.12 x 0.0001 x 88 ≈ 1 m°C
with 1% duty cycle, the small amount of heat deposited at the focal zone during each 0.1 ms pulse is likely removed by thermal conduction during the 9.9 ms spanned between two consecutive pulses. Hence, the robust calcium signals seen in Figure 5B, 5C, 5D are most likely induced by non-thermal mechanism(s).
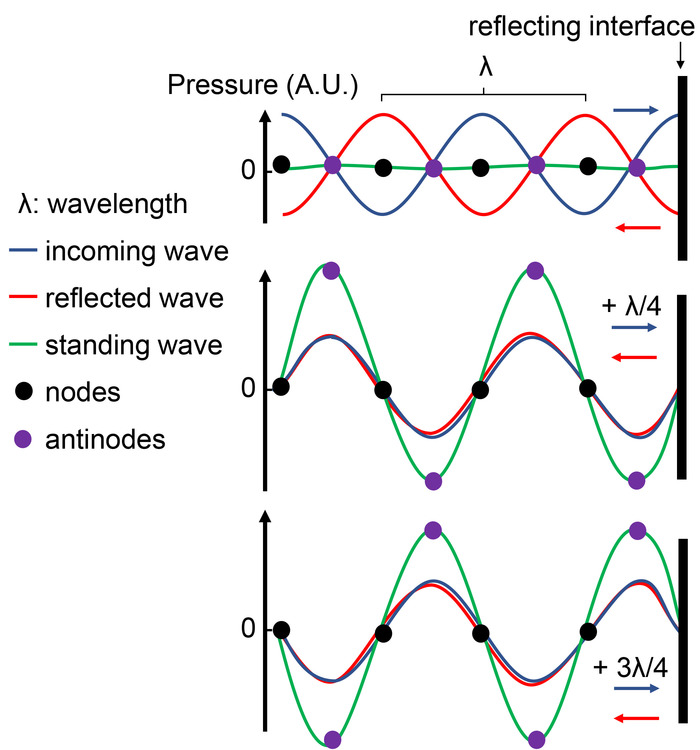
Figure 1: Standing wave formation at a reflecting interface. The presence of an interface between materials with different acoustic impedance reflects an incoming pressure wave (blue) with a wavelength λ. Since both waves travel in opposite directions, an oscillating phase shift is established. Top: at this time, the phase shift is 180°, producing destructive interference of the standing wave (green wave). Middle: After the waves have moved a distance corresponding to λ/4 with respect to the top panel, the phase shift is null and both waves amplify via constructive interferences, producing a standing wave of higher amplitude. Bottom: After the waves have moved an additional distance of λ/2 (hence a total of λ/4+λ/2 = 3/4 λ from the top reference), the phase shift becomes null again, producing a standing wave of high amplitude but with inverse polarity. Note that some positions within the path have a constant null pressure (node, black circles) while other positions constantly oscillate between minimum and maximum pressures (antinodes, green circles). Please click here to view a larger version of this figure.
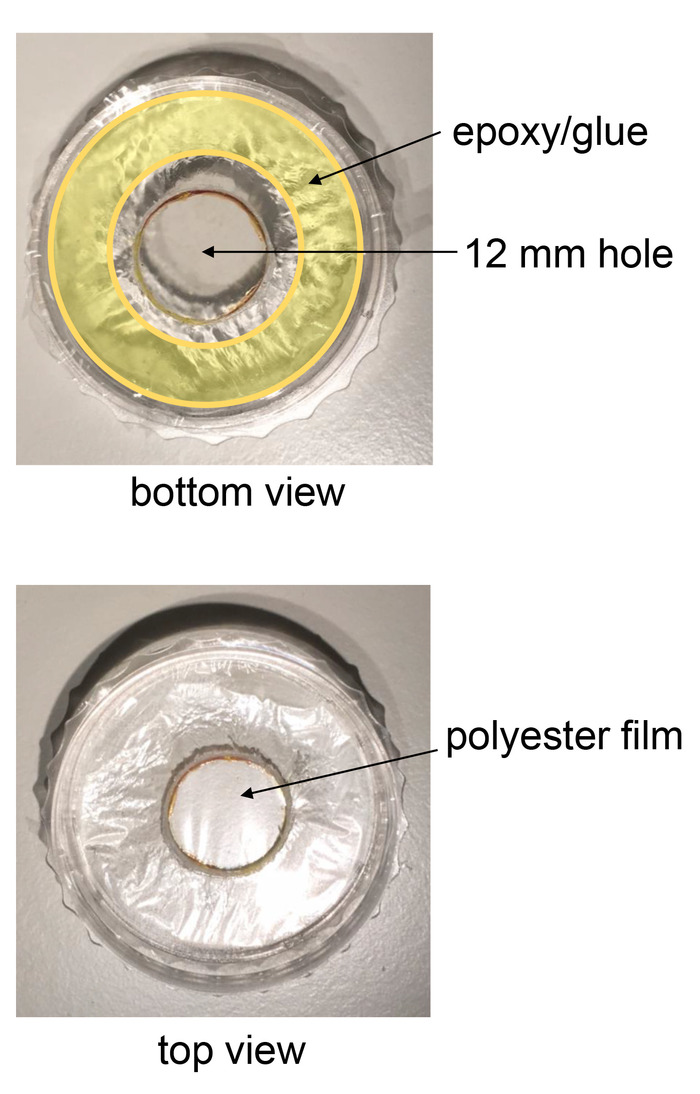
Figure 2: Growing cells on acoustically-transparent polyester film. The figure shows the bottom part of a 35 mm dish with a large 12 mm hole in its center. The hole is subsequently covered with a thin polyester film. The film is firmly glued to the external bottom of the dish using marine grade epoxy. Please click here to view a larger version of this figure.
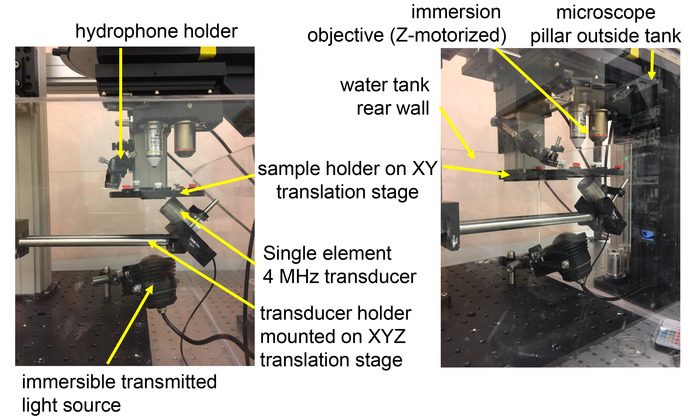
Figure 3: Implementation of an ultrasound set-up to an upright fluorescence microscope. A custom-made water tank is placed under an upright fluorescence microscope without transmitted illumination hardware. A motorized sample holder positioned under the objective is attached to optomechanical components fixed to the vibration table outside the tank. The transducer is positioned underneath the sample and attached to a translation stage affixed to optomechanical components inside the tank. Please click here to view a larger version of this figure.
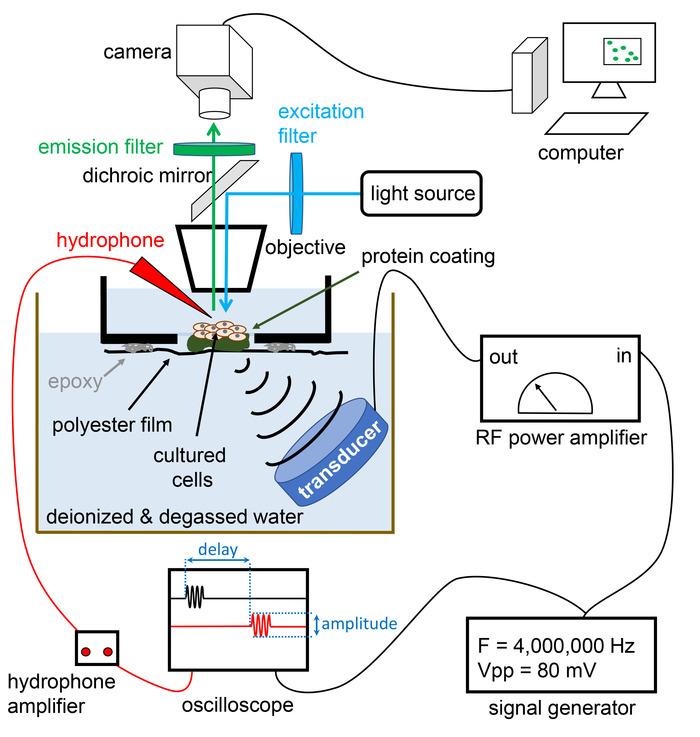
Figure 4: Schematic diagram of the entire set-up. The set-up includes an epi-fluorescence upright fluorescence microscope to enable fluorescence imaging of cultured cells. The transducer is shown with an oblique orientation with respect to the optical path. This configuration avoids backward reflection of acoustic waves along the acoustic path, thus preventing standing wave formation and/or repetitive stimulation of the sample with multiple ultrasound echoes. A desired waveform is produced by a function generator which can be manually switched on or electronically triggered by a computer interface (Transistor-Transistor-Logic or Universal Serial Bus). The amplitude and delay of the signal measured by the hydrophone needle (red) can be analyzed using an oscilloscope. Please click here to view a larger version of this figure.
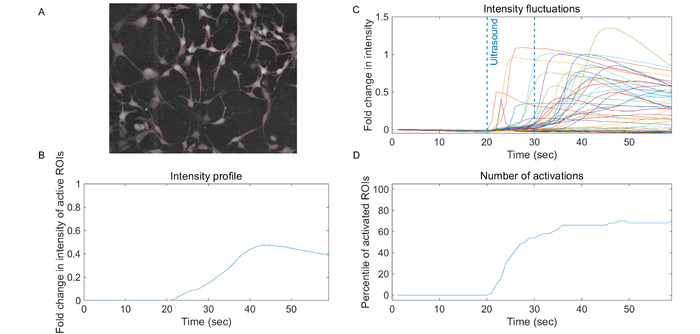
Figure 5: Example of LIPUS-induced calcium signals in human glioblastoma cells A-172. (A) Raw fluorescence image of A-172 cells grown on a polyester-bottom 35 mm culture dish and loaded with a cell-permeant version of the calcium-indicator Fluo-4. The red traces represent cell boundaries automatically identified by a computer program and labeled as regions of interest (ROI). (B) Time course of relative fluorescence change (Ft-F0/F0, or ΔF/F0) for each ROI during a LIPUS experiment. Images were acquired at a speed of 1 frame per second by a standard CCD camera and LIPUS was applied for 10 s between frames 20 and 30. The LIPUS waveform consists of 100 µsec pulses containing 400 cycles at 4 MHz and repeated every 10 ms for 10 s. (C) Plot showing the time course of the mean ΔF/F0 calculated for all ROIs (error bars not shown). (D) Plot showing the percentile of ROI exhibiting ΔF/F0 above a user-defined activation threshold. Please click here to view a larger version of this figure.
Discussion
A main advantage of focused ultrasound is its ability to non-invasively deliver mechanical and/or thermal energy to biological samples with high spatio-temporal precision. Other techniques intended to mechanically stimulate cells usually employ invasive physical probes (e.g., cell-poking) or requires the interaction of high energy laser beams with foreign objects (e.g., optical tweezers). Magnetic heating can heat specific spatial locations inside biological samples but requires the presence of foreign magnetic nanoparticles. On the other hand, precise non-invasive heating of small sample (e.g., cell cultures) in aqueous media is possible using infrared or microwave excitation17,18,19.
Since commercial systems able to perform ultrasound excitation in conjunction with fluorescence microscopy are not available, many biophysicists have created customized systems tailored to their specific applications8,12,13,20. The implementation of such systems can, however, be difficult for non-experts. This article described the basic operation to drive a focused ultrasound beam toward the focal plane of an upright epi-fluorescence microscope objective while limiting acoustic reflections and standing waves artefacts that are produced at mismatch acoustic impedance interfaces. These acoustic artifacts are not usually explicitly taken into consideration in the published literature.
If using an acoustic beam perpendicular to the optical path, the beam will ultimately encounter the liquid-solid interface formed by the front lens of an immersed objective or, if an air objective is being used, the water-air interface above the sample. These interfaces will reflect the acoustic waves back to the sample, producing standing waves (see Figure 1). To mitigate or avoid these reflections, it is recommended to position the transducer with an oblique angle with respect to the optical path.
The use of polyester-bottom culture dishes not only minimize acoustic reflections produced by the thick plastic culture dishes bottom, they also enable the experimentalist to stimulate the sample from underneath with an upright microscope. This is a preferable configuration as compared to an inverted microscope to position an immersed transducer with an oblique orientation with respect to the optical path.
This protocol is designed for use with focused ultrasound transducers, but experimentalist may also use non-focused (planar) ultrasound transducers as well. Note that, since LIPUS is intended for precise stimulation of desired areas within tissue, focused ultrasound transducers are generally preferred for both in vitro and in vivo applications. Acoustic beams produced by planar transducers are also broader, making it more difficult to reduce reflections and other mechanical artefacts.
The focal zone of a single element focused ultrasound transducer depends on many parameters such as the element diameter, the acoustic frequency and the sound velocity of the propagating material. For a standard MHz transducer, the focal area is typically confined in a millimetric or sub-millimetric region. A trade-off exists between the size of the focal zone and the ability of the acoustic beam to penetrate inside tissue without too much loss due to attenuation: the higher the frequency, the smaller the focal zone but the weaker the penetration.
To effectively stimulate cells visualized under a microscope, the acoustic focal zone of the transducer must overlap with the optical focal plane of the objective. To this aim, it is necessary to precisely align the acoustic beam using a commercially available hydrophone. There are two main types of hydrophones: hydrophone needles and fiber optic systems. Both types can be used. During alignment, it is important to make sure that the acoustic intensity is within the pressure limits of the hydrophone and that the frequency of the transducer is within the frequency range of the hydrophone's sensitivity.
Use the calibration provided by the manufacturer (if available) to convert the voltage amplitude output of the hydrophone into actual acoustic pressure (hydrophones are usually calibrated with a number in V Pa-1 or similar units). Hydrophones also usually have a preferential orientation (directionality) with respect to the acoustic beam, hence it is preferred to position the hydrophone in the same direction of the acoustic axis. If not possible, hydrophones may have a chart of attenuation vs. directional angle, allowing directional post-correction after the measurements have been taken.
Beam alignment can be a frustrating task, especially when operating a transducer with a narrow focal zone. The hydrophone signal should appear with a delay with respect to the pulse driving the transducer. This delay corresponds to the time taken by the ultrasound to travel from the transducer's surface to the hydrophone probe (in water, sound waves travel at a speed of approximately 1,500 m s-1) (see Figure 4). Note that the delay of RF signals traveling through electrical cables is small, only about 3 ns m-1, which, in the present case, can be safely ignored. A way to test if the beam is well aligned is to calculate the distance between transducer and hydrophone using the delay measured at the oscilloscope and the known sound velocity in water. For instance, a delay of approximately 17 µs is expected for a transducer with a focal length of 25 mm.
If the use of commercial hydrophone is not possible (e.g., unusual transducer frequency with not matching hydrophones or limited space for placing the hydrophone), the acoustic beam can be aligned with the pulse-echo method20. This is usually done by first placing a small reflecting object of a size similar or smaller than the beam diameter of the transducer into the focus within the microscope field of view. The transducer is then used both as acoustic emitter (to send ultrasound pulses) and receiver (to detect the echo from the reflecting object). Note that in this configuration, a dedicated amplifier is necessary to amplify the rather small echo signal coming out of the transducer.
For smooth operation of RF instruments, it is important that all the cables and connections to instruments have matching electrical impedance, otherwise undesired electrical reflections will occur and alter the electrical waveform. This is usually the case with most Bayonet Neill-Concelman (BNC) cables and RF equipment having 50 Ω impedance. Some oscilloscopes, however, have a high input impedance of 1 MΩ and thus will reflect a RF signal fed through a 50 Ω BNC cable. In this case, a simple 50 Ω feed-through terminator should be inserted between the end of the BNC cable and the oscilloscope's input to properly terminate the signal without loss.
This method is technically limited by the instrumentation used for delivering ultrasound pulses and to perform fluorescence imaging. For example, a standard single-photon fluorescence microscope would only enable imaging of two-dimensional samples. However, it can perform more complex LIPUS imaging of three-dimensional samples like brain slices or small organs using multi-photon excitation.
Another challenge with LIPUS experiments is to distinguish mechanical vs. thermal effects imparted by acoustic beams. A mechanical displacement oblique to the optical path can be detected if it displaces the image in the plane axis (x,y) or defocuses the sample in the z-axis. These displacements depend on the combined optical resolution of the microscope camera and objective. In addition, as these motions would only occur during sonication, one would need to use a frame rate high enough to synchronize the temporal overlap between camera exposure and pulse duration.
To investigate ultrasound-induced thermal effects, the use conventional physical probes to measure temperature is not recommended because of unavoidable vibrations of the probe. However, the technique described here is well suited to measure temperature changes using genetically-encoded thermosensitive fluorescence reporters or thermosensitive dyes21,22. In the future, as more biocompatible fluorescent reporters become available, this technique will enable the study of ultrasound effects on many other biophysical parameters.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Drs. Mikhail Shapiro and Nikita Reznik for fruitful discussions. This work was supported by start-up funds from Western University of Health Sciences and NIH grant R21NS101384.
Materials
| upright microscope with large working volume | Thorlabs | CERNA |
| upright microscope with large working volume | Scientifica | SliceScope |
| optomechanical components | Thorlabs | n/a |
| needle hydrophone | ONDA Corporation | HNP/C/R/A/T series + AH/G pre-amplifier |
| needle hydrophone | Precision Acoustics | n/a |
| fiber optic hydrophone | ONDA Corporation | HFO series |
| fiber optic hydrophone | Precision Acoustics | n/a |
| oscilloscope | Keysight Technology | DSOX2004A (4-channels 70MHz) |
| function generator | Keysight Technology | 33500B (20MHz single-channel) |
| RF power amplifier | Electronic Navigation Industries (ENI) | 325LA, 525LA, 240L, 350L, A075, 2100L, 3100LA |
| RF power amplifier | Electronics & Innovation (E&I) | |
| immersion ultrasound transducer | Olympus | focused immersion transdcuers |
| immersion ultrasound transducer | Benthowave Instrument | HiFu transducer BII-76 series |
| immersion ultrasound transducer | Precision Acoustics | Piezo-ceramic or HiFu transducers |
| immersion ultrasound transducer | Ultrasonic-S-lab | HiFu transducers made to order |
| high-density Matrigel | Corning | VWR 80094-330 |
| Mylar film 2.5 microns | Chemplex | CAT.NO:107 |
Riferimenti
- Elhelf, I. A. S., et al. High intensity focused ultrasound: The fundamentals, clinical applications and research trends. Diagnostic and Interventional Imaging. 99 (6), 349-359 (2018).
- Toccaceli, G., Delfini, R., Colonnese, C., Raco, A., Peschillo, S. . Emerging strategies and future perspective in neuro-oncology using Transcranial Focused Ultrasound Technology. , (2018).
- Duck, F. A. Medical and non-medical protection standards for ultrasound and infrasound. Progress in Biophysics and Molecular Biology. 93 (1-3), 176-191 (2007).
- Legon, W., et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nature Neuroscience. 17 (2), 322-329 (2014).
- Tyler, W. J. The mechanobiology of brain function. Nature Reviews: Neuroscience. 13 (12), 867-878 (2012).
- Tyler, W. J. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist. 17 (1), 25-36 (2011).
- Tufail, Y., et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 66 (5), 681-694 (2010).
- Tyler, W. J., et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PloS One. 3 (10), e3511 (2008).
- Suarez Castellanos, I., et al. Calcium-dependent ultrasound stimulation of secretory events from pancreatic beta cells. Journal of Therapeutic Ultrasound. 5, 30 (2017).
- Suarez Castellanos, I., Jeremic, A., Cohen, J., Zderic, V. Ultrasound Stimulation of Insulin Release from Pancreatic Beta Cells as a Potential Novel Treatment for Type 2 Diabetes. Ultrasound in Medicine and Biology. 43 (6), 1210-1222 (2017).
- Ibsen, S., Tong, A., Schutt, C., Esener, S., Chalasani, S. H. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nature Communications. 6, 8264 (2015).
- Prieto, M. L., Firouzi, K., Khuri-Yakub, B. T., Maduke, M. Activation of Piezo1 but Not NaV1.2 Channels by Ultrasound at 43 MHz. Ultrasound in Medicine and Biology. 44 (6), 1217-1232 (2018).
- Kubanek, J., et al. Ultrasound modulates ion channel currents. Scientific Reports. 6, 24170 (2016).
- Prieto, M. L., Omer, O., Khuri-Yakub, B. T., Maduke, M. C. Dynamic response of model lipid membranes to ultrasonic radiation force. PloS One. 8 (10), e77115 (2013).
- Sato, T., Shapiro, M. G., Tsao, D. Y. Ultrasonic Neuromodulation Causes Widespread Cortical Activation via an Indirect Auditory Mechanism. Neuron. 98 (5), 1031-1041 (2018).
- O’Brien, W. D. Ultrasound-biophysics mechanisms. Progress in Biophysics and Molecular Biology. 93 (1-3), 212-255 (2007).
- Shapiro, M. G., Homma, K., Villarreal, S., Richter, C. P., Bezanilla, F. Corrigendum: Infrared light excites cells by changing their electrical capacitance. Nature Communications. 8, 16148 (2017).
- Shapiro, M. G., Homma, K., Villarreal, S., Richter, C. P., Bezanilla, F. Infrared light excites cells by changing their electrical capacitance. Nature Communications. 3, 736 (2012).
- Shapiro, M. G., Priest, M. F., Siegel, P. H., Bezanilla, F. Thermal mechanisms of millimeter wave stimulation of excitable cells. Biophysical Journal. 104 (12), 2622-2628 (2013).
- Hwang, J. Y., et al. Investigating contactless high frequency ultrasound microbeam stimulation for determination of invasion potential of breast cancer cells. Biotechnology and Bioengineering. 110 (10), 2697-2705 (2013).
- Nakano, M., et al. Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response. PloS One. 12 (2), e0172344 (2017).
- Donner, J. S., Thompson, S. A., Kreuzer, M. P., Baffou, G., Quidant, R. Mapping intracellular temperature using green fluorescent protein. Nano Letters. 12 (4), 2107-2111 (2012).

