Xylem Water Distribution in Woody Plants Visualized with a Cryo-scanning Electron Microscope
Summary
Observing the water distribution within the xylem provides significant information regarding water flow dynamics in woody plants. In this study, we demonstrate the practical approach to observe xylem water distribution in situ by using a cryostat and cryo-SEM, which eliminates artifactual changes in the water status during sample preparation.
Abstract
A scanning electron microscope installed cryo-unit (cryo-SEM) allows specimen observation at subzero temperatures and has been used for exploring water distribution in plant tissues in combination with freeze fixation techniques using liquid nitrogen (LN2). For woody species, however, preparations for observing the xylem transverse-cut surface involve some difficulties due to the orientation of wood fibers. Additionally, higher tension in the water column in xylem conduits can occasionally cause artifactual changes in water distribution, especially during sample fixation and collection. In this study, we demonstrate an efficient procedure to observe the water distribution within the xylem of woody plants in situ by using a cryostat and cryo-SEM. At first, during sample collection, measuring the xylem water potential should determine whether high tension is present in the xylem conduits. When the xylem water potential is low (< ca. −0.5 MPa), a tension relaxation procedure is needed to facilitate better preservation of the water status in xylem conduits during sample freeze fixation. Next, a watertight collar is attached around the tree stem and filled with LN2 for freeze fixation of the water status of xylem. After harvesting, care should be taken to ensure that the sample is preserved frozen while completing the procedures of sample preparation for observation. A cryostat is employed to clearly expose the xylem transverse-cut surface. In cryo-SEM observations, time adjustment for freeze-etching is required to remove frost dust and accentuate the edge of the cell walls on the viewing surface. Our results demonstrate the applicability of cryo-SEM techniques for the observation of water distribution within xylem at cellular and subcellular levels. The combination of cryo-SEM with non-destructive in situ observation techniques will profoundly improve the exploration of woody plant water flow dynamics.
Introduction
Availability of water resources (i.e., precipitation, soil water content) strictly determines the mortality and geographic distribution of plant species, since they need to absorb water from the soil and transport it to the leaves for photosynthetic production. Plants must maintain their water transport system under fluctuating water supplies. In particular, woody plants generate high tensions in their conduits along the transpiration streams as, in some cases, they need to hold their crown more than ~100 m above ground. To maintain water columns under such high negative pressure, xylem conduits consist of a continuum of tubular cells with rigid and hydrophobic-lignified cell walls1. The vulnerability to xylem dysfunction of xylem conduits in each species is a good determinant of the species survival under fluctuating water supply2. In addition, studying the water status of xylem conduits is important for the evaluation of the health condition of individual trees subjected to abiotic or biotic stresses. Measuring sap flow or water potential can provide estimates of a woody plant's water status due to the integrated hydraulic function of xylem conduits. Furthermore, visualizing the distribution of water in xylem cells can clarify the condition of individual components of the xylem hydraulic system.
Several techniques for visualizing the water status of xylem conduits exist3. The classical and useful methods for observing water pathways in woody tissue involve staining the water column by immersing the ends of cut branches into a dye or by injecting a dye into standing tree stems4. Soft X-ray photography also allows the visualization of water distribution of sliced wood disks due to the differential X-ray absorption intensity of the moisture in xylem5,6. These methods, however, only provide tracks of water movement or demonstrate macroscopic distributions of water. Recently, non-destructive observation techniques, such as micro focus X-ray computed tomography (µCT)7,8,9,10and magnetic resonance imaging (MRI)11,12, have been significantly improved to allow observation of water in xylem conduits within intact saplings. These non-destructive methods have great advantages in that we can observe the xylem's water status without artificial cutting effects, and we can track water flow dynamics by sequential imaging or introducing a contrast agent10. However, we need to use a customized MRI for plant imaging or a specialized facility for synchrotron-based µCT in order to obtain the images which can identify cellular level water content. In addition, although the synchrotron-based µCT system enabled to obtain fine images with high spatial resolution, which is comparable to light microscopy7,8,9, living cells can be injured by the radiation of high energy X-ray13,14. Employing a scanning electron microscope in which cryo-units are installed (cryo-SEM) is a very useful method for precisely locating the water in xylem at a cellular level, although this requires destructively harvesting the sample for observation. To fix the water in xylem conduits, a portion of the stems (i.e., twigs, branches or stems) are frozen in situ by liquid nitrogen (LN2). Observations of the surface of trimmed, frozen specimens by cryo-SEM provide highly-magnified images of the xylem structure from which we can identify the water in xylem conduits as ice. A significant limitation of this method is that sequential observation of water movability within the same sample is impossible. However, the application of µCT or MRI for sequential observation of trees that live in a field is extremely challenging because these instruments are not portable. In contrast, cryo-SEM has a potential for using this technique on big trees in field experiments to clearly visualize water contents at not only the cellular level but also at a finer structure level, e.g., water in intervascular pits15, water in intercellular spaces16, or bubbles in water column17.
Many studies observing xylem water by cryo-SEM have been reported 5,12,18,19,20,21,23. Utsumi et al. (1996) initially established the protocol for observation of xylem in situ by freeze-fixation of a living trunk via filling LN2 into a container set onto the stem21. The temperature of the sample was maintained below -20 °C during sample collection and during cryo-SEM preparation in order to avoid melting the ice within xylem conduits. This method has been used to observe the water in xylem in order to clarify water distribution under changing water regime11,12,24,25,26,27,28, the seasonal variation of water distribution21,29,30, the effect of freeze-thaw cycles17,31,32, the distribution of water in wet wood5, changes in the water distribution during the transition from sapwood to heartwood20, seasonal time course of cambial activity and differentiation of vessels33, and cavitation induced by certain biotic stresses23,34. Hydraulic conductivity and conduits vulnerability to cavitation have also been verified using cryo-SEM35,36. Cryo-SEM equipped with energy dispersive X-ray spectrometry (EDX or EDS) has been used to study element distribution over the surface of a specimen containing water37.
Freeze-fixation of a living trunk which contains conduits under high hydraulic tension sometimes causes artificial cavitations which are observed by cryo-SEM as fractured ice crystals in the lumen of conduits38,39. In particular, broadleaved species with longer and wider conduits are vulnerable to tension-induced artifacts, such as cavitation caused by sample cutting, even if conducted under water3,40. Cavitation artifacts become conspicuous after sampling of a transpiring tree (i.e., sampling during the day time) or under severe drought conditions and they can mislead to an overestimation of cavitation occurrence3,38,39. Therefore, the tension working in the conduits has to be released in order to avoid the artifactual cavitation3,12,39.
The freeze-fracture technique using a knife installed in a specimen chamber is often employed to expose specimen surface for cryo-SEM observation. However, freeze-fractured planes of woody plant tissues, especially transverse sections of secondary xylem, are too rough to clearly observe the anatomical features and water in the tissue6. The application of a cryostat for trimming a specimen allows rapid and high-quality preparation of sample surfaces20,23. The overall goal of this method is providing evidence with electron microscopy resolution of the water distribution in various kinds of xylem cells in situ without the occurrence of sampling artifacts. We introduce our updated procedure, which has been steadily improved since we first adopted it, regarding the sampling, trimming and cleaning the specimen surface for obtaining high-quality electron micrographs of cryo-fixed samples of xylem.
Protocol
NOTE: A schematic chart of this protocol is shown in Figure 1.
1. Sampling: Tension Relaxation within Water Column of Xylem Conduits
NOTE: The following tension relaxation treatment is recommended before the LN2 application to avoid both freezing and tension–induced artifacts in the xylem water distribution.
- Enclose a branch and leaves for sampling with a black plastic bag to equilibrate the water potential between xylem and leaves more than two hours before sampling.
- Determine the water potential of at least two leaves from the sample using a pressure chamber or a psychrometer. When the water potential is higher than ca. −0.5 MPa (i.e., no or very-low tension exists), a sample can be harvested after freezing (refer to section 2: Freeze fixation). When the water potential is lower than −0.5 MPa, a treatment for relaxation is needed as described below.
- Fix a watertight collar around the stem in order to be filled with water. A plastic cup without bottom can serve as a watertight collar. Care should be taken to tightly seal the spaces between the stem and the collar using a sticky tape for preventing leakage of liquid media which are used subsequently. For harvesting flexible stems such as thin branches or twigs, sink a cutting portion into a water-filled bucket by bending the stem. Cut under the water surface using pruning shears or a saw. Transfer the sample to another container of water as quickly as possible to minimize exposing the cut end to air.
- Keep the cut end of the sample under water. For broadleaved species, ensure that the length from the spot where a cryo-sample for SEM will be obtained to the cut edge of the harvested stem is longer than the samples' maximum vessel length in order to prevent tension–induced artifacts within the cryo sample.
- Cover the sample containing leaves with a black plastic bag to reduce transpiration. Keep the cut end of the sample in the water and maintain this condition for approximately 30 min in order to relax the xylem tension. Avoid a longer relaxation time (>1 h) due to possible artificial refilling of cavitated conduits12.
- Measure the water potential again to confirm the relaxation of the xylem tension (nearly 0 MPa).
NOTE: Prior to sampling, maximum vessel length of the target species should be researched or determined with similar samples by air injection method. When sampling a large tree, or a large branch, it is difficult to conduct the tension-relaxation procedures described above. Therefore, samples from large trees must be collected during the predawn period when the xylem water potential is higher.
2. Freeze Fixation with LN2
- Cut and open one side of a watertight collar with scissors or a utility knife. Tightly attach the collar around the stem with an adhesive tape while holding the aperture horizontally.
- Wear insulating gloves/mitten, but make sure to hold the bottle of LN2 safely, and run LN2 into the collar to fill it with LN2. Keep it filled by steadily adding LN2 to completely freeze the water in the xylem. The time required for freezing is dependent on the sample size; 1 min after the boiling of poured LN2 has stopped is sufficient for a small twig or a seedling, while more than 20 min is needed for a stem of a larger tree20. Add LN2 continuously during freezing as it evaporates rapidly due to large temperature differences between LN2 and ambient temperatures.
- Detach the collar from the frozen portion of the sample stem in order to remove LN2 after the sufficient freezing time. Make sure to wear insulating gloves to avoid contact with potential LN2 spills caused by detaching the collar.
- Harvest the sample with a fine handsaw immediately.
- Cover the frozen sample with a piece of aluminum foil or put it into a sample tube, on which sample ID numbers are written. Rapidly place the harvested sample into a container filled with LN2 or pack into an insulated box filled with dry ice.
- Store the samples in a deep freezer until observation. The preferred storage temperature is −80 °C in order to prevent ice sublimation and its recrystallization during storing.
3. Specimen Preparation
NOTE: For observation, a sample must be trimmed and its surface for observation must be planed at subzero temperature in order to keep the water distribution in its xylem in situ. A biological microtome with a cryostat system (cryostat) is ideal for trimming and exposing the surface of a specimen in this type of observation by cryo-SEM.
- Set the temperature of the specimen chamber of the cryostat to −30 °C, which is usually cold enough to keep the xylem sap of most plants in a frozen state.
- Trim a sample into a small piece (< ca. 2 cm in height and < ca. 1 cm in width or diameter) that can be adjusted for the specimen holder of a cryo-SEM. Use a sharp knife or a fine-toothed saw for trimming in order to prevent breaking the ice in the specimen. In the case of a larger sample that cannot be cut with a knife, quickly pre-cut with a cooled saw in a freezer box.
- Attach the trimmed piece to a chuck, a holder for a cryostat by mounting onto a tissue freezing embedding medium (e.g., OCT compound) for cryo-sectioning. Then, attach the chuck to a specimen holder of a microtome of the cryostat.
- Trim the surface by repeatedly shaving with 5–7 µm sections in thickness. Trimming by cutting away more than 1,000 to 2,000 µm, in total depth from the initial surface at sample collection, is useful for eliminating the damaged portion of the sample caused by pre-cutting with a knife or saw as described in the step 3.2.
- After roughly trimming a surface of the sample, adjust an unused portion of the microtome blade above the specimen's surface. Do not allow the blade to touch the sample which would exceed the thickness setting.
- Before the first cut by the unused blade portion, slightly widen the distance between the surface of the specimen and the blade.
- Cut the surface of the sample only once or twice. Further, slide the blade again adjusting an unused blade portion on the specimen's surface.
- Repeat steps 3.6 and 3.7 three or four times. This is important in order to obtain a clear surface without “knife marks” (Figure 4).
- After the final cut, set the blade's position far from the sample to prevent dust from sticking onto the sample.
- Detach the chuck from the specimen holder and detach the specimen from the chuck by removing the frozen embedding medium with a sharp knife. Ensure that the specimen is placed in the cryostat chamber to prevent its planed surface from frost dust.
- Attach the specimen to a cryo-SEM specimen holder with a tissue freezing embedding medium in the cryostat chamber.
4. Transfer to the Cryo-SEM Specimen Chamber
NOTE: The surface–prepared specimen must be protected from an increase of temperature or accumulation of frost during the transfer from the cryostat chamber to the observation stage in the cryo-SEM specimen chamber.
- Maintain the cold stage temperature in the cryo-SEM specimen chamber at lower than –120 ˚C with LN2 according to the equipment's user manual.
- Place the specimen holder with the prepared specimen into an insulating container filled with LN2 in the cryostat chamber.
- Hold the specimen holder with a specimen exchanging rod beneath the LN2. Avoid exposing the specimen holder to air whenever possible.
- Rapidly set the specimen holder to the pre-evacuation chamber of the cryo-SEM specimen chamber as soon as starting evacuation of the pre-evacuation chamber. Then, place the specimen holder on the cold stage after air is fully evacuated. Although a bit of frost accumulation is unavoidable, the "freeze-etching" procedure (step 6) can remove it.
5. Setting in SEM
NOTE: Typical setting for the observation is described below. Some modifications are required depending on the vacuum condition or electron beam.
- Set the SEM parameters for observation as follows:
Acceleration voltage: 3–5 kV
Temperature of the specimen stage: < −120 °C
Detector: Secondary emission
6. Freeze-etching
NOTE: Freeze-etching is the procedure for accentuating the edge of the sample's cell walls by slight ice crystal sublimation. Freeze-etching also involves removing surface frost dusts.
- Turn on the acceleration voltage of the electric gun during freeze-etching. It is better to conduct freeze-etching while observing the specimen.
- Raise the temperature of the specimen stage to −100 °C.
- Wait for several minutes until frost dust is removed and the surface level of the ice in xylem cells has decreased slightly by comparison to the cell walls.
- Lower the temperature of the specimen stage to −120 °C.
NOTE: If there is no installed temperature controller for the specimen stage, hold the specimen using the exchange rod and detach it from specimen stage for a few minutes. Observe the specimen several times during this freeze-etching process to verify the specimen's sublimation status.
7. Metal Coating (Optional)
NOTE: Recent improvements to the SEM instrument and image processing can provide high quality images of electric insulating specimens without metal coating. However, non-conductive specimens, such as biological materials, are sometimes subject to charge; higher brightness at specific positions of the specimen due to accumulation of electrons (“charging”). Exposing the specimen to electron beams for a longer period of time or for a high magnification, increases the charging effects. Coating the surface of the specimen with electric-conductive metal materials prevents the occurrence of charging. Use the vacuum coating system which is installed within the cryo-SEM unit in order to prevent the temperature of the specimen from increasing during coating.
- Ensure that coating material is installed at a designated evaporator head of the coating system.
- Maintain the temperature of the cold stage in the coating system below −170 °C.
- Place the specimen holder on the cold stage of the coating system after sufficient freeze-etching.
- Open a partition between the cold stage and the evaporator head.
- Set the current value and the voltage value of the evaporator head at ca. 30 mA and ca. 5 V, respectively.
- Evaporate coating material for ca. 30 s to coat the surface of the specimen.
- Set both of the current and voltage values of the evaporator head at zero and close the partition.
- Place the specimen holder on the cold stage of the specimen chamber for observation.
Representative Results
Representative images of transverse-cut surfaces of coniferous and broadleaved tree xylem, observed by cryo-SEM, are shown in Figure 2. At low magnification, the black area in the images indicates the cavities from which water entirely or partly disappears, and the gray area indicates xylem cell walls, cytoplasm, and water (Figure 2A). At high magnification, it is apparent that the water is not entirely lost from the lumina of three tracheids, indicating the occurrence of macro bubbles in the xylem sap in situ (Figure 2B). With respect to broadleaved species, cavitation occurrence is easily detected within vessels, while water existence is hard to distinguish within fibers, especially at low magnification (Figure 2C). Cytoplasm in parenchyma cells can be distinguished from water within trahceids or vessels through ice plain textures (e.g., Figure 2B).
The effect of temperature on the freeze-etching process is shown in Figure 3. Frost dust is gradually removed and intertracheary pit membranes become clearer through the progression of sublimation with increasing temperature. Remaining large frost dust particles can be eliminated by further freeze-etching but this can be problematic as it unnecessarily decreases the surface-level of ice in xylem conduits.
The high quality of observation is largely achieved through accurate specimen preparation; especially important is smoothing the surface with a sharp blade of the microtome. Insufficient smoothing by a used-blade can sometimes cause rough surface (called "knife marks", Figure 4) or numerous occurrences of dust from the cuts. After carefully planning the specimen's surface, the quick transfer of the specimen to the specimen chamber is also crucial for eliminating contaminations caused by frost formation.
Sample freezing without the relaxation of negative water column pressure will cause artifactual induction of cavitation in xylem conduits (Figure 5). Clustered ice crystals were observed in vessels of specimens from which the sample was not relaxed (arrowheads in Figure 5A), contrastingly, no clustered ice crystals were observed in relaxed sample specimens with a similar water potential (Figure 5B). This tends to be more significant in xylem of broadleaved trees rather than in coniferous trees (unpublished data).
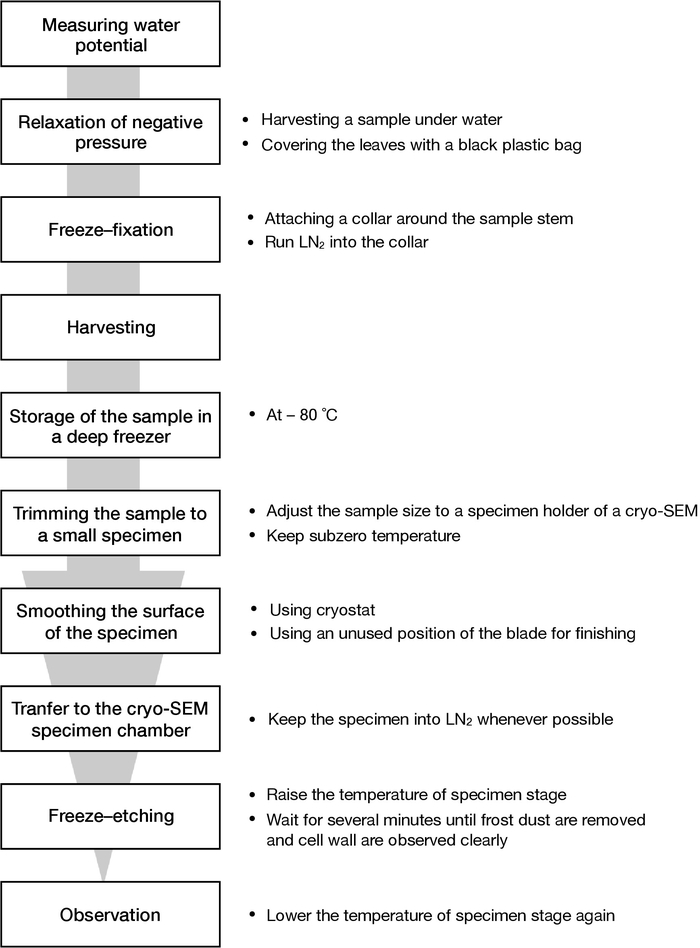
Figure 1: A schematic chart of this protocol. The flow of procedures from sampling to SEM observation described in this paper is shown. Please click here to view a larger version of this figure.
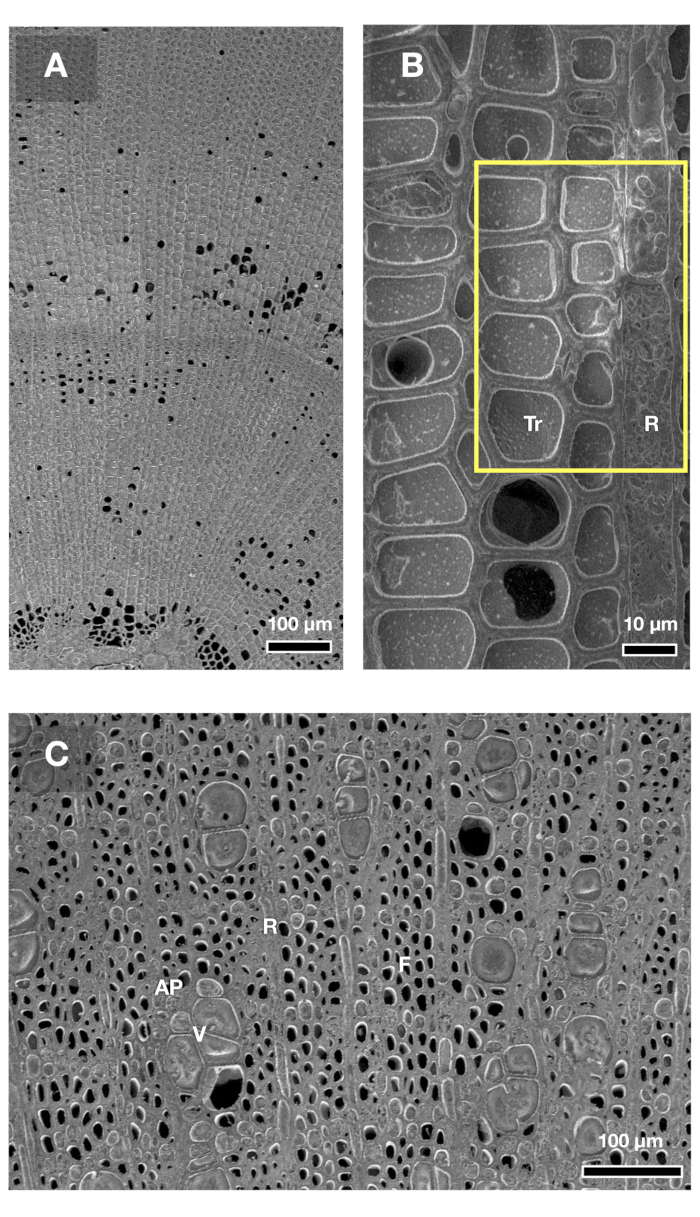
Figure 2: Example cryo-SEM micrographs of the transverse-cut surfaces of angiosperm and gymnosperm tree species. Gray and black areas in xylem cells correspond to the water and cavities in the water column of xylem cells, respectively. Since samples were freeze-fixed with liquid nitrogen prior to sample collection, the images by cryo-SEM show the plant's water status and native embolism at the moment of sampling. (A) and (B): two-year-old twig of an adult tree of Cryptomeria japonica (coniferous wood). The diameter of the twig was 3 mm, and water potential was −0.39 MPa at predawn harvesting. (C): two-year-old shoot of Carpinus tschonoskii (diffuse-porous wood) seedling (1.4 m in height and 1.1 cm in basal diameter). The seedling was sampled after tension-relaxation procedure following prolonged limitation of irrigation for four days. The water potential was −1.78 MPa after a prolonged drought and was −0.15 MPa after the tension-relaxation procedure. Tr: tracheid, R: ray parenchyma, V: vessel, F: fiber, AP: axial parenchyma. Please click here to view a larger version of this figure.
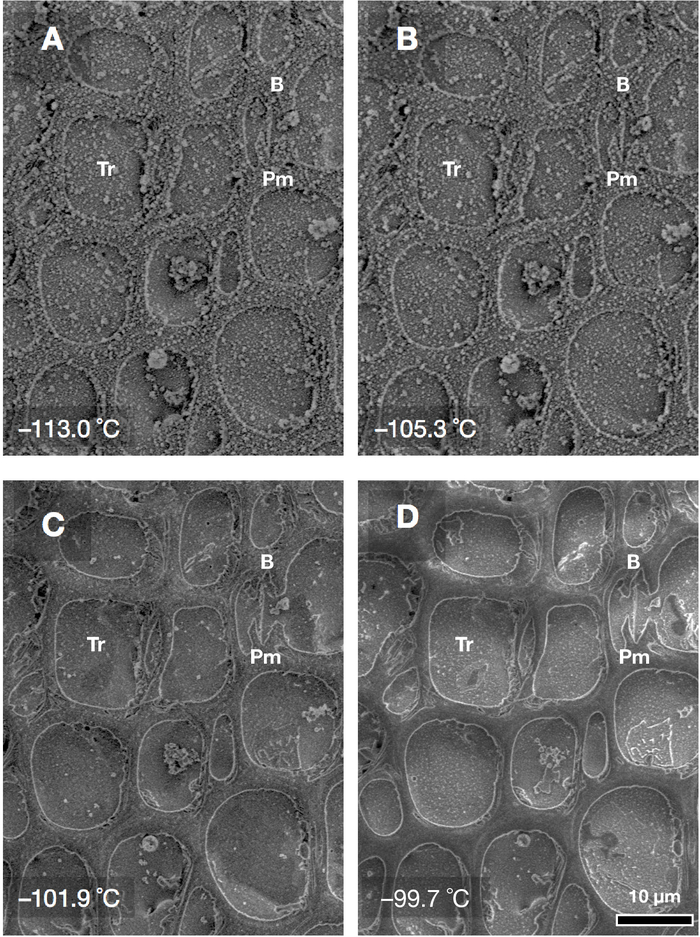
Figure 3: Defrost and etching procedure progress by raising the temperature of the cold specimen stage. Transverse-cut surface of xylem of a Cryptomeria japonica twig (coniferous wood). The decreasing temperatures of the specimen stage are (A) −113.0 °C, (B) −105.3 °C, (C) −101.9 °C and (D) −99.7 °C. Each image was obtained approximately 5 min after the temperature of the cold specimen stage was set at the respective temperature value. Ice sublimation progresses if the temperature of the stage is maintained above approximately −120 °C (for our equipment). Tr: tracheid, B: bordered pit pair, Pm: pit membrane. Please click here to view a larger version of this figure.
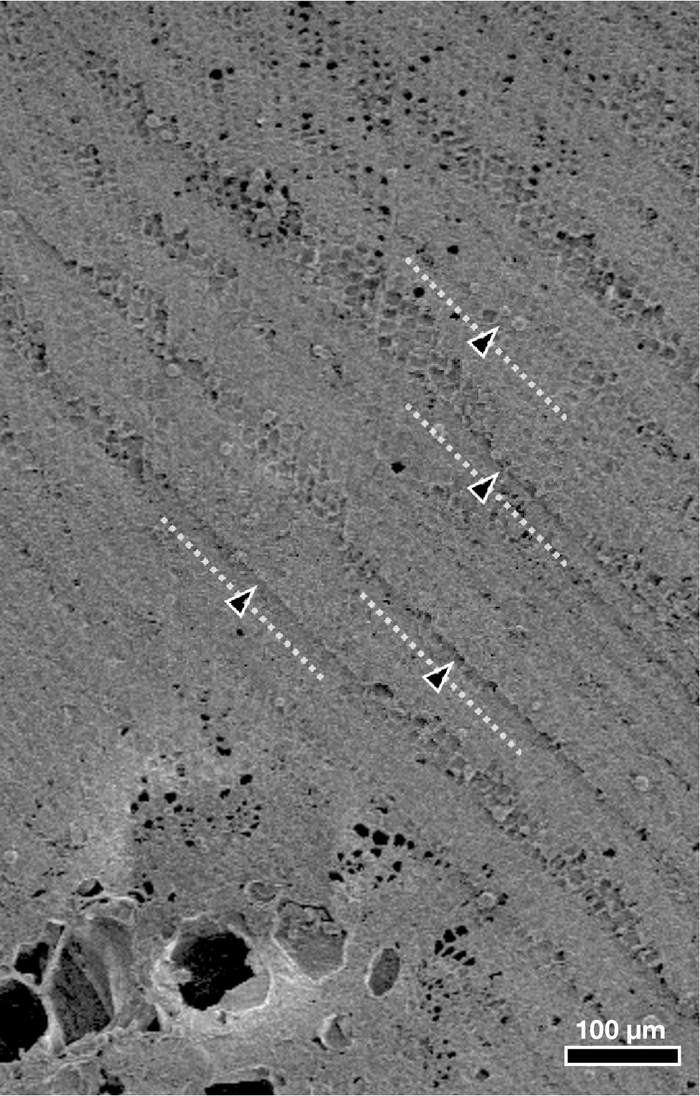
Figure 4: Knife mark example. Transverse-cut surface of xylem of a Cryptomeria japonica twig (coniferous wood) showing so-called knife marks. Arrowheads and dashed lines represent typical knife marks. Clearing the surface of a specimen by a cryostat should be completed by an unused portion of the knife blade. Please click here to view a larger version of this figure.
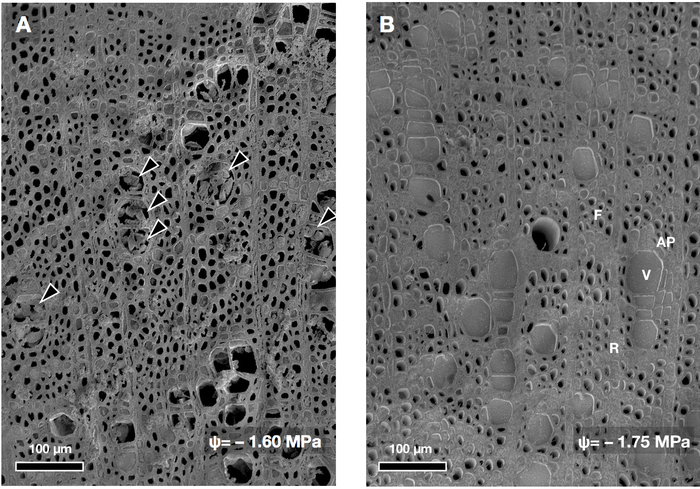
Figure 5: Example of the effect of relaxation of the water column tension in conduits. Transverse-cut surface of stem of a Carpinus tschonoskii seedling (diffuse-porous wood) observed by cryo-SEM. The water potential during the daytime was similar in both seedlings. The stem of the transpiring seedling was frozen intact (A), while the stem of another seedling was frozen after relaxation of the existing hydraulic tension (B). The water potential of (B) at harvesting was −0.5 MPa after the tension-relaxation procedure. Arrowheads in panel (A) are freezing artifacts of ice crystals within vessels. Please click here to view a larger version of this figure.
Discussion
The cryo-SEM observation methods introduced in this paper are practical for clearly visualizing water distribution on a cellular scale. Through this method, exploring the changes in the distribution of water within xylem can potentially help clarify the mechanism of tree species tolerance to abiotic stress (water shortage or freezing) or biotic stress (tree disease).
The most crucial step in this method is preserving the water distribution characteristic of the native water status during sample collection and subsequent sample preparation. Xylem tissue of species with long conduits (especially earlywood vessels of ring-porous trees) can easily lose water during harvesting and freezing. Cochard et al. (2000) have discussed freezing artifacts due to high tension in the water column along xylem conduits38,41. Umebayashi et al. (2016) confirmed the validity of cryo-SEM observations of tension-relaxed samples by comparison to non-destructive MRI observations39. Both observation techniques showed a similar pattern of water distribution. Although we still need to verify the species-specific robustness against air-entry induced by freezing under high hydraulic tension, relaxation procedures should be conducted to provide reliable estimations of water distributions, and in particular for water-stressed plants.
Frost and ice particles are significant obstacles to detailed observation. To prevent frost accumulation, the specimen should not be exposed to the atmosphere until it is attached to the cryo-SEM specimen chamber. Although exposure to the atmosphere cannot be entirely prevented during the transfer of the sample to the specimen chamber, the transfer time should be kept short. The insulating transfer container of the specimen holder should be well dried after removing the specimen holder and LN2 in order to prevent frost and ice originated from dew condensation.
The appropriate length of time for freeze-etching depends on the performance of the instruments used. Important factors in determining this are the vacuum level in the specimen chamber and the stability of the temperature controller of the specimen stage. The extent of sublimation corresponding to the temperature should be primarily assessed before formal use. Excessive freeze-etching will attenuate the presence of water in xylem conduits and make it difficult to be identified, especially in the lumina of narrow cells.
It takes specific steps and efforts to ensure that destructive methods of sampling are free from artifact occurrence during the freezing, harvesting and trimming procedures. Although the significance of the occurrence of artifacts is frequently pointed out, the degree of occurrence of freezing artifacts in xylem conduits has not been sufficiently validated38,39. Further validation by non-destructive methods is desirable for confirming the accuracy of the tension-relaxation procedure and the freeze-fixation. Since synchrotron-based µCT or MRI systems have not been sufficiently prevalent in the study of plant-water relations by comparison to the cryo-SEM system, application of a commercial µCT system can possibly progress the validation of cryo-SEM results42.
Hydraulic tree traits, such as sap flux, hydraulic conductivity of stem segments, percentage loss of conductivity (PLC), or xylem capacitance provide estimates of tree water use and resistance to drought. Comparison of water use among species is needed for predicting tree survival under water stress caused by anthropogenic climate change2. Cryo-SEM observation has many advantages in providing anatomical knowledge for clarifying the cause of changes within hydraulic features. The recent improvements of both destructive and non-destructive methods of plant anatomical and hydraulic studies can together deepen our understanding of the nature of tree water use.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by JSPS KAKENHI (No. 20120009, 20120010, 19780129, 25292110, 23780190, 23248022, 15H02450, 16H04936, 16H04948, 17H03825, 18H02258)
Materials
| coating material | JOEL Ltd., Japan | Gold wire, 0.50 × 1000 mm, 99.99 %, Parts No. 125000499 | |
| cryo scanning electron microscope | JOEL Ltd., Japan | JSM-6510 installed with MP-Z09085T / MP-51020ALS | |
| cryostat | Thermo Scientific | CryoStar NX70 | |
| microtome blade | Thermo Scientific | HP35 ULTRA Disposable Microtome Blades, 3153735 | |
| tissue freezing embedding medium | Thermo Scientific | Shandon Cryomatrix embedding resin, 6769006 |
Riferimenti
- Tyree, M. T., Zimmermann, M. H. . Xylem structure and the ascent of sap. , (2002).
- Choat, B., Jansen, S., et al. Global convergence in the vulnerability of forests to drought. Nature. 491 (7426), 752-755 (2012).
- Klein, T., Zeppel, M. J. B., et al. Xylem embolism refilling and resilience against drought-induced mortality in woody plants: processes and trade-offs. Ecological Research. 33 (5), 839-855 (2018).
- Sano, Y., Okamura, Y., Utsumi, Y. Visualizing water-conduction pathways of living trees: selection of dyes and tissue preparation methods. Tree Physiology. 25 (3), 269-275 (2005).
- Sano, Y., Fujikawa, S., Fukazawa, K. Detection and features of wetwood in Quercusmongolica var. grosseserrata. Trees – Structure and Function. 9 (5), 261-268 (1995).
- Utsumi, Y., Sano, Y. Freeze stabilization and cryopreparation technique for visualizing the water distribution in woody tissues by X-ray imaging and cryo-scanning electron microscopy. Electron Microscopy. (Chapter 30), 677-688 (2014).
- Brodersen, C. R., McElrone, A. J., Choat, B., Matthews, M. A., Shackel, K. A. The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography). Plant Physiology. 154 (3), 1088-1095 (2010).
- Brodersen, C. R., McElrone, A. J., Choat, B., Lee, E. F., Shackel, K. A., Matthews, M. A. In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiology. 161 (4), 1820-1829 (2013).
- Choat, B., Badel, E., Burlett, R. E. G., Delzon, S., Cochard, H., Jansen, S. Noninvasive measurement of vulnerability to drought-induced embolism by X-ray microtomography. Plant Physiology. 170 (1), 273-282 (2016).
- Pratt, R. B., Jacobsen, A. L. Identifying which conduits are moving water in woody plants: a new HRCT-based method. Tree Physiology. 38 (8), 1200-1212 (2018).
- Fukuda, K., Kawaguchi, D., et al. Vulnerability to cavitation differs between current-year and older xylem: nondestructive observation with a compact MRI of two deciduous diffuse-porous species. Plant, Cell and Environment. 38 (12), 2508-2518 (2015).
- Ogasa, M. Y., Utsumi, Y., Miki, N. H., Yazaki, K., Fukuda, K. Cutting stems before relaxing xylem tension induces artefacts in Vitis coignetiae, as evidenced by magnetic resonance imaging. Plant, Cell and Environment. 39 (2), 329-337 (2016).
- Petruzzellis, F., Pagliarani, C., et al. The pitfalls of in vivo imaging techniques: evidence for cellular damage caused by synchrotron X-ray computed micro-tomography. New Phytologist. 220 (1), 104-110 (2018).
- Savi, T., Miotto, A., et al. Drought-induced embolism in stems of sunflower: A comparison of in vivo micro-CT observations and destructive hydraulic measurements. Plant Physiol Biochem. 120, 24-29 (2017).
- Choat, B., Jansen, S., Zwieniecki, M. A., Smets, E., Holbrook, N. M. Changes in pit membrane porosity due to deflection and stretching: the role of vestured pits. Journal of Experimental Botany. 55 (402), 1569-1575 (2004).
- Nakaba, S., Hirai, A., et al. Cavitation of intercellular spaces is critical to establishment of hydraulic properties of compression wood of Chamaecyparis obtusa seedlings. Annals of Botany. 117 (3), 457-463 (2016).
- Utsumi, Y., Sano, Y., Funada, R., Fujikawa, S., Ohtani, J. The progression of cavitation in earlywood vessels of Fraxinus mandshurica var japonica during freezing and thawing. Plant Physiology. 121 (3), 897-904 (1999).
- McCully, M., Canny, M. J., Huang, C. X. Cryo-scanning electron microscopy (CSEM) in the advancement of functional plant biology. Morphological and anatomical applications. Functional Plant Biology. 36 (2), 97-124 (2009).
- Canny, M. J. Vessel contents of leaves after excision – A test of Scholander’s assumption. American Journal of Botany. 84 (9), 1217-1222 (1997).
- Kuroda, K., Yamashita, K., Fujiwara, T. Cellular level observation of water loss and the refilling of tracheids in the xylem of Cryptomeria japonica during heartwood formation. Trees – Structure and Function. 23 (6), 1163-1172 (2009).
- Utsumi, Y., Sano, Y., Ohtani, J., Fujikawa, S. Seasonal changes in the distribution of water in the outer growth rings of Fraxinus mandshurica var. Japonica: A study by cryo-scanning electron microscopy. IAWA Journal. 17 (2), 113-124 (1996).
- Ohtani, J., Fujikawa, S. Cryo-SEM observations on vessel lumina of a living tree: Ulmus davidiana var. japonica. IAWA Journal. 11 (2), 183-194 (1990).
- Yazaki, K., Takanashi, T., et al. Pine wilt disease causes cavitation around the resin canals and irrecoverable xylem conduit dysfunction. Journal of Experimental Botany. 69 (3), 589-602 (2018).
- Tyree, M. T., Salleo, S., Nardini, A., Lo Gullo, M. A., Mosca, R. Refilling of embolized vessels in young stems of laurel. Do we need a new paradigm?. Plant Physiology. 120 (1), 11-21 (1999).
- Melcher, P. J., Goldstein, G., et al. Water relations of coastal and estuarine Rhizophora mangle: xylem pressure potential and dynamics of embolism formation. Oecologia. 126 (2), 182-192 (2001).
- Yazaki, K., Sano, Y., Fujikawa, S., Nakano, T., Ishida, A. Response to dehydration and irrigation in invasive and native saplings: osmotic adjustment versus leaf shedding. Tree Physiology. 30 (5), 597-607 (2010).
- Yazaki, K., Kuroda, K., et al. Recovery of physiological traits in saplings of invasive Bischofia tree compared with three species native to the Bonin Islands under successive drought and irrigation cycles. PLoS ONE. 10 (8), e0135117 (2015).
- Umebayashi, T., Morita, T., et al. Spatial distribution of xylem embolisms in the stems of Pinus thunbergii at the threshold of fatal drought stress. Tree Physiology. 36 (10), 1210-1218 (2016).
- Utsumi, Y., Sano, Y., Funada, R., Ohtani, J., Fujikawa, S. Seasonal and perennial changes in the distribution of water in the sapwood of conifers in a sub-frigid zone. Plant Physiology. 131 (4), 1826-1833 (2003).
- Utsumi, Y., Sano, Y., Fujikawa, S., Funada, R., Ohtani, J. Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiology. 117 (4), 1463-1471 (1998).
- Ball, M. C., Canny, M. J., Huang, C. X., Egerton, J. J. G., Wolfe, J. Freeze/thaw-induced embolism depends on nadir temperature: the heterogeneous hydration hypothesis. Plant, Cell and Environment. 29 (5), 729-745 (2006).
- Mayr, S., Cochard, H., Ameglio, T., Kikuta, S. B. Embolism formation during freezing in the wood of Picea abies. Plant Physiology. 143 (1), 60-67 (2007).
- Kudo, K., Utsumi, Y., et al. Formation of new networks of earlywood vessels in seedlings of the deciduous ring-porous hardwood Quercus serrata in springtime. Trees – Structure and Function. 32 (3), 725-734 (2018).
- Crews, L., McCully, M., Canny, M. J., Huang, C., Ling, L. Xylem feeding by spittlebug nymphs: Some observations by optical and cryo-scanning electron microscopy. American Journal of Botany. 85 (4), 449-460 (1998).
- Hukin, D., Cochard, H., Dreyer, E., Le Thiec, D., Bogeat-Triboulot, M. B. Cavitation vulnerability in roots and shoots: does Populus euphratica Oliv., a poplar from arid areas of Central Asia, differ from other poplar species?. Journal of Experimental Botany. 56 (418), 2003-2010 (2005).
- Mayr, S., Cochard, H. A new method for vulnerability analysis of small xylem areas reveals that compression wood of Norway spruce has lower hydraulic safety than opposite wood. Plant, Cell and Environment. 26 (8), 1365-1371 (2003).
- Kuroda, K., Yamane, K., Itoh, Y. Cellular level in planta analysis of radial movement of artificially injected caesium in Cryptomeria japonica xylem. Trees – Structure and Function. 100 (8), 1-13 (2018).
- Cochard, H., Bodet, C., Ameglio, T., Cruiziat, P. Cryo-scanning electron microscopy observations of vessel content during transpiration in walnut petioles. Facts or artifacts?. Plant Physiology. 124 (3), 1191-1202 (2000).
- Umebayashi, T., Ogasa, M. Y., Miki, N. H., Utsumi, Y., Haishi, T., Fukuda, K. Freezing xylem conduits with liquid nitrogen creates artifactual embolisms in water-stressed broadleaf trees. Trees – Structure and Function. 30 (1), 305-316 (2016).
- Wheeler, J. K., Huggett, B., Tofte, A. N., Rockwell, F. E., Holbrook, N. M. Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant, Cell and Environment. 36 (11), 1938-1949 (2013).
- Canny, M. J., Huang, C. X. The cohesion theory debate continues. Trends In Plant Science. 6 (10), 454-456 (2001).
- Suuronen, J. -. P., Peura, M., Fagerstedt, K., Serimaa, R. Visualizing water-filled versus embolized status of xylem conduits by desktop x-ray microtomography. Plant Methods. 9 (1), 11 (2013).

