Examining the Effect of Pesticides on Caenorhabditis elegans Neurons
Summary
Young adult Caenorhabditis elegans nematodes are exposed to different concentrations of commercial pesticides or other toxicants for 2-24 h. Then, different neurons can be visualized using fluorescent-expressing strains. This paper demonstrates how to expose nematodes to pesticides and assess neuron damage.
Abstract
Caenorhabditis elegans is a powerful model organism used in many research laboratories to understand the consequences of exposure to chemical pollutants, pesticides, and a wide variety of toxic substances. These nematodes are easy to work with and can be used to generate novel research findings, even in the undergraduate biology laboratory. A multi-week laboratory series of authentic, student-driven research projects trains students in a toolkit of techniques and approaches in behavioral measurements, cell biology, and microscopy that they then apply to their projects. One technique in that toolkit is quantifying the percentage of neurons exhibiting neurodegenerative damage following exposure to a chemical toxicant like a pesticide. Young adult C. elegans nematodes can be exposed to different concentrations of commercially available pesticides or other types of toxicants for 2-24 h. Then, undergraduate students can visualize different neuron subtypes using fluorescent-expressing strains of C. elegans. These techniques do not require sophisticated image processing software and are effective at even low magnifications, making the need for expensive confocal microscopy unnecessary. This paper demonstrates how to treat the nematodes with pesticides and how to image and score the neurons. It also provides a straightforward protocol for the microscopy and analysis of neuron morphology. The materials used for this technique are inexpensive and readily available in most undergraduate biology departments. This technique can be combined with behavioral measures like locomotion, basal slowing, or egg-laying to conduct a potentially publishable series of experiments and give undergraduate students an authentic research experience at a very low cost.
Introduction
Caenorhabditis elegans is an excellent model organism for laboratory course training in biological science courses for introductory- and intermediate-level students. This laboratory procedure can be used as part of a multi-week module that explores various effects of commonly used pesticides on C. elegans behavior and cell biology. Students can learn how to design and carry out independent projects that teach them data analysis and presentation skills. This paper focuses on the protocols for exposing C. elegans to pesticide mixtures and then observing and analyzing the effects on neuron morphology.
Lawn chemical pesticide mixtures are widely used for residential and agricultural use and can be purchased at any local garden store. There is increasing concern about the safety of these chemicals for humans and wildlife1,2,3. Students can read the scientific literature and select a pesticide for experimental evaluation and, in so doing, can learn about basic biology and neurobiology, as well as important laboratory skills like experimental design and analysis, and general lab skills like pipetting and serial dilutions, dissecting microscopy, fluorescent microscopy, digital photography, and figure production.
The protocols described in this paper can stand alone in an intermediate-level course in biology or neuroscience or be part of a multi-week module that could also include measurements of behaviors governed by particular groups of neurons. For example, described in this protocol is an assessment of the morphology of cholinergic neurons that govern locomotion using a strain of nematode that expresses GFP (LX 929) in cholinergic neurons4. These strains can be obtained for very low prices from the Caenorhabditis elegans Genetics Center (https://cgc.umn.edu/). Strains expressing GFP in dopaminergic neurons (OH 7457), cholinergic neurons (LX 929), or mCherry expressed in all neurons (PVX4) are all good choices. Students could also measure locomotion and obtain data to accompany the assessment of morphology. A full description of a multi-week student group project can be found in Susman5.
This student group project is quite inexpensive and easy to set up for groups of four students. The materials needed include a dissecting microscope, access to a fluorescence compound microscope that can have an attached digital camera, Petri plates and access to nematode growth agar, growth-limited bacteria (strain OP50, from the CGC), a gas flame Bunsen burner or an alcohol lamp, an autoclave, platinum wire, and general laboratory supplies like micropipetters, microscope slides, coverslips, and glass Pasteur pipettes. Depending on the chemical toxicant being examined by the student groups, the steps in the protocol might need to occur under a fume hood or with gloves. This protocol uses chemical mixtures that are water-soluble (not volatile), and all safe-handling procedures recommended by the manufacturer are followed.
Protocol
All use of invertebrate animals was in compliance with the animal care and use guidelines of the institution.
1. Preparation of pesticide-coated Petri plates
- Prepare agar Petri dishes (6 cm diameter work best) using standard procedures6.
NOTE: These can be made months in advance and stored in the refrigerator until use. Bring plates to room temperature (RT) on the benchtop. In most cases, the student groups should be provided with a plate for each dilution of the pesticide mixture, including a water control. - Prepare 1 mL of each dilution of the chosen pesticide in labeled 1.5 mL microfuge tubes. For safe handling, be sure to read the instructions and provide gloves, or work in a fume hood, if suggested.
NOTE: Suggested dilutions to test: 1 mL of full-strength pesticide, 1:10 dilution in water (100 µL of pesticide, 900 µL of distilled water), 1:100 dilution (10 µL of pesticide, 990 µL of water), 1:1000 dilution (10 µL of the 1:10 dilution, 990 µL of water), etc. - Make a solution spreader by carefully bending a glass Pasteur pipette (9 ml size) in a Bunsen burner flame. Use the flame to close the opening of the pipette. Then ethanol-sterilize the spreader before each spreading on the Petri plate.
- Using a micropipetter, place 100 µL of the dilution (or water control) on the center of the agar and spread gently across the surface with the sterilized spreader. Re-sterilize the spreader prior to each use.
- Set aside the Petri dishes covered, and let the solution soak into the surface until "dry."
NOTE: Depending on the pesticide and humidity levels in the laboratory, this may take some time. Students might need to prepare their plates the day before the exposure period. - For exposure periods longer than 12 h, add 50 µL of an overnight culture of Escherichia coli to the plates prior to adding the nematodes. For acute experiments (12 h or less), there is no need to have E. coli on the plates.
2. Adding nematodes to pesticide-coated plates
NOTE: Students will need to have a plate of adult nematodes (5 day cultures are best) of the fluorescent strain LX929, which is available from the Caenorhabditis elegans Genetics Center. There are two possible procedures for this. If students have had prior experience working with nematodes (for example, if this procedure is part of a multi-week exercise and they have already learned how to pick nematodes with a worm pick), use Step 2.1. If they have not, use Step 2.2.
- Pick nematodes with a worm pick. Fashion a worm pick from a 1 in piece of platinum wire that is melted to a Pasteur pipette using a Bunsen burner flame. Pick up a small blob of sticky E. coli onto the pick and then pick up adult nematodes using the sticky blob. For a decent sample size, pick 10 nematodes for each treatment plate.
NOTE: Students without much experience can follow Steps 2.2-2.3. - Use a micropipette to place 1 mL of sterile water onto the plate of nematodes. Swish the water around to lift up the nematodes, then remove the liquid with the nematodes to a 1.5 mL microfuge tube.
- Let the nematodes settle by gravity for about 10 min, then carefully remove at least 500 µL of the water and discard. Resuspend the nematodes and, using a cut off yellow pipette tip, remove 50-100 µL of suspended worms to each treatment plate. Be sure that each plate has at least 10 adult worms.
- Set a timer, or note the time, and expose the nematodes at RT for the chosen time period. During the chosen exposure time, prepare microscope slides for wet mounts. The slides should be made the same day as the microscopy.
NOTE: Student groups will have chosen their time of exposure during the planning for their independent study.
3. Preparing microscope slides for wet mounts
NOTE: Steps 3.1-3.3 can be performed by each student group. Prepare three slides each.
- Prepare two slides by placing a piece of label tape on only one side of the slide (Figure 1, left panel). The tape is used to create the correct thickness for the agarose to form a pad of uniform thickness. Then position a clean, unused slide in between them on the benchtop, as illustrated in the figure.
- Use a micropipetter to place a 10 µL drop of melted agarose (3% in ddH2O, heated using a 65 °C dry block heater) onto the center of the slide that is in the middle.
- Flatten the drop by placing another clean slide on top, perpendicular to the slide with the drop, as shown in the figure (Figure 1, right panel). Flatten by applying pressure, so the agarose drop forms the thickness of the labeling tape.
- The agarose will solidify within about 1 min. Then, apply steady pressure to separate the two slides. The agar circle will adhere to one of the slides. Rest this slide, agar side up, on the benchtop, and allow it to dry for 1-2 min before using.
4. Mounting live nematodes to microscope slides
- To add nematodes to the prepared slide with the agarose pad, add a 5 µL drop of water containing 1 M sodium azide (NaN3) to the agar pad. This solution anesthetizes the worms and immobilizes them.
CAUTION: Sodium azide stock solution can cause illness if ingested. - Then transfer nematodes (at least 10 per treatment slide) to the droplet using a worm pick. Sterilize the pick before and after using it to transfer nematodes.
- Use forceps to add a coverslip by placing it at an angle and slowly lowering it. Prevent the coverslip from slipping or lowering too quickly by using a pencil or metal probe.
- Place the prepared wet mount on a fluorescent microscope to observe the worms, first under 10x magnification and phase contrast, then under 40x magnification with phase contrast and fluorescent illumination.
5. Fluorescent microscopy
NOTE: Student groups should make a wet mount of several worms of each type on separate microscope slides, which they should label appropriately. The instructions that follow are general tips about the use of a standard fluorescence compound microscope that can have an attached trinocular setup, digital camera, and computer system. The setup used for this manuscript is seen in Figure 2. Students should be familiar with the settings of the microscope, including the objectives, types of light filters, ability to switch between bright field and fluorescence light, how to send the light signal to the digital camera, etc.
- Place one prepared wet mount at a time on the microscope stage and secure it in place using the microscope stage clips.
- Begin viewing the wet mounts by first viewing with the lowest magnification objective, which is typically 10x, and use bright field or phase contrast to visualize and focus on the nematodes.
- Once a nematode is located in the field of view, focus on it using the fine focus knob on the microscope. Switch the illumination to the fluorescence by turning the dial. Then direct the light to the camera to capture the digital images. Adjust the illumination using the imaging software so that the neurons are brightly illuminated but not oversaturated.
- At some point, obtain a separate image of a ruler at the same magnification as the cell images to provide a magnification scale for their figure. Place a short section of a transparent ruler, or a micrometer slide, onto the microscope stage, focus on it using the fine focus knob, and obtain an image using the imaging software.
- Obtain additional images (from at least 10 separate nematodes) at higher magnifications, if possible. The intense light will likely bleach the area from which it is being imaged, so carefully monitor the imaging and do not allow the light to remain on the same area of the slide for long periods of time. Be sure to name the acquired images to keep track of the nematode and region, as well as the treatment and magnification.
- Make a folder on the computer and move the images into the folder for use in analysis and figure preparation.
6. Morphological integrity assessment scale
NOTE: A key cytological characteristic of neurodegenerative damage in neurons is a change to the soma morphology. There are three morphologies that can easily be viewed and counted.
- Count the neurons with smooth, uniformly fluorescent processes and non-round cell bodies as normal, as shown in Figure 3.
- Often the soma becomes rounded and swollen-looking compared with healthy, young neurons. Count the neurons that look like this as "rounded."
- Neurons with long neural processes can exhibit blebbing, where there are rounded puncta or even gaps in the processes. Count the neurons that show these features as "blebbed."
7. Data analysis and figure preparation
- If the percentage healthy (no rounding, no blebbing) and the percentage impaired (rounding and/or blebbing) for at least ten nematodes for each treatment were counted (Figure 3), perform statistical analysis using available statistical software.
- Count all of the neurons that are fluorescently tagged, keeping a tally of those exhibiting normal morphology, blebbing morphology, or rounding morphology, and then calculate the percentage of each morphology out of the total count for each nematode analyzed.
- To prepare figures, import the images into an appropriate software like Powerpoint and then add labels and a scale bar based on the ruler or micrometer image acquired.
Representative Results
The methods and protocols described in this paper provide important laboratory skills for intermediate-level undergraduate students in biology or neuroscience. Students can gain important experience in developing an independent project and conducting an experiment of their own design that could provide novel results. Figure 3 shows an optimal result of a student project that eventually became part of a published paper3. While the majority of student projects do not result in publishable results, most students are able to create a sophisticated figure and figure legend, and most also are able to obtain cell counts, conduct statistical analysis, and create a figure or data table. Particular neurons govern specific behaviors. Thus, when this procedure is part of a multi-week independent project that includes other measurements like assays of locomotion or chemotaxis, the resulting project yields multiple figures and a class presentation of which students can be proud.
Student groups can choose the type of pesticide they wish to explore. Depending on their literature research on the active ingredient of the pesticide they have selected, the students might evaluate different neurons. As all of the neurons in C. elegans are known and their functions well-described, students can choose specific neuronal types, like dopamine neurons or cholinergic neurons, to study in an independently designed experiment. In the example provided in this manuscript, the students examined a neonicotinoid pesticide and focused on cholinergic neurons using a fluorescent strain of nematode expressing GFP in cholinergic neurons. Another example, shown in Figure 4, shows a more typical result from a student group that examined the effects of a manganese-containing pesticide on dopaminergic neurons using a strain in which dopamine neurons express GFP (OH7457). This particular strain shows a bright signal in dopamine neurons, and the neurons were readily visualized by students. However, over time, the fluorescent signal bleaches if the neurons are exposed to the light for long periods of time, as occurred with this student group.
If the students in the course have had very little experience with fluorescent microscopy, it is a good idea to build in an extra week to give the students a chance to repeat their experiment since their skills will dramatically improve with practice.
A challenging aspect for the laboratory instructor is if there are multiple small groups, each assessing the morphology of different neuron types. The different fluorescent strains are not expensive to obtain or maintain, but there is more effort required to have numerous different fluorescent strains on hand. However, it is possible to limit the types of projects to the available strains and resources. Strains that work very well with undergraduate students include the LX929 and OH7547 strains. PVX4, which is a pan-neuronal expressing mCherry, is also a very easy strain to work with7,8.
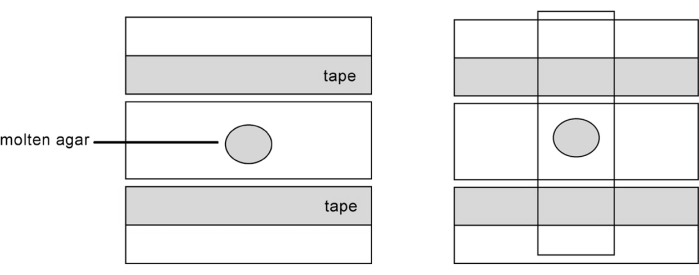
Figure 1: Diagram of how to prepare microscope slides for wet mounts of C. elegans. The left panel shows the placement of label tape and the droplet of 2%-3% agarose (10 µL). Then, in the right panel, a second slide is placed on top at a 90° angle to flatten the droplet of agarose to the thickness of the tape. Once the agarose has solidified, the slides are carefully separated. The agarose pad will adhere to one of the slides. To prevent drying out, the nematodes are placed into a droplet of buffer containing sodium azide (which paralyzes the nematodes) within an hour of the production of the slide with pad. Please click here to view a larger version of this figure.

Figure 2: The fluorescence microscope with a digital camera used in this study. The microscope sits at a table that can accommodate small groups (three to four) of students. The microscope has a digital camera connected by a USB cable to a computer with appropriate digital image capture software. Please click here to view a larger version of this figure.
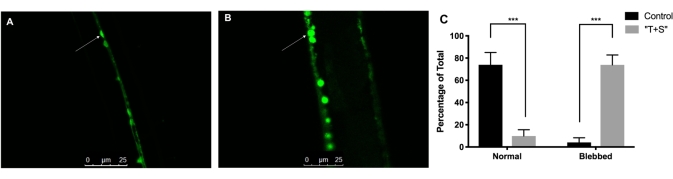
Figure 3: Evaluation of the effects of a neonicotinoid-containing pesticide on neuron morphology. (A) Untreated cholinergic neurons from the fluorescent strain LX929. (B) Pesticide-exposed (T+S) cholinergic neurons. (C) Percentage of neurons exhibiting blebbing in the two conditions. Error bars are the standard error of the mean. *** indicates p < 0.001 from a one-way ANOVA. A total of 113 neurons from 12 individual nematodes were analyzed. This figure has been modified from Bradford, et al.3. Please click here to view a larger version of this figure.
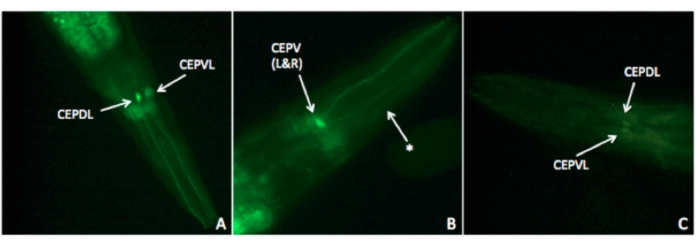
Figure 4: Effects of a manganese-containing pesticide on dopamine neuron morphology. The student group did not provide a scale bar for their figure but took the image at 40x magnification. (A) Putative CEPDL and CEPVL neuronal soma (labeled) in control worms. The intact head neuron somata and their processes indicate a lack of degeneration. (B) Putative CEPD L&R processes (asterisk) and CEPV L&R neuronal soma (labeled) in acute exposed worms. The soma and processes expressed GFP, but there appears to be a dampening of GFP expression in dorsal processes. (C) Putative CEPDL and CEPVL neuronal soma (labeled) in chronic exposed worms. The soma did not express GFP, and the processes were not visible, indicating significant degeneration. Please click here to view a larger version of this figure.
Discussion
The protocols described in this manuscript work successfully alone or as part of a multi-week independent student group project. The protocols are also amenable to stand-alone, one-week exploratory experiences. The nematode strains are cheap and easy to maintain in the research laboratory. Students can readily learn how to pick worms with a worm pick or how to move them by flushing plates with water and allowing them to settle by gravity. The experiments can be performed over the course of a single lab period or over multiple weeks. Students can independently design their experiments, or a procedure can be provided to them. Dissecting microscopes do not have to be expensive. The most expensive item is the fluorescent microscope with a digital camera. This is a limitation for teaching laboratories that do not have access to a fluorescent microscope or digital camera.
If a fluorescent microscope and digital camera and computer imaging system are not available for use in the laboratory, the students could measure specific simple behaviors that are governed by particular neurons. Many behavioral assays are available and can be found in Wormbook9. The behaviors can be assessed using the dissecting microscope.
A critical step in this protocol is preparing the microscope slides for the wet mounts of the nematodes. Another critical step is adding the nematodes to the droplet. A final critical step in the protocol is making sure not to expose the nematodes to the fluorescent illumination for so long that it bleaches the signal. It would be important for the instructor to point out these critical steps so that student groups practice and pay attention closely to the instructions they are being given.
The most exciting aspect of this methodology is that students can design and carry out novel and authentic experiments that could yield publishable results or become the basis for more advanced research, like a senior research project. The procedures can be used to study a variety of pesticides, mixtures of pesticides, and many other different environmental chemicals10, which provides students with a truly relevant research experience and training in experimental design, data analysis, figure preparation, and presentation of results.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The work described in this manuscript was done for an intermediate-level class in neuroscience. Funds for the reagents and supplies were provided by the Biology Department at Vassar College. The microscopes and digital imaging system were also provided by the Biology Department at Vassar College. The author thanks all the many students who took this course.
Materials
| Agar | Fisher Scientific | BP97445 | |
| Agarose | Fisher Scientific | MP1AGAH0250 | |
| Alcohol lamp | Fisher Scientific | 17012826 | |
| Bunsen burner | Fisher Scientific | 17-012-820 | |
| C. elegans strains | C. elegans Genetics Center | ||
| CaCl | Fisher Scientific | 10035-04-8 | |
| Cholesterol | Fisher Scientific | AAA1147030 | |
| Coverslips | Fisher Scientific | 12-545-AP | |
| Digital camera | Nikon | These can vary depending on the requirement | |
| Dissecting scope | Nikon | SMZ745 | |
| E. coli strain (OP50) | C. elegans Genetics Center | ||
| Ethanol | Fisher Scientific | BP2818100 | |
| Fluorescent scope | Nikon | These can vary depending on the requirement | |
| Imaging software | Nikon | These can vary depending on the requirement | |
| Inoculation loop | Fisher Scientific | 131045 | |
| LB Broth Base | Fisher Scientific | BP9723-500 | |
| MgSO4 | Fisher Scientific | 10034-99-8 | |
| Microfuge tubes | Fisher Scientific | 05408129 | |
| Microscope slides | Fisher Scientific | 22-265446 | |
| Pasteur pipets | Fisher Scientific | 13-678-20A | |
| Petri dishes | Fisher Scientific | AS4050 | |
| Pipette tips | Fisher Scientific | 94060316 | |
| Pipetters | Fisher Scientific | 14-386-319 | |
| Platinum wire | Genesee Scientific | 59-1M30P | |
| Potassium Phosphate buffer | Fisher Scientific | AAJ61413AP | |
| Sodium azide | Fisher Scientific | AC447810250 |
Riferimenti
- Duzguner, V., Erdogan, S. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats. Pesticide Biochemistry and Physiology. 104 (1), 58-64 (2012).
- Catae, A. F., et al. Exposure to a sublethal concentration of imidacloprid and the side effects on target and nontarget organs of Apis mellifera (Hymenoptera, Apidae). Ecotoxicology. 27 (2), 109-121 (2018).
- Bradford, B. R., Whidden, E., Gervasio, E. D., Checchi, P. M., Raley-Susman, K. M. Neonicotinoid-containing insecticide disruption of growth, locomotion, and fertility in Caenorhabditis elegans. PLOS One. 15 (9), 0238637 (2020).
- Jospin, M., et al. A neuronal acetylcholine receptor regulates the balance of muscle excitation and inhibition in Caenorhabditis elegans. PLoS Biology. 7 (12), 1000265 (2009).
- Susman, K. . Discovery-Based Learning in the Life Sciences. , (2015).
- Stiernagle, T. Maintenance of C. elegans. WormBook. , (2006).
- Brody, H. A., Chou, E., Gray, J. M., Pokyrwka, N. J., Raley-Susman, K. M. Mancozeb-induced behavioral deficits precede structural neural degeneration. NeuroToxicology. 34, 74-81 (2013).
- Chen, P., Martinez-Finley, E. J., Bornhorst, J., Chakraborty, S., Aschner, M. Metal-induced neurodegeneration in C. elegans. Frontiers in Aging Neuroscience. 5, (2013).
- Hart, A. Behavior. WormBook. , (2006).
- Harlow, P. H., et al. The nematode Caenorhabditis elegans as a tool to predict chemical activity on mammalian development and identify mechanisms influencing toxicological outcome. Scientific Reports. 6 (1), 22965 (2016).

