Xenograft Skin Model to Manipulate Human Immune Responses In Vivo
Summary
The present protocol describes how to graft human skin onto non-obese diabetic (NOD)-scid interleukin-2 gamma chain receptor (NSG) mice. A detailed description of the preparation of human skin for transplant, preparation of mice for transplant, transplantation of split-thickness human skin, and post-transplantation recovery procedure are included in the report.
Abstract
The human skin xenograft model, in which human donor skin is transplanted onto an immunodeficient mouse host, is an important option for translational research in skin immunology. Murine and human skin differ substantially in anatomy and immune cell composition. Therefore, traditional mouse models have limitations for dermatological research and drug discovery. However, successful xenotransplants are technically challenging and require optimal specimen and mouse graft site preparation for graft and host survival. The present protocol provides an optimized technique for transplanting human skin onto mice and discusses necessary considerations for downstream experimental aims. This report describes the appropriate preparation of a human donor skin sample, assembly of a surgical setup, mouse and surgical site preparation, skin transplantation, and post-surgical monitoring. Adherence to these methods allows for maintenance of xenografts for over 6 weeks post-surgery. The techniques outlined below allow maximum grafting efficiency due to the development of engineering controls, sterile technique, and pre- and post-surgical conditioning. Appropriate performance of the xenograft model results in long-lived human skin graft samples for experimental characterization of human skin and preclinical testing of compounds in vivo.
Introduction
Mouse models are frequently used to make inferences about human biology and disease, partly due to their experimental reproducibility and capacity for genetic manipulation. However, mouse physiology does not completely recapitulate human organ systems, particularly skin, and therefore has limitations for use as a preclinical model in drug development1. Anatomical differences between mouse and human skin include differences in epithelial thicknesses and architecture, lack of murine eccrine sweat glands, and variations in hair cycling2. Furthermore, both the innate and adaptive arms of the immune system are divergent between the two species3. Mouse skin contains a unique immune population of dendritic epidermal T cells (DETCs), has a higher abundance of dermal γδ T cells, and varies in immune cell subset localization in comparison to human tissue4. Therefore, experimental findings regarding human skin biology and inflammation benefit from validation with human tissue. While in vitro and organoid culture systems are widely utilized tools to study human tissue, these systems are limited by absent or incomplete immune reconstitution and a lack of connection to peripheral vasculature5. The humanized xenograft skin transplant model aims to allow for therapeutic or biological manipulation of immune and non-immune pathways in human tissues in vivo.
The human skin xenograft model has been utilized to study skin physiology and pharmacology, analyze immune rejection and responses, dissect human skin cancer mechanisms, and understand skin diseases and wound healing6. While applicable to multiple fields of skin research, the xenograft model has lower throughput than in vitro studies and lacks the ease of genetic manipulation employed in mouse models. Time points within this model may range from weeks to months, and successful grafting requires appropriate facilities and equipment to perform these surgeries. However, the xenograft model supplies biological and physiological context to experiments, while organoid culture systems, such as tissue explants, often require replicating a myriad of moving parts, such as exogenous signals, at specific time intervals7. Therefore, this model is best utilized to further validate findings observed in vitro and within mouse models, or for work that is not otherwise biologically feasible. Appropriate use of the xenograft model provides a unique opportunity to study and manipulate intact human tissue in vivo.
Optimization of the xenograft skin transplant model has relied on decades of research to preserve graft integrity over time. Critical to this process is utilizing the non-obese diabetic (NOD)-scid interleukin-2 gamma chain receptor (NSG) mouse, which lacks B and T adaptive immune cells, functional NK cells, and has deficiencies in macrophage and dendritic cells8. The immunodeficient nature of these NSG hosts allows for the transplant of human hematopoietic cells, patient-derived cancers, and skin8,9,10. Despite this immunosuppressive host environment, additional suppression of mouse neutrophilic immune responses by anti-GR1 administration is necessary for graft success10. The major roadblocks in transplanting intact tissue are infection, rejection, and difficulty in re-establishing blood flow to the graft, sometimes leading to loss of dermal and epidermal integrity11. Techniques including administration of anti-FR1 and use of appropriate graft depth improve graft survival10. Meticulous optimization makes it possible to perform human xenograft skin transplants on NSG mice with high efficiency and survival rates, ranging from 90%-100%.
Protocol
The present study was approved and performed in compliance with UCSF IACUC (AN191105-01H) and IRB (13-11307) protocols. Skin samples, discarded as part of routine elective surgical procedures, such as hernia repair, were used for the present research. The skin samples are either de-identified and certified as Not Human Subjects Research or, if clinically identifying information is required for downstream analyses, patients provided written consent under IRB protocol 13-11307. No other inclusion or exclusion criteria were utilized. NSG mice of either sex, 8-10 weeks of age, were employed in the study. The mice were obtained from commercial sources (see Table of Materials).
1. Processing of donor human skin sample
NOTE: The human skin sample used in this transplant was a large sample collected from the abdomen of a healthy patient. The sample must be at least 15 cm x 7.5 cm. Size limitations may affect the number of mice for which skin is available and the choice of graft size.
- Maintain the skin sample at cold temperatures (on ice; 4°C) before preparation and grafting. Keep the sample moist in a closed specimen collection cup with the gauze soaked in phosphate-buffered saline (PBS).
NOTE: Storing the skin sample at 4 °C for longer than 2 days is not recommended. However, reports exist where the skin samples are stored for a longer time12.
CAUTION: Treat all human tissue with standard biohazard precautions. - Prepare to dermatome the human skin sample in a sterilized negative pressure tissue culture hood on a sterilized dissection board.
- Place the skin sample, epidermis side up, on the dissection board. Wipe the epidermis with a sterile alcohol prep pad and then with PBS.
- Pin the closer edge of the skin in place with a 1.5 in dissecting T-pin (see Table of Materials).
- Dermatome the skin specimen at a 400 µm thickness, applying steady pressure while cutting forward at a 30°-45° angle. Follow all instrument-specific instructions and safety measures (see Table of Materials).
NOTE: For details on the dermatome technique, please see a previously published report13. - Prepare a 100 mm x 20 mm Petri dish by placing a sterile gauze soaked in sterile PBS at the bottom of the dish. Place the skin, epidermis side up, onto the wet gauze.
- Seal and cover the plate edges with a semitransparent sealing film (see Table of Materials) to ensure the sample is not contaminated. Store the sample at 4° C prior to grafting.
2. Pre-surgery conditioning and preparation
- Prepare the sterile instruments and a sterile surgical station for grafting. Use autoclaved paper towels as sterile surfaces for instrument and mouse placement.
NOTE: Mice may be grafted after weaning but are preferably grafted between 8-10 weeks of age. Mice of either sex may be grafted. - Perform the surgical preparation, such as hair removal, in an area physically separated from the surgical station.
- Prepare the anti-GR1 (see Table of Materials) by diluting it to 1 mg/mL in sterile saline. Dose each mouse with 100 µg/100 µL of the anti-GR1 solution intraperitoneally following anesthesia induction.
- Anesthetize the mice, one at a time, with isoflurane or other institutionally approved anesthetics.
NOTE: Isoflurane needs to be given at a 3%-5% concentration during induction. Once the mouse is immobile, lower the isoflurane concentration to 1%-3% to effect for the duration of the surgery.- Monitor the mouse for appropriate depth of anesthesia by observing respiratory rate, absence of toe-pinch response, and appropriate pink coloration of ears and mouth.
CAUTION: Use appropriate anesthetic machinery and scavenging methods, and avoid exposure to isoflurane vapors.
- Monitor the mouse for appropriate depth of anesthesia by observing respiratory rate, absence of toe-pinch response, and appropriate pink coloration of ears and mouth.
- Transfer the mouse to a heating pad or another heat source (see Table of Materials).
- Administer the ophthalmic ointment by dabbing a small drop of ointment on the eye with a gloved finger.
- Administer analgesics Buprenorphine (0.08 mg/kg) and Carprofen (5 mg/kg) (see Table of Materials) subcutaneously by pinching the skin and injecting at an angle parallel to the body.
NOTE: Prepare the pre-treatment analgesia following institutional protocols. Follow institutional guidelines for the selection and administration of analgesics. The method of analgesia used in this study is outlined in step 2.7 and Supplementary Figure 1. - Administer the anti-GR1 (prepared in step 2.4) intraperitoneally by slightly lifting the mouse by the tail, exposing the abdomen, and injecting at a 30° angle using an insulin 1 mL (12.7 mm) syringe.
- Use animal-safe electric clippers (see Table of Materials) to shave the middle and upper portions of the dorsal side of the mouse.
- Clear all the hair and apply a generous amount of hair removal ointment onto the shaved skin for 30 s to 1 min.
- Completely wipe away hair removal ointment with a paper towel and PBS.
3. Transplantation procedure
- Transfer the mouse to a secondary surgical location, away from the hair removal station.
- Sterilize the surgical site with the iodine swab stick in a circular motion, starting in the middle and working out toward the edge of the depilated area.
- Place a piece of sterile plastic wrap over the mouse and cut a window in the plastic slightly larger than the size of the area to be grafted.
- Cut a rectangle-shaped 10 mm x 10 mm portion of donor skin to be grafted with a scalpel. Do this by firmly holding the donor skin in place with the backside of the forceps and cutting alongside the forceps with the scalpel.
- Using the surgical scissors, snip a rectangular area of mouse skin matching the size of the donor skin piece, creating a graft bed. Use forceps to pull the skin away from the body, and cut the skin with the scissors angled away from the body to avoid cutting deeply into the facia.
- Place the donor skin piece, epidermis side up, onto the prepared graft bed.
- Using the back of the forceps, manipulate the skin, sliding back and forth until the donor skin lies completely flat against the graft bed.
- Add drops of surgical glue tissue adhesive (see Table of Materials) where the donor skin meets the mouse skin and hold the mouse and donor skin together with forceps for 1-2 s so that the glue adheres to the tissues. Completely seal the edge of the graft and allow for the glue to dry fully.
- Bandage the mice (Figure 1) following the steps below.
- Cut a piece of petrolatum gauze (see Table of Materials) large enough to cover the graft area completely.
- Cover the graft with the petrolatum gauze, and lightly press the gauze against the skin using forceps.
- Cut a strip of a transparent film dressing lengthwise so that the width is large enough to cover the mouse's wound.
- Firmly press the transparent film dressing, adhesive side down, over the gauze. Quickly roll the mouse to wrap the dressing completely around the torso, ensuring it fits tightly without impeding respiration and all limbs are free for movement.
- Place the mouse in a recovery cage and monitor it until it is alert and moving around. Provide a heat source on part of the cage for at least 15 min following recovery.
NOTE: The animals are expected to recover within 1-5 min after placing them in the recovery cage.
- Singly house the mice following grafting.
- Administer post-surgical analgesia as required by institutional protocols.
NOTE: Buprenorphine (0.08 mg/kg) was administered subcutaneously 4-6 h later during the present study.
4. Post-surgical procedures
- Inject 100 µL (100 µg) of anti-GR1 intra-peritoneally at 4 days, 7 days, and 11 days post-grafting to prevent graft rejection (Supplementary Figure 1).
- Replace bandages as necessary and if removed by mice.
- Rebandage all the mice on day 7.
- Remove the bandages on day 14.
- Monitor the mice for signs of graft rejection and systemic inflammation (weight loss, hair loss, extreme lethargy).
- Harvest the mice between 3 and 6 weeks post-graft.
- Euthanize the mice via regulator-controlled carbon dioxide (CO2) administration followed by cervical dislocation.
NOTE: Follow institutional guidelines for euthanasia. - Dissect the skin grafts from the mice14. Place a portion of the graft in 10% formalin for 24 h before paraffin embedding and sectioning for histology staining9.
- Mince the skin grafts with scissors and digest enzymatically with 250 IU/mL of Collagenase IV and 0.02 mg/mL of DNase in cell culture media overnight. Stain the cells for surface and intracellular markers following the previously described procedure14.
- Euthanize the mice via regulator-controlled carbon dioxide (CO2) administration followed by cervical dislocation.
Representative Results
Human skin xenografts were performed on NSG mice inside a super-barrier animal facility. Success was defined by the prolonged graft and mouse survival and behavioral health of mice post-transplant. Poor survival during the week following surgery was initially observed as the biggest barrier to experimental success, with up to 50% of mice requiring euthanasia. Improving sterile technique and better support of mouse body temperatures during and immediately after surgery increased surgical survival consistently to over 80% and often to 90%-100% survival. While the addition of antibiotics in the mouse drinking water was trialed, it was not determined to improve outcomes and was discontinued as a potential confounder of results. Successfully grafted mice should appear active and healthy in the days following the transplant. Mice were moving around their cage, exploring, building nests, and eating and drinking as usual. An unwell mouse may seem lazy, non-reactive, and may not be eating or drinking enough to maintain its weight. Supplementation of lethargic mice with soft-feed and oral hydration options may assist post-surgical recovery.
A xenograft that is healing correctly will first scab and then recover within about 50 days of surgery. Grafts may contract over time but remain adherent around the edges where the donor skin meets the mouse's skin (Figure 2). While graft scabbing should subside, grafts will remain thicker and more inflamed than healthy human skin (Figure 2). Excessive contraction of grafts can occur, reducing available tissue for endpoint analysis. Utilization of consistently sized grafts and appropriate graft placement is expected to mitigate such issues. All grafts throughout five independent experiments survived to the time of harvest, up to 50 days post-transplant.
Tissue analysis may include immunohistochemistry and single-cell analyses following enzymatic digestion. A portion of skin was processed by overnight digestion in 250 IU/mL of Collagenase Type IV and 0.02 mg/mL of DNase in cell culture media (see Table of Materials), and stained for surface and intracellular markers as previously described14. Analysis by flow cytometry revealed the sustained presence of human immune cells within most grafts but counts varied between xenografts (Figure 3A). Figure 3B shows representative results from a successful graft (left) and one that has failed to maintain human immune cells (right). Analysis of hematoxylin and eosin-stained (H&E) sections taken from the center of the graft revealed intact, non-devitalized skin composed of human dermis and epidermis for both 35 and 50 day time points (Figure 4).
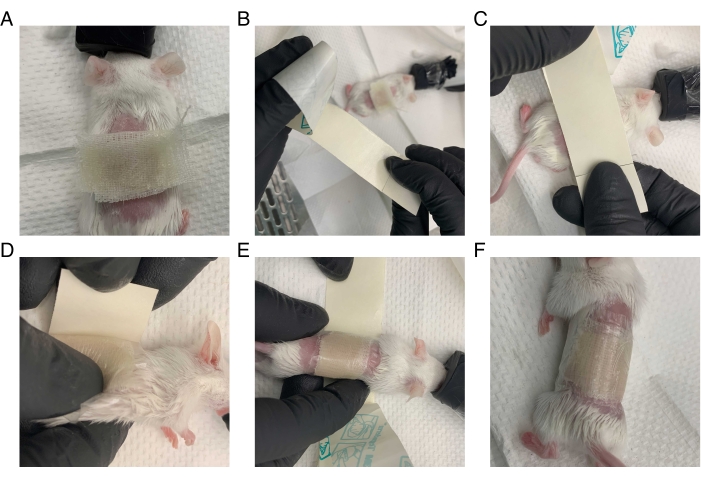
Figure 1: Method for bandaging mouse after surgery. The mouse is wrapped tightly in petrolatum gauze and transparent film dressing while under anesthesia. (A) Petrolatum gauze is placed over an area slightly larger than the surgical wound. (B) The transparent film dressing is prepared: a long strip is cut slightly wider than the petrolatum gauze. The backing covering the adhesive side of the transparent film dressing is partially removed. (C) The adhesive side of the film is firmly pressed against the back of the mouse, leaving an inch of space on the front end of the film. (D) The mouse is turned on its back. The overhanging front portion of the film is pressed onto the mouse, and the backing is removed. (E) The mouse is tightly wrapped in the film, and the remaining support is removed as the mouse is wrapped. (F) The mouse is successfully wrapped in the dressing, and its arms and legs are unconstrained. Please click here to view a larger version of this figure.
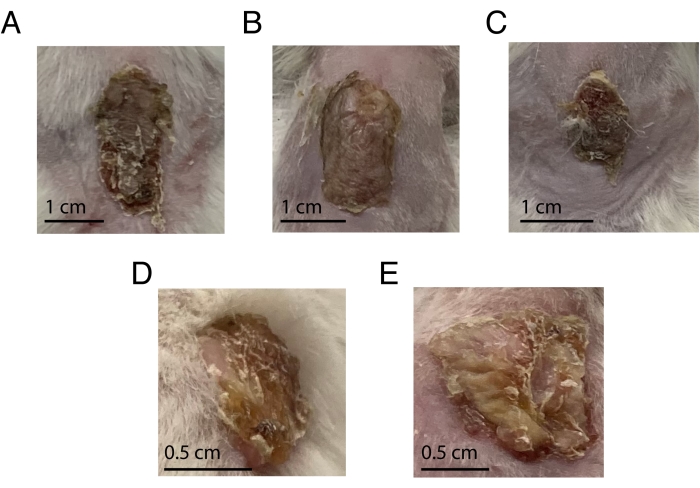
Figure 2: Representative images from transplanted animals from Day 10 to 21 post-transplant. (A–C) Three different mice with optimal transplants at day 10. (D–E) Two different mice with transplants on day 21. Please click here to view a larger version of this figure.
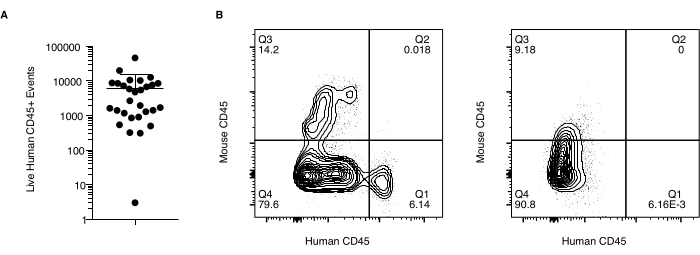
Figure 3: Immune cell chimerism in grafted skin. Flow cytometry data of human immune cell recovery from xenografts. Data is gated on the events of singlet, live, human CD45+ mouse CD45-. (A) Number of cells (Live Human CD45+ Events) recovered from two independent experiments. (B) Representative plots of human and mouse CD45+ immune cell staining in a successful graft (left) compared to graft with unsuccessful maintenance of the human immune compartment (right). Please click here to view a larger version of this figure.
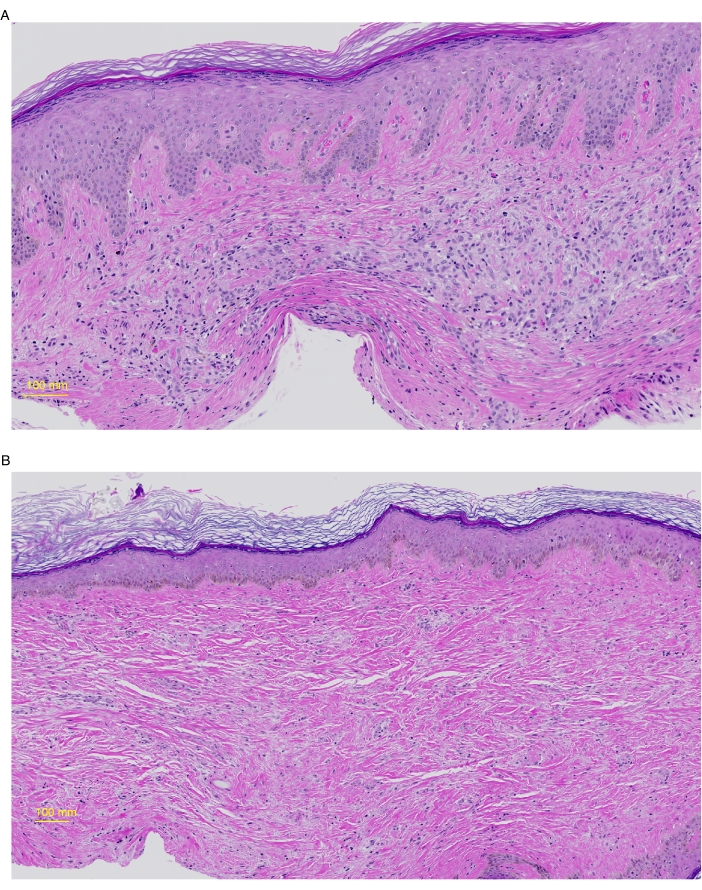
Figure 4: Histology of grafted human skin at day 35 and 50. Grafted skin was harvested from mice on day 35 (A) or 50 (B), preserved in 10% formalin, and paraffin-embedded and stained for Hematoxylin and Eosin (H&E). (A) The epidermis shows moderate hyperplasia (acanthosis), slight hyper-granulosis, and compact orthokeratosis. In the papillary dermis, there are occasional dilated and congested capillaries. Melanocytes and melanin pigment are present in the basal layer of the epidermis. The dermis is moderately cellular and is composed of plump oval fibroblasts with pale syncytial cytoplasm and scattered lymphocytes. (B) The epidermis comprises stratified squamous epithelium, basket-weave orthokeratosis in the cornified layer, and is of normal thickness. Melanocytes and melanin pigment are present in the basal layer of the epidermis. The dermis shows oval fibroblasts and slightly enhanced extracellular matrix deposition. Please click here to view a larger version of this figure.
Supplementary Figure 1: Timeline of the protocol adopted for the xenograft study. Please click here to download this File.
Discussion
The mouse xenograft skin transplant model is a key technique to mechanistically dissect human skin immune responses in an in vivo setting14. Successful skin xenograft transplants rely upon appropriate preparation of mice and skin specimens and mice and adherence to aseptic rodent surgery methods15. Rapid cooling and proper storage of skin samples at cold temperatures in media (such as sterile saline) are important to ensure continued tissue health prior to transplant12. Collection of split-thickness samples, using an instrument such as a dermatome, allows consistency in sample depth between mice and increases the provision of nutrients from the host to improve graft survival10. Loss of graft integrity with epidermal sloughing may be observed, particularly in full-thickness grafts with disrupted blood supply16. Mouse survival through and after surgery is aided by sterile technique, provision of heat during and after surgery, and techniques that minimize surgical time, such as utilizing surgical glue15. Administration of anti-GR1 following transplant allows for suppression of the remaining mouse immune responses in the immunodeficient host to promote graft survival10. These methods increase the likelihood of mouse and graft survival during the xenograft transplant model and improve experimental numbers and reproducibility from one experiment to the next.
Although extremely useful, there are some key limitations to the xenograft skin transplant model, as demonstrated. First, the graft exists in an inflammatory environment, most likely secondary to transient ischemia and infiltration of human grafted tissue with murine myeloid cells. Thus, it does not reflect human skin at a steady state and may not fully reflect all human inflammatory skin diseases10,14. Studying human inflammatory disorders by grafting clinical biopsies from lesional skin is an alternative strategy, but limitations in the numbers of biopsies available and inconsistent control of grafting depth make this option less favorable. Additionally, xenograft transplants do not recapitulate the human peripheral immune architecture. Reconstituting the mouse periphery with human cells may also bear caveats, as the NSG mouse lymphoid structure is atrophic prior to human cell engraftment; this may lead to preferential cell selection in some settings10,17. Additionally, skin engraftment and immune cell recovery from skin specimens may vary from mouse to mouse, even in the absence of treatment. At least 10 replicates per condition are recommended for this model to observe consistent differences between groups. The appropriate duration of the assay may need to be adjusted for the experimental question, as graft healing and re-vascularization occur over the initial weeks following transplant, and the effect of interventions may depend upon the stage of engraftment.
Despite these drawbacks, the human xenograft skin transplant model remains one of the few assays to study human skin immune responses in the intact organ in vivo. While transgenic mouse models may allow for mechanistic pathway dissection, differences in anatomy, immune cell composition, and dominant immune pathways limit translation to human disease1. Ex vivo culture of human-derived cells, skin organoid models, and human tissue explant studies may be alternatively used but are also subject to limitations, including disruption of immune cell networks and egress of immune cells from the intact tissue18,19. Therefore, the xenograft skin transplant models remain a key method for studying therapeutic candidates and human skin responses to inflammation in a preclinical in vivo setting.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was funded in part by sponsored research agreements from TRex Bio and grants from the NIH (1R01AR075864-01A1). JMM is supported by the Cancer Research Society (grant 26005). We acknowledge the Parnassus Flow Cytometry Core supported in part by grants NIH P30 DK063720, S10 1S10OD021822-01, and S10 1S10OD018040-01.
Materials
| 10% Neutral Buffered Formalin | Fisher | SF100-20 | Fixative for histology |
| 3M Vetbond Tissue Adhesive | 3M | 1469SB | surgical glue |
| Alexa 700 CD45 monoclonal antibody (Clone 30F11) | Thermo Fischer | 56-0451-82 | Flow cytometry analysis: Surface protein staining |
| Anti-GR1 clone RB6-8C5 | BioXcell | BE0075 | Anti-rejection |
| APC mouse anti-human CD25 (Clone 2A3) | BD Biosciences | 340939 | Flow cytometry analysis: Surface protein staining |
| APC-eFluor 780 anti-human HLA-DR (Clone LN3) | eBioscience | 47-9956-42 | Flow cytometry analysis: Surface protein staining |
| Autoclave pouches | VWR | 89140-800 | For autoclaving tools and paper towels |
| Brilliant Violet 60 anti-human CD4 antibody (Clone OKT4 | Biolegend | 317438 | Flow cytometry analysis: Surface protein staining |
| Brilliant Violet 65 anti-human CD8a antibody (Clone RPA-T8) | Biolegend | 301042 | Flow cytometry analysis: Surface protein staining |
| Brilliant Violet 711 anti-human CD3 antibody (Clone OKT3) | Biolegend | 317328 | Flow cytometry analysis: Surface protein staining |
| Buprenex 0.3 mg/mL | Covetrus | 059122 | Analgesia |
| Carprofen 50 mg/mL | Zoetis | NADA # 141-199 | Analgesia |
| Collagenase Type IV | Worthington | 4188 | Skin digestion |
| D42 Dermatome blade | Humeca | 5.D42BL10 | dermatome (1 blade per sample) |
| Dermatome D42 | Humeca | 4.D42 | dermatome |
| Disposable Scalpel | Bard-Parker | 371610 | skin preparation |
| Dissecting T-Pins; 1-1/2 inch, 1000/CS 1.5 | Cole-Parmer | UX-10915-03 | To pin skin specimen for dermatome |
| Dissection scissors | medicon | 02.04.10 | sample preparation and mouse dissection |
| DNAse | Sigma-Aldrich | DN25-1G | Skin digestion |
| eBioscience Foxp3 / Transcription Factor Fixation/Permeabilization Concentrate and Diluent | eBioscience | 00-5521-00 | Flow cytometry analysis: Cell Fixation and Permeabilization |
| eFluor-450 FOXP3 monoclonal antibody (Clone PCH101) | eBioscience | 48-4776-42 | Flow cytometry analysis: Intracellular protein staining |
| Electric clippers | Kent | CL8787-KIT | hair removal |
| Epredia Shandon Instant Eosin | Fisher Scientific | 6765040 | H&E |
| Epredia Shandon Instant Hematoxylin | Fisher Scientific | 6765015 | H&E |
| FITC anti-human CD45 (Clone HI30) | Tonbo Biosciences | 35-0459-T100 | Flow cytometry analysis: Surface protein staining |
| Forceps | medicon | 07.60.07 | sample preparation and mouse dissection |
| Gauze | Fisherbrand | 22-362-178 | Sample preparation |
| Heating lamp | Morganville Scientific | HL0100 | Post-surgical care |
| Heating pads 4" x 10" | Pristech | 20415 | Surgical heat supply |
| Insulin 1cc 12.7 mm syringes | BD | 329410 | drug administration |
| Isoflurane | United States Pharmacopeia (USP) | NDC 66794-013-25 | Anesthesia |
| Isoflurane machine | VetEquip | 911103 | Anesthesia |
| Nair for Men | Nair | 10022600588556 | hair removal |
| Neomycin and Polymyxin Bisulfates and Bacitracin Zinc Ophthalmic ointment | Dechra | NDC 17478-235-35 | eye ointment to prevent drying |
| NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice | The Jackson Laboratory | 005557 | Mice |
| Paper towels | Kleenex | 100848 | May be autoclaved for sterile surfaces |
| Parafilm | Fisher Scientific | 13-374-12 | Semitransparent sealing film |
| PE mouse anti-human CD127 (Clone HIL-7R-M21) | BD Biosciences | 557938 | Flow cytometry analysis: Surface protein staining |
| PE-Cy-7 mouse anti-Ki-67 (Clone B56) | BD Biosciences | 561283 | Flow cytometry analysis: Intracellular protein staining |
| PerCP-eFluor-710 CD152 (CTLA-4) monoclonal antibody (Clone 14D3) | eBioscience | 46-1529-42 | Flow cytometry analysis: Intracellular protein staining |
| Permeabilization Buffer 10x | eBioscience | 00-8333-56 | Flow cytometry analysis: Intracellular protein staining buffer |
| Petri Dish 150 mm | Corning | 430597 | Sample storage |
| Plastic Wrap | Fisherbrand | 22-305-654 | Site preparation |
| Providone-Iodine Swab stick | PDI | S41350 | Site sterilization |
| Soft-Feed and Oral Hydration (Napa Nectar) | Se Lab Group Inc | NC9066511 | For supplementing poorly recovering mice post-surgery |
| Specimen Collection Cups | Fisher Scientific | 22-150-266 | sample storage |
| Sterile alcohol prep pad | Fisherbrand | 22-363-750 | skin preparation |
| Sterile PBS | Gibco | 14190-144 | Media for sample storage |
| Sterile saline | Hospira | NDC 0409-4888-02 | For drug dilution |
| Tegaderm Film 4” x 43/4” | 3M | 1626 | transparent film wound dressing |
| Vaseline Petrolatum Gauze 3” x 8” | Kendall | 414600 | wound dressing |
| Violet 510 Ghost Dye | Tonbo Biosciences | 13-0870-T100 | Flow cytometry analysis: Viability dye |
Riferimenti
- Zomer, H. D., Trentin, A. G. Skin wound healing in humans and mice: Challenges in translational research. Journal of Dermatological Science. 90 (1), 3-12 (2018).
- Wong, V. W., Sorkin, M., Glotzbach, J. P., Longaker, M. T., Gurtner, G. C. Surgical approaches to create murine models of human wound healing. Journal of Biomedicine & Biotechnology. 2011, 969618 (2011).
- Mestas, J., Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. The Journal of Immunology. 172 (5), 2731-2738 (2004).
- Pasparakis, M., Haase, I., Nestle, F. O. Mechanisms regulating skin immunity and inflammation. Nature Reviews Immunology. 14 (5), 289-301 (2014).
- Sun, H., Zhang, Y. -. X., Li, Y. -. M. Generation of skin organoids: potential opportunities and challenges. Frontiers in Cell and Developmental Biology. 9, 3176 (2021).
- Cristóbal, L., et al. Mouse models for human skin transplantation: a systematic review. Cells Tissues Organs. 210 (4), 250-259 (2021).
- Rossi, G., Manfrin, A., Lutolf, M. P. Progress and potential in organoid research. Nature Reviews Genetics. 19 (11), 671-687 (2018).
- Ito, M., et al. NOD/SCID/γcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 100 (9), 3175-3182 (2002).
- Meraz, I. M., et al. An improved patient-derived xenograft humanized mouse model for evaluation of lung cancer immune responses. Cancer Immunology Research. 7 (8), 1267-1279 (2019).
- Racki, W. J., et al. NOD-scid IL2rgamma(null) mouse model of human skin transplantation and allograft rejection. Transplantation. 89 (5), 527-536 (2010).
- Meehan, G. R., et al. Developing a xenograft model of human vasculature in the mouse ear pinna. Scientific Reports. 10 (1), 2058 (2020).
- Gokkaya, A., et al. Skin graft storage in platelet rich plasma (PRP). Dermatologic Therapy. 33 (1), 13178 (2020).
- . The Humeca D42 and D80 battery operated cordless dermatomes Available from: https://www.youtube.com/watch?v=YCRowX-TdA (2021)
- Rodriguez, R. S., et al. Memory regulatory T cells reside in human skin. The Journal of Clinical Investigation. 124 (3), 1027-1036 (2014).
- Hoogstraten-Miller, S. L., Brown, P. A. Techniques in rodent aseptic surgery. Current Protocols in Immunology. 82 (1), 12-14 (2008).
- Karim, A. S., et al. Evolution of ischemia and neovascularization in a murine model of full thickness human wound healing. Wound Repair and Regeneration: Official Publication of the Wound Healing Society [and] the European Tissue Repair Society. 28 (6), 812-822 (2020).
- Ali, N., et al. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PloS One. 7 (8), 44219 (2012).
- Souci, L., Denesvre, C. 3D skin models in domestic animals. Veterinary Research. 52 (1), 21 (2021).
- Holtkamp, S. J., et al. Circadian clocks guide dendritic cells into skin lymphatics. Nature Immunology. 22 (11), 1375-1381 (2021).

