Recording Electrical Activity from Identified Neurons in the Intact Brain of Transgenic Fish
Summary
In this video, we will demonstrate how to record electrical activity from identified single neurons in a whole brain preparation, which preserves complex neural circuits. We use transgenic fish in which gonadotropin-releasing hormone (GnRH) neurons are genetically tagged with a fluorescent protein for identification in the intact brain preparation.
Abstract
Understanding the cell physiology of neural circuits that regulate complex behaviors is greatly enhanced by using model systems in which this work can be performed in an intact brain preparation where the neural circuitry of the CNS remains intact. We use transgenic fish in which gonadotropin-releasing hormone (GnRH) neurons are genetically tagged with green fluorescent protein for identification in the intact brain. Fish have multiple populations of GnRH neurons, and their functions are dependent on their location in the brain and the GnRH gene that they express1 . We have focused our demonstration on GnRH3 neurons located in the terminal nerves (TN) associated with the olfactory bulbs using the intact brain of transgenic medaka fish (Figure 1B and C). Studies suggest that medaka TN-GnRH3 neurons are neuromodulatory, acting as a transmitter of information from the external environment to the central nervous system; they do not play a direct role in regulating pituitary-gonadal functions, as do the well-known hypothalamic GnRH1 neurons2, 3 .The tonic pattern of spontaneous action potential firing of TN-GnRH3 neurons is an intrinsic property4-6, the frequency of which is modulated by visual cues from conspecifics2 and the neuropeptide kisspeptin 15. In this video, we use a stable line of transgenic medaka in which TN-GnRH3 neurons express a transgene containing the promoter region of Gnrh3 linked to enhanced green fluorescent protein7 to show you how to identify neurons and monitor their electrical activity in the whole brain preparation6.
Protocol
1. Dissection of Brains from Adult Medaka
- Anesthetize adult male or female (Figure 1A) by immersing in 5 ml MS-222 (150 mg/L, pH 7.4); wait a couple of minutes after gills movements have ceased before decapitating. All procedures were approved by the Institutional Animal Care and Use Committee of University of California-Los Angeles.
- Decapitate the fish in fish saline at the caudal end of the operculum with scissors in a 60-mm diameter Petri dish.
- Transfer fish head to a 35-mm diameter Petri dish, half filled with fish saline and lined with Sylgard that has a depression in it to position and secure the head for brain dissection. Position and secure the head dorsal-side up with insect pins.
- Carefully cut the dorsal part of the skull around its perimeter with fine scissors and gently remove it with forceps, exposing the dorsal part of brain completely including the anterior part of the spinal cord.
- Lift the spinal cord gently with fine-tip forceps and sever the bilateral connective nerves with fine-tipped scissors: cranial nerves, spinal nerves, and optic nerves on the ventral side of the brain, and the olfactory nerves at the anterior part of the brain.
- Place the fully dissected brain in a fresh Petri dish filled with fish saline, and check carefully under the microscope to ensure the whole brain is intact (including the pituitary gland) without any cuts or punctures. Damaged brains are not used for experimentation.
2. Mounting the Brain in a Recording Chamber
- Place a small drop of cyanoacrylate or some other fast-acting glue with a needle into the center of the recording chamber and spread the glue about 1 cm2.
- Quickly transfer the brain and glue it down in a ventral-side up position.
- Add 1 ml of fish saline so that it fills the recording chamber and covers the brain. Continuously perfuse the brain with aerated fish saline at a rate of at least 200 μl/min.
- Using the fine-tip forceps carefully remove the meninges over the surface of the recording area of the brain.
3. Electrophysiology
- Loose patch recording of action potential firing
- Glass microelectrodes (6-10 MΩ) are constructed from borosilicate glass pipettes (1B150F-4, World Precision Instruments) using an electrode puller (e.g. Sutter P-87), and back filled with the loose-patch recording solution half-way up the electrode.
- Using an upright fluorescent microscope with a cooled charge-coupled device (CCD) camera, find the GFP-expressing TN-GnRH3 neurons through 10x (Figure 1B), then 40x water immersion objectives (Figure 1C). They are located at or near the ventral surface of the olfactory bulbs, just anterior to the telencephalon.
- Switch to a video monitor that is providing real-time images from the microscope to locate the TN-GnRH3 neuron cluster (Figure 1C).
- Position the microelectrode into the bath so that the tip of the electrode is above the neuron target. Apply constant slight positive pressure to the microelectrode so that it doesn’t become clogged, and gently approach the target neuron with the tip of the electrode.
- Check and monitor the electrode resistance in voltage-clamp mode using AxoGraph software and amplifier Axoclamp 200B with Axograph software (Axon Instruments). When you can see from the video monitor that the tip of the electrode is on the surface of the neuron and from the AxoGraph display that the resistance changes slightly, release the positive pressure and apply slight negative pressure if necessary to make a low resistance seal on the neuron (<100 MΩ).
- Switch to current-clamp mode, and record the membrane voltage continuously without any current input, adjusting the scale of voltage and rate of recording if necessary. Data is collected and analyzed by Powerlab software (ADInstruments Inc.).
- Record baseline electrical activity in normal fish saline for about 5 min before any treatment (Figure 2A). Bath perfusion of drugs, hormones, peptides, etc. added to fish saline continues at the rate of 200 μl/min, followed by a washout period in normal fish saline 5, 6, 11.
- Whole cell recording of membrane potential
The whole cell patch clamp electrophysiology procedure is the same as that of the extracellular loose-patch recording procedure described above, from steps 3.1.1 – 3.1.4. Then the procedures deviate accordingly:- Check and monitor the electrode resistance in voltage-clamp mode. When you can see from the video monitor that the tip of the electrode is on the surface of the neuron and from the oscilloscope that the resistance changes slightly, release the positive pressure and apply a little negative pressure to make a high resistance seal on the neuron (~ 3 Giga Ω). Apply gentle suction by mouth to rupture the patched membrane; you will see a sudden drop of the resistance to about 120-250 MΩ.
- Switch to current-clamp mode, and record the membrane voltage continuously without any current input, adjusting the scale of voltage and rate of recording if necessary.
- Record baseline electrical activity in normal fish saline for about 5 min before any treatment, as described above in 3.1.7 (Figure 2B).
Representative Results
An example of bilateral clusters of GFP-labeled TN-GnRH3 neurons from the excised brain of medaka fish are shown in Figures 1B and 1C. Each cluster contains about 8-10 GnRH neurons. The spontaneous neuronal activities of the target TN-GnRH3 were recorded in current-clamp mode (I=0) with typical firing rates of 0.5-6 Hz. The pattern of action potential firing is typically a tonic or beating pattern, with a fairly regular interspike interval. Sample traces are shown in Figure 2 (2A: loose-patch; 2B: whole-cell).
This experimental approach is also successful in recording from GFP-labeled GnRH neurons located in the forebrain of adult zebrafish (Figures 3A and 3B). Neuron electrical activity in the excised, intact brain was recorded in the similar way as described above in medaka by loose-patch (Figure 3C) and whole-cell recording (Figure 3D). Unlike with medaka TN-GnRH neurons, the pattern of spontaneous action potential firing of zebrafish GnRH neurons in the preoptic area, ventral telencephalon, and hypothalamus is often irregular.
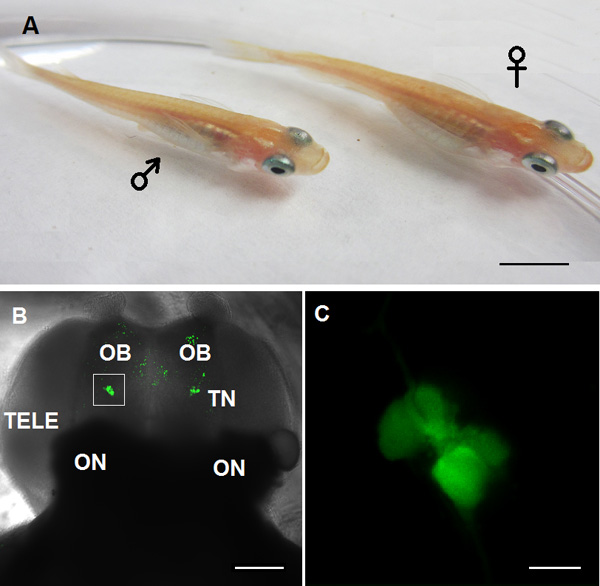
Figure 1. Animal model used for the TN-GnRH3 neuron study in this video. A: Adult medaka GnRH3:GFP transgenic fish, left: male; right: female. B: Confocal image of the ventral view of the forebrain. OB: olfactory bulb; TN: Terminal nerve GnRH3 neurons; TELE: telencephalon; ON: optic nerve. C: High magnification of TN-GnRH3 neurons shown in white box of panel B. (Scale bar: A: 5 mm; B: 100 μm; C: 20 μm).
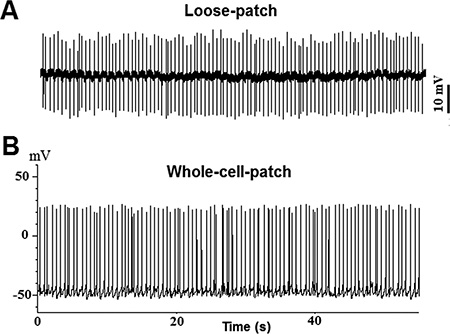
Figure 2. Spontaneous action potentials recorded from TN-GnRH3 neurons in current-clamp mode (I=0). A: Sample trace from loose-patch recording; B: Sample trace from whole-cell recording.
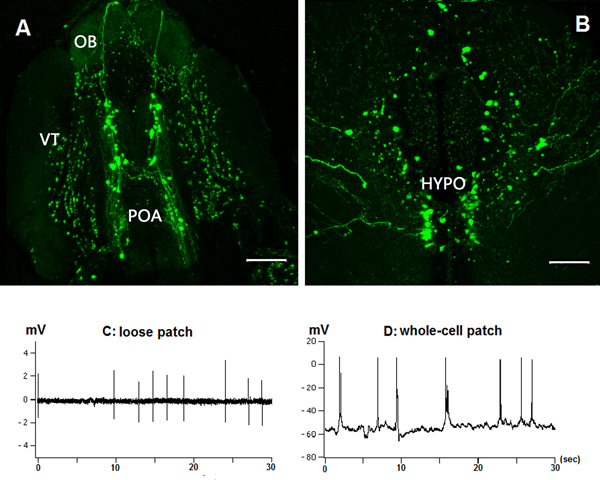
Figure 3. Recording of GnRH3:EMD (Emerald green fluorescent protein) neuron activity from intact brain preparation of adult transgenic zebrafish9 using the same experimental approach as described for the medaka brain preparation. A: GnRH3:EMD neurons located in ventral telencephalon (VT) and preoptic area (POA) in forebrain of adult zebrafish. B: GnRH3:EMD neurons located in hypothalamus (HYPO). Scale bars: 100 μm. C: Sample trace of loose-patch recording from GnRH3:EMD neuron in VT. D: Sample trace of whole-cell patch recording from VT.
Discussion
GnRH3:GFP transgenic fishes provide unique models to study the neurophysiological mechanisms underlying neuronal integration and regulation in central control of behaviors that are both directly and indirectly involved in reproduction3, 8-10. One of the significant advantages of this model system is that many GnRH3 neurons expressing GFP are close to the ventral surface of the brain, allowing for relatively easy access to the neurons for electrophysiological recording without disrupting neural circuits6, 9, 11, 12 (Figures 1B and 1C; Figures 3A and 3B). In this video, we have shown both loose-patch and whole-cell recordings from TN-GnRH3 neurons in the intact adult brain of medaka fish. TN-GnRH3 neurons exhibit a tonic or beating pattern of spontaneous action potential firing with a frequency of 0.5-6Hz.There were no detectable sex differences in spontaneous firing rate 5, but there were dramatic developmental changes in GnRH3 neuron electrical activities during embryogenesis9. Loose-patch and whole-cell electrophysiology have different methodological advantages and disadvantages. Loose-patch is technically easier than whole-cell recording, but only shows the neuron firing pattern and frequency. On the other hand, whole-cell recording can provide much more information: resting membrane potential, firing pattern and frequency, action potential analysis, membrane capacitance and resistance. Because loose-patch recording does not break the cell membrane and disrupt the intracellular ion and second messenger concentrations, it is ideal for long-term recording of neuronal activity. Whole cell recordings rupture the cell membrane and eventually lead to dialysis of intracellular molecules, thereby potentially altering neuron properties with more prolonged recordings. Because of the advantages and disadvantages of both electrophysiological methods, it can be important to study neuron properties using both techniques. Because both pH and osmolarity are very important for neuron survival and function, all the solutions in use need to be adjusted properly.
To confirm the identity of the recorded neuron, negative pressure is applied after completion of the recording, and then fluorescence at the tip of the electrode is confirmed using microscopic fluorescence imaging. Small molecular dyes or probes (such as Alexa Fluor 568 dye or biocytin) may be included in the intracellular solution for the whole-cell recording to mark the recorded neuron for further morphological analysis.
To record successfully from target neurons in intact brain, the depth of location of the neurons is crucial — the deeper the neurons, the harder to patch (both loose-patch and whole-cell). Generally, the feasible depth for successful patching is about 5-6 cell layers from the surface. Medaka TN-GnRH3 neurons are typically at the ventral surface of the brain, while zebrafish hypothalamic GnRH3 neurons can be up to 6 cell layers from the surface. Positive pressure is very helpful to prevent the recording electrode from clogging while approaching the cells. This type of experimental approach can be used for any neuron that is genetically labeled with fluorescent protein. To study GnRH neuron activity in the adult brain, we routinely use medaka fish and zebrafish of 4-6 months of age. The younger fish with smaller brains or older fish with more calcified skulls, will make the dissection more challenging. The TN:GnRH3 neurons are easily accessible for electrophysiology from adult medaka brain, but this is not the case for adult zebrafish in which TN:GnRH3 neurons are encased in a tough connective sheath. On the other hand, GnRH3 neurons in POA, VT, and hypothalamus are more readily accessible for electrophysiology in adult zebrafish brain than from medaka. There are no sex differences based on the difficulties of dissection and electrophysiology recording of either species.
Although this is not an in vivo experimental approach, recording from the relatively intact neuronal circuits with minimum damage is an attractive way to explore the physiological function and regulation of the neuronal system of interest. The intact brain preparation lasts for hours, and a good recording can last for over an hour 6. This protocol is suitable for researchers who already have basic knowledge of and experience with electrophysiology techniques 13, and as well as the specialized equipment required to do the work.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Meng-Chin Lin and Ms. Yuan Dong for technical assistance. This work was supported by a grant from the National Institutes of Health HD053767 (subcontract to NLW), and by funds from the Department of Physiology and Office of the Vice Chancellor for Research, University of California-Los Angeles (NLW).
Materials
| Name | Company | Catalog Number | Comments |
| Microscope | Olympus | BX50W (Upright) | |
| Amplifier | Axon Instruments | Axoclamp 200B | |
| A-D converter | Computer Interference Corp. | Digidata ITC-18 | |
| Cooled CCD camera | PCO Computer Optics | Sensicam | |
| Xenon lamp | Sutter Instruments Co. | ||
| GFP filter set | Chroma Technologies | ||
| Imaging Software | Intelligent Imaging Innovations | Slidebook software | |
| Electrophysiology Data Acquisition Software | Axon Instruments | Axograph software | |
| Electrophysiology Data Acquisition Software | AD Instruments Inc. | PowerLab | |
| Headstage for electrophysiology | Axon Instruments | CV 203BU | |
| Micromanipulator | Sutter Instrument Co | MP-285 | |
| Recording Chamber Platform | Warner Instrument Corp. | P1 | |
| Recording Chamber | Warner Instrument Corp. | RC-26G | |
| Electrode Puller | Sutter instruments | P87 | |
| Filament for electrode puller | Sutter Instruments | FB330B | 3.0 mm wide trough filament |
| 1.5 mm glass capillaries | World Precision Instruments | 1B150-4 | Microelectrode for recording |
| Syringe | Becton Dickinson | 309586 | 3 ml |
| MS-222 | Sigma | E10521-10G | Ethyl 3-aminobenzoate methanesulfonate salt |
| Fish saline | mM: 134 NaCl; 2.9 KCl; 2.1 CaCl2; 1.2 MgCl2; 10 HEPES | ||
| Electrode solution (loose-patch) | mM: 150 NaCl; 3.5 KCl; 2.5 CaCl2; 1.3 MgCl2; 10 HEPES; 10 glucose | ||
| Electrode solution (whole-cell patch) | mM: 112.5 K-gluconate; NaCl; 17.5 KCl; 0.5 CaCl2; 1 MgCl2; 5 MgATP; 1 EGTA; 10 HEPES; 1 GTP; 0.1 leupeptin;10 phospho-creatine |
Riferimenti
- Kah, O., Lethimonier, C., Lareyre, J. J. Gonadotrophin-releasing hormone (GnRH) in the animal kingdom. J. Soc. Biol. 198 (1), 53-60 (2004).
- Ramakrishnan, S., Wayne, N. L. Social cues from conspecifics alter electrical activity of gonadotropin-releasing hormone neurons in the terminal nerve via visual signals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297 (1), R135-R141 (2009).
- Abe, H., Oka, Y. Mechanisms of neuromodulation by a nonhypophysiotropic GnRH system controlling motivation of reproductive behavior in the teleost. 57 (6), 665-674 (2011).
- Oka, Y. Tetrodotoxin-resistant persistent Na+ current underlying pacemaker potentials of fish gonadotrophin-releasing hormone neurones. J. Physiol. 482 (Pt. 1), 1-6 (1995).
- Zhao, Y., Wayne, N. L. Effects of Kisspeptin1 on Electrical Activity of an Extrahypothalamic Population of Gonadotropin-Releasing Hormone Neurons in Medaka. PLoS One. 7 (5), e37909 (2012).
- Wayne, N. L., et al. Whole-cell electrophysiology of gonadotropin-releasing hormone neurons that express green fluorescent protein in the terminal nerve of transgenic medaka (Oryzias latipes). Biol. Reprod. 73 (6), 1228-1234 (2005).
- Okubo, K., et al. Forebrain gonadotropin-releasing hormone neuronal development: insights from transgenic medaka and the relevance to X-linked Kallmann syndrome. Endocrinology. 147 (3), 1076-1084 (2006).
- Okubo, K., et al. A novel form of gonadotropin-releasing hormone in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 276 (1), 298-303 (2000).
- Ramakrishnan, S., et al. Acquisition of spontaneous electrical activity during embryonic development of gonadotropin-releasing hormone-3 neurons located in the terminal nerve of transgenic zebrafish (Danio rerio). Gen. Comp. Endocrinol. 168 (3), 401-407 (2010).
- Abraham, E., et al. Targeted gonadotropin-releasing hormone-3 neuron ablation in zebrafish: effects on neurogenesis, neuronal migration, and reproduction. Endocrinology. 151 (1), 332-340 (2010).
- Wayne, N. L., Kuwahara, K. Beta-endorphin alters electrical activity of gonadotropin releasing hormone neurons located in the terminal nerve of the teleost medaka (Oryzias latipes. Gen. Comp. Endocrinol. 150 (1), 41-47 (2007).
- Oka, Y. Three types of gonadotrophin-releasing hormone neurones and steroid-sensitive sexually dimorphic kisspeptin neurones in teleosts. J. Neuroendocrinol. 21 (4), 334-338 (2009).
- Molleman, A. . Patch Clamping: An Introductory Guide To Patch Clamp Electrophysiology. , (2003).

