高分辨率呼吸测定法,使用基于腔室和板式呼吸计评估细胞和组织中的生物能量学
Summary
使用高分辨率呼吸计评估氧化磷酸化已成为线粒体和细胞能量代谢功能分析的一个组成部分。在这里,我们提出了使用基于腔室和微孔板的高分辨率呼吸计分析细胞能量代谢的方案,并讨论了每种设备的主要优点。
Abstract
高分辨率呼吸测定法(HRR)允许实时监测氧化磷酸化,以分析单个细胞能量状态,并使用多样化的底物 – 解耦 – 抑制剂滴定(SUIT)方案评估呼吸复合物。在这里,演示了两种高分辨率呼吸测定仪的使用,并介绍了适用于分析培养细胞,骨骼和心肌纤维以及软组织(如大脑和肝脏)的基本方案集合。为基于腔室的呼吸计提供培养细胞和组织方案,为基于微孔板的呼吸计提供培养细胞,两者都包含标准呼吸方案。为了进行比较,CRISPR工程的HEK293细胞在线粒体翻译不足导致多种呼吸系统缺陷,与这两种设备一起使用,以证明呼吸中的细胞缺陷。两种呼吸计都允许全面测量细胞呼吸,其各自的技术优点和适用性取决于所研究的研究问题和模型。
Introduction
线粒体履行能量的关键提供,是一种分区化的细胞器,有助于基本的细胞生物能量和代谢过程,如核苷酸,脂质和氨基酸的合成代谢,铁硫簇生物发生,并与信号传导有关,如受控细胞死亡1,2,3.通过氧化磷酸化的线粒体生物能量有助于细胞内几乎所有的细胞过程,因此,原发性或继发性来源的线粒体功能障碍与广泛的疾病条件有关4,5。线粒体功能障碍不仅涉及结构或线粒体密度的改变,还涉及呼吸系统的质量和调节6。该定性元素包括底物控制,偶联特性,翻译后修饰,cristae动力学和呼吸超复合物7,8。因此,准确分析线粒体生物能量学对于评估细胞能量代谢的实验和诊断方法在健康和疾病中是重要的。
线粒体氧化磷酸化(OXPHOS)是呼吸系统或电子转移系统(ETS)内的一系列反应,用于通过三磷酸腺苷(ATP)产生细胞能量9。利用从电子流经络合物I和II到络合物IV的能量的多酶步骤通过线粒体内膜产生电化学质子梯度,随后用于通过络合物V(F1FO ATP合酶)将腺苷二磷酸(ADP)磷酸化为ATP(图1A)。
首先,在三羧酸循环(TCA),糖酵解和丙酮酸氧化期间产生双电子载体:烟酰胺腺嘌呤二核苷酸(NADH)和二氢黄嘌呤二核苷酸(FADH2)。NADH在复合物I(NADH脱氢酶)处被氧化,在此期间,两个电子被转移到辅酶Q(醌被还原为喹诺尔),而质子被泵入膜间空间(IMS)。其次,复合物II(琥珀酸脱氢酶)氧化FADH2 并将电子供给辅酶Q而无需泵送质子。第三,在复合物III(细胞色素c氧化还原酶)处,来自辅酶Q的电子被转移到细胞色素c,而质子被泵入IMS。第四,细胞色素c将电子转移到复合物IV(细胞色素c氧化酶),最终复合物泵送质子,其中氧作为电子受体吸收质子,最终形成水。线粒体消耗的正是这种氧气,可以通过氧化法测量。最后,使用由配合物I,复杂III和复杂IV产生的质子旋转复合物V,从而产生ATP9。
重要的是,电子转移不仅以线性方式发生,否则表示为电子传递链。相反,电子可以通过多个呼吸途径转移到辅酶Q池中,并促进收敛电子流。例如,NADH底物和琥珀酸盐可以分别通过复合物I和复合物II进入。来自脂肪酸氧化的电子可以通过电子转移黄素蛋白复合物捐赠。事实上,对OXPHOS的全面分析需要采用具有适当燃料基质的整体方法(图1A)。
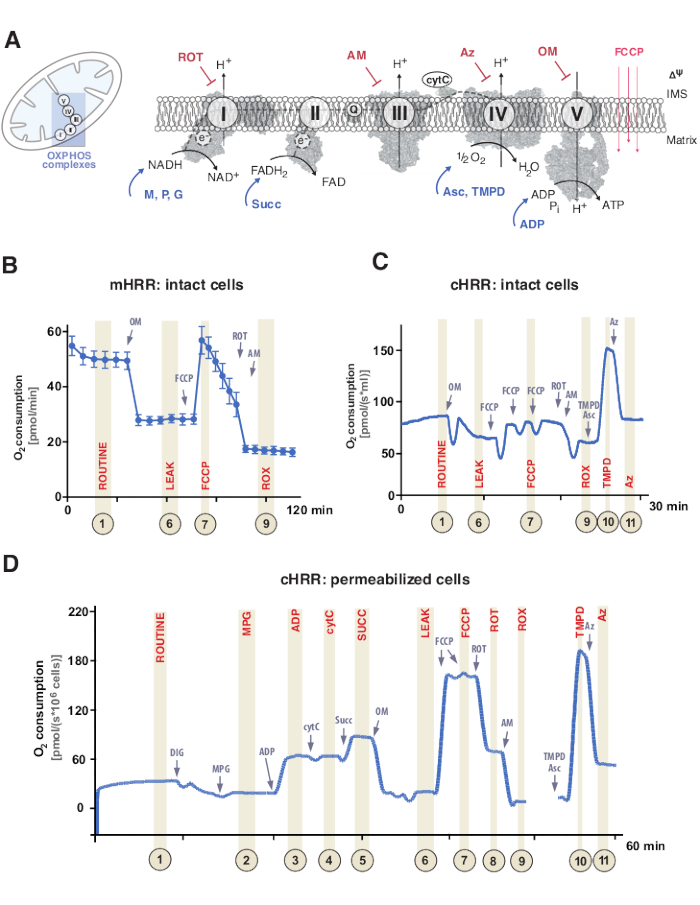
图1:线粒体氧化磷酸化和特定底物和抑制剂方案。 (A)线粒体和电子转移系统(CI-CIV)和线粒体F1F0 ATP合酶(CV)的方案。所有结构均来自 PDB。这些图仅描述了本研究中描述的底物和抑制剂)。(B)在mHRR设备中使用标准方案在完整的HEK293细胞中样品痕量氧通量。(C)在chHRR设备中使用标准方案在完整的HEK293细胞中样品痕量氧通量。(D)来自具有相应SUIT方案的健康供体的透化人成纤维细胞中氧通量的样本痕迹。缩写:1 =完整细胞的常规呼吸;2 = 状态 2;3 = 状态 3(I);4 = 状态 3(I) 与 cytC;5 = 状态 3 (I+II);6 = 泄漏(OM);7 = ETS 容量;8 = S(腐烂);9 = ROX;10 = TMPD;11 = Az. ROT = 鱼藤酮, AM = 抗霉素, ATP = 三磷酸腺苷, Az = 叠氮化物, OM = 寡霉素, FCCP = 羰基氰化物对三氟甲氧基苯腙;Asc = 抗坏血酸,TMPD = N,N,N′,N′-四甲基对苯二胺,Succ = 琥珀酸盐,M = 苹果酸盐,P = 丙酮酸盐,ADP = 二磷酸腺苷,NAD = 烟酰胺腺嘌呤二核苷酸,IMS = 膜间空间,FAD = 黄素腺嘌呤二核苷酸。 请点击此处查看此图的大图。
使用HRR分析线粒体OXPHOS容量已成为一种仪器生化方法,不仅对原发性线粒体缺陷具有诊断价值10,11 ,而且扩展到所有其他生物学领域,如癌症和衰老12。HRR允许通过分析线粒体OXPHOS容量来确定细胞呼吸,其直接反映了单个或组合的线粒体呼吸复合物缺乏,并且间接与细胞功能障碍和能量代谢改变有关9。在方法学上,呼吸测量使用细胞,组织或分离的线粒体11,13,14进行,冷冻材料仅部分适合15,16。冷冻组织被证明具有完整的ETS,并维持超复杂稳定性15。因此,与传统的TCA中间体相反,相应的基板直接送入ETS。然而,ETS和ATP合成之间的偶联会丢失,因为膜的完整性会因冻结损伤(冰晶形成)而受到损害。
呼吸实验通常在37°C的生理温度下进行,用于非透化或透化细胞或组织中的吸热。前者考虑的是胞质代谢背景,而后者通过添加特定底物(和抑制剂)来提供单个OXPHOS复合物和ATP酶的能量贡献。底物和抑制剂的序列和变异导致了各种SUIT方案17和测定18的开发,以解决OXPHOS功能的各种科学问题(在12下综述)。细胞呼吸的基本方案评估四种不同的状态:i)常规呼吸 – 在各自的呼吸介质中呼吸,而不添加任何消耗内源性底物的底物或抑制剂。这种状态可以揭示一般的OXPHOS或继发性呼吸缺陷,例如,由代谢物谱改变引起的。接下来,添加ATP酶抑制剂寡霉素揭示了线粒体内膜对质子的通透性,定义为ii)泄漏呼吸。随后对质子团(例如解偶联羰基氰化物)的滴定,可以确定在开路跨膜质子电路模式(定义为iii)未耦合呼吸)中ETS容量最大的状态。重要的是,通过对线粒体膜的过度机械损伤进行实验干预,也可以发生未耦合状态。相反,非耦合状态是指通过生理控制的内在机制进行呼吸分离。最后,通过添加复合物III抑制剂抗霉素和复合物I抑制剂鱼藤酮来完全抑制ETS,从而确定非线粒体耗氧过程中的残余氧消耗量(ROX)(图1A-C)。
线粒体生物能量学由五种不同的呼吸状态19,20组成。状态 1 呼吸不含任何额外的底物或 ADP,但内源性可用呼吸除外。添加ADP后,仍无底物,实现状态2呼吸。当添加底物时,允许电子转移和ATP合成,达到状态3呼吸。在这种状态下,OXPHOS容量可以在ADP,无机磷酸盐,氧,NADH-和琥珀酸盐连接的底物的饱和浓度下定义。状态4呼吸或泄漏呼吸可以定义为没有ADP或化学抑制ATP合成酶的状态,同时具有足够的底物。最后,当所有氧气在封闭室设置中耗尽(缺氧)时,观察到状态5呼吸。
有几种方法可以评估细胞能量状态14 ,其中两种设备通过分析氧消耗量来主导当前OXPHOS的实时评估,测量为封闭室系统中氧气随时间减少的功能,其适用性取决于实验模型和研究问题:Oroboros 2k高分辨率呼吸计和Seahorse XF细胞外通量分析仪。两种装置都将耗氧率记录为每秒氧(O2)的微摩尔(pmol)的减少,作为腔室或微孔板孔内的绝对值。每质量的特定耗氧量是通过对特定缓冲液配方中每个细胞数(百万),组织重量(mg)或蛋白质量的相应耗氧量进行归一化而获得的。
O2k(Oroboros仪器)是一个封闭的双室系统,配有极谱法氧传感器(缩写为基于腔室的高分辨率呼吸计:cHRR)。每个实验室可容纳2 mL液体,通过磁力搅拌器保持均匀。极谱法氧传感器利用电化学方法来测量氧气:它包含一个金阴极,一个银/氯化银阳极,中间是KCI溶液,形成一个施加电压(0.8 V)的电化学电池。来自测定介质的氧气通过25μm氟化乙烯丙烯膜(O2渗透)扩散,并在阴极处发生还原,产生过氧化氢。在阳极,银被过氧化氢氧化,产生电流。该电流(安培)与氧分压呈线性关系。氧气的分压和测定介质的氧溶解度因子用于计算氧气浓度。由于氧分压取决于实验温度,而极谱法测量对温度敏感,因此温度波动需要通过帕尔贴加热块进行精确(±0.002°C)调节。温度可控制在4°C和47°C范围内。
Seahorse XF 细胞外通量分析仪 (Agilent) 是一种基于板的系统,具有 24 孔或 96 孔微孔板格式,其中三个荧光电极测量每个孔中随时间变化的氧消耗量(缩写为基于微孔板的高分辨率呼吸计:mHRR)。检测管中最多有四个端口可用于检测过程中的自动进样。测定包含多个循环,每个循环有三个阶段:1)混合,2)等待和3)测量。在测量阶段,传感器探针被降低到微孔板中,形成一个包含7-10μL体积的临时封闭室,以测量发射的光。该光由传感器探针尖端上的聚合物包埋荧光团发射,其基于磷光猝灭感O2 。荧光信号的强度与O2 成正比,并受传感器和测定介质温度的影响。因此,精确的氧气估计需要一种没有任何样品的背景阱的相对方法。恢复氧浓度发生在混合阶段,当传感器上下移动以混合临时腔室上方的体积时。每个周期计算一个耗氧率。温度可控制在16°C和42°C范围内。
HRR是评估原发性和线粒体相关疾病以及一般细胞代谢中细胞生物能量的金标准。在这项研究中,提供了HRR的基本方案来评估细胞和组织中的OXPHOS功能。
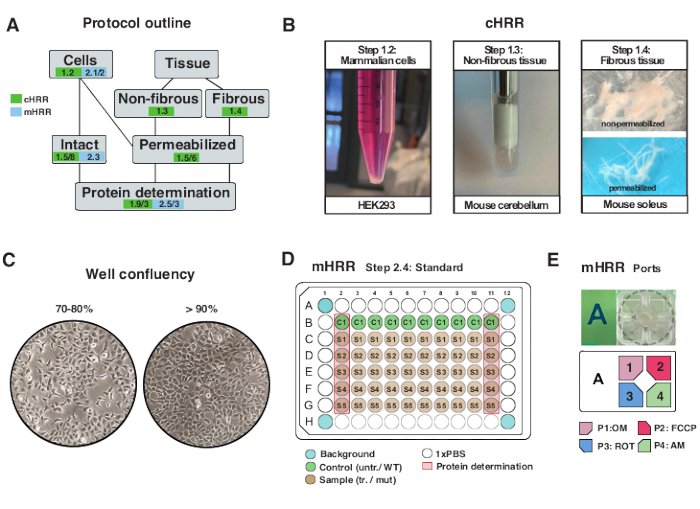
图2:用于chRR的细胞和组织制备的工作流程,以及用于mHRR呼吸测定法的细胞制备的工作流程。(B)哺乳动物细胞(步骤1.2):HEK293沉淀等于3×106个细胞(左图)。非纤维组织(步骤1.3):在2mL特氟龙陶器(中间面板)中制备小鼠小脑裂解物。皂苷诱导的骨骼肌透化(步骤1.4)右图)用于chRR呼吸测定法。(C)标准微孔板接种布局(步骤2.4)和汇合检查,用于分析真核细胞(HEK293),用于mHRR呼吸测定。(D、 E)用于mHRR呼吸测定的注射口负载方案(步骤2.4)。请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
传统上,线粒体生物能量学已经用克拉克型氧电极进行了研究。然而,缺乏分辨率和吞吐量是技术进步的必要条件。迄今为止,O2k(称为chRR)和海马XF96通量分析仪(称为mHRR)已在细胞生物能量学领域得到广泛采用。在这里,我们提出了一个可理解的协议集合,用于通过使用chRR或mHRR评估线粒体呼吸来分析细胞能量代谢,讨论每种设备的主要益处并提供实用指导。此处提供的方案包括哺乳动物细?…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
这项工作得到了芬兰科学院(C.B.J),Magnus Ehrnroot基金会(C.B.J)和综合生命科学研究生院(R.A.)博士奖学金的资助。
Materials
| 2 mL Potter-Elvehjem Glass/PTFE Tissue Grinder/Homogenizer | Omni International | 07-358029 | |
| 95% O2, 5% CO2 medical gas mixture | Potter for tissue grinding | ||
| ADP | Sigma | A 4386 | |
| Antimycin A | Sigma | A 8674 | Chemical |
| Ascorbate | Merck | PHR1279-1G | Chemical, dissolve in ethanol |
| BSA (fatty accid free) | Sigma | A 6003 | Chemical |
| CaCO3 | Sigma | C 4830 | Chemical |
| Cytochrome c | Sigma | C 7752 | Chemical |
| Digitonin | Sigma | D 5628 | Chemical |
| Dithiothreitol | Sigma | D 0632 | Chemical, dissolve in DMSO |
| D-Sucrose | Roth | 4621.1 | Chemical |
| Dulbecco’s modified Eagle’s medium (High glucose) | Fisher Scientific | 41965-039 | Chemical |
| Dulbecco’s modified Eagle’s medium (No Glucose) | Fisher Scientific | A14430-01 | |
| EGTA | Sigma | E 4378 | |
| Etomoxir | Sigma | E1905 | Chemical |
| Falcon 15 ml Conical Centrifuge Tubes | Fisher Scientific | AM12500 | Chemical |
| Falcon 50 ml Conical Centrifuge Tubes | Fisher Scientific | AM12501 | |
| FCCP | Sigma | C 2920 | |
| Glucose | Sigma | G7021 | Chemical, dissolve in ethanol |
| Glutamate | Sigma | G 1626 | Chemical |
| GlutaMax (100x) (200 nM L-alanyl-L-glutamine dipeptide) | Fisher Scientific | 35050061 | Chemical |
| HEK293 cells | ATTC | CRL-1573 | |
| Hemocytometer | Fisher Scientific | 0267151B | Instrument for cell counting |
| Hepes | Sigma | H 7523 | Chemical |
| Imidazole | Fluka | 56750 | Chemical |
| KCl | Merck | 1.04936 | Chemical |
| L-carnitine | Sigma | C0283 | Chemical |
| Malate | Sigma | M 1000 | Chemical |
| MES hydrate | Sigma | M8250 | Chemical |
| MgCl2 | Sigma | M 9272 | Chemical |
| Na2ATP | Sigma | A 2383 | Chemical |
| Na2Phosphocreatine | Sigma | P 7936 | Chemical |
| Na-pyruvate (100 mM) (100x) | Fisher Scientific | 11360070 | |
| NEAA (Non-essential amino acids) 100x | Fisher Scientific | 11140035 | |
| Normal FBS (10x) | Fisher Scientific | 10500064 | |
| O2k-Core: Oxygraph-2k | Oroboros Instruments | 10000-02 | High-resolution respirometry instrument |
| O2k-Titration Set | Oroboros Instruments | 20820-03 | Hamilton syringes for chemical injections |
| Oligomycin | Sigma | O 4876 | Chemical, dissolve in ethanol |
| Palmitoylcarnitine | Sigma | P 4509 | Chemical |
| Penicillin-Streptomycin | Fisher Scientific | 15140122 | |
| Pierce BCA Protein Assay Kit | Fisher Scientific | 23227 | |
| Pyruvate | Sigma | P 2256 | Chemical |
| RIPA-Buffer | Fisher Scientific | 89900 | Chemical |
| Rotenone | Sigma | R 8875 | Chemical, dissolve in ethanol |
| Saponin | Sigma | S7900 | Chemical |
Seahorse XF DMEM assay medium pack, pH 7.4 |
Agilent, Santa Clara, CA |
103680-100 | |
| Seahorse XFe96 Extracellular Flux Analyzer | Agilent, Santa Clara, CA |
High-throughput respirometry instrument | |
| Seahorse XFe96 FluxPak | Agilent, Santa Clara, CA |
Includes assay plates, cartridges, loading guides for transferring compounds to the assay cartridge, and calibrant solution. |
|
| Small scissors | Fisher Scientific | 08-951-20 | |
| Sodium azide | Sigma | S2002 | Chemical |
| Succinate | Sigma | S 2378 | Chemical |
| Taurine | Sigma | T 8691 | Chemical |
| TMPD | Sigma | T 3134 | Chemical |
| Trypan Blue solution | Merck | 72-57-1 | Chemical |
| Trypsin 0.25% EDTA | Fisher Scientific | 25200056 | |
| Two thin-edged forceps | Fisher Scientific | 12-000-122 | |
| Uridine stock (500x) | Sigma | U3750 | Chemical |
Riferimenti
- McBride, H. M., Neuspiel, M., Wasiak, S. Mitochondria: More than just a powerhouse. Current Biology. 16 (14), 551-560 (2006).
- Mehta, M. M., Weinberg, S. E., Chandel, N. S. Mitochondrial control of immunity. Beyond ATP. Nature Reviews Immunology. 17 (10), 608-620 (2017).
- Spinelli, J. B., Haigis, M. C. The multifaceted contributions of mitochondria to cellular metabolism. Nature Cell Biology. 20 (7), 745-754 (2018).
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. International Journal of Biochemistry and Cell Biology. 41 (10), 1837-1845 (2009).
- Gorman, G. S., et al. Mitochondrial diseases. Nature Reviews Disease Primers. 2, 1-23 (2016).
- Boushel, R., Gnaiger, E., Schjerling, P., Skovbro, M., Kraunsøe, R., Dela, F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 50 (4), 790-796 (2007).
- Cogliati, S., et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 155 (1), 160-171 (2013).
- Kühlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biology. 13, 1-11 (2015).
- Gnaiger, E. Mitochondrial pathways and Respiratory control. An introduction to OXPHOS analysis. Bioenergetics communications. 5th ed. , (2020).
- Jackson, C. B., et al. Mutations in SDHD lead to autosomal recessive encephalomyopathy and isolated mitochondrial complex II deficiency. Journal of Medical Genetics. 51 (3), 170-175 (2014).
- Pesta, D., Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods in Molecular Biology. 810, 25-58 (2012).
- Horan, M. P., Pichaud, N., Ballard, J. W. O. Review: Quantifying mitochondrial dysfunction in complex diseases of aging. Journals of Gerontology – Series A Biological Sciences and Medical Sciences. 67 (10), 1022-1035 (2012).
- Doerrier, C., Garcia-Souza, L. F., Krumschnabel, G., Wohlfarter, Y., Mészáros, A. T., Gnaiger, E. High-resolution fluorespirometry and oxphos protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods in Molecular Biology. 1782, 31-70 (2018).
- Zhang, J., et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nature Protocols. 7 (6), 1068-1085 (2012).
- García-Roche, M., Casal, A., Carriquiry, M., Radi, R., Quijano, C., Cassina, A. Respiratory analysis of coupled mitochondria in cryopreserved liver biopsies. Redox Biology. 17, 207-212 (2018).
- Acin-Perez, R., et al. A novel approach to measure mitochondrial respiration in frozen biological samples. The EMBO Journal. 39 (13), 1-18 (2020).
- Cell metabolism assay kits. Seahorse assay kits and media Available from: https://www.agilent.com/en/product/cell-analysis/real-time-cell-metabolic-analysis/xf-assay-lits-reagents-cell-assay-media (2021)
- Chance, B., Williams, G. R. A method for the localization of sites for oxidative phosphorylation. Nature. 176 (4475), 250-254 (1955).
- Gnaiger, E., et al. Mitochondrial respiratory states and rates. MitoFit Preprint Arch. , (2019).
- Gnaiger, E. O2k-procedures: SOP O2k quality control 1: Polarographic oxygen sensors and accuracy of calibration Section Page. Oroboros. 03 (18), 1-21 (2020).
- Robinson, B. H., Petrova-Benedict, R., Buncic, J. R., Wallace, D. C. Nonviability of cells with oxidative defects in galactose medium: A screening test for affected patient fibroblasts. Biochemical Medicine and Metabolic Biology. 48 (2), 122-126 (1992).
- King, M. P., Attardi, G. Human cells lacking mtDNA: Repopulation with exogenous mitochondria by complementation. Science. 246 (4929), 500-503 (1989).
- Makrecka-Kuka, M., Krumschnabel, G., Gnaiger, E. High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. Biomolecules. 5 (3), 1319-1338 (2015).
- Fasching, M., Gnaiger, E. O2k quality control 2: Instrumental oxygen background correction and accuracy of oxygen flux. Mitochondrial Physiology Network. 14 (06), 1-14 (2016).
- Gnaiger, E., Lassnig, B., Kuznetsov, A., Rieger, G., Margreiter, R. Excess capacity of cytochrome c oxidase. Journal of Experimental Biology. 1139, 1129-1139 (1998).
- Gnaiger, E., et al. Mitochondria in the Cold. Life in the Cold. , 431-442 (2000).
- Fontana-Ayoub, M., Fasching, E., Gnaiger, Selected media and chemicals for respirometry with mitochondrial preparations. Mitochondrial Physiology Network. 02 (17), 1-9 (2014).
- Gerencser, A. A., et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Analytical Chemistry. 81 (16), 6868-6878 (2009).
- Krumschnabel, G., Eigentler, A., Fasching, M., Gnaiger, E. Use of safranin for the assessment of mitochondrial membrane potential by high-resolution respirometry and fluorometry. Methods in Enzymology. 542, 163-181 (2014).
- Nászai, A., Terhes, E., Kaszaki, J., Boros, M., Juhász, L. Ca(2+)N it be measured? Detection of extramitochondrial calcium movement with high-resolution fluorespirometry. Scientific Reports. 9 (1), 1-13 (2019).
- Pajak, B., et al. 2-Deoxy-d-Glucose and its analogs: From diagnostic to therapeutic agents. International Journal of Molecular Sciences. 21 (1), 234 (2019).
- Mercier-Letondal, P., Marton, C., Godet, Y., Galaine, J. Validation of a method evaluating T cell metabolic potential in compliance with ICH Q2 (R1). Journal of Translational Medicine. 19 (1), 1-15 (2021).
- Sauerbeck, A., et al. Analysis of regional brain mitochondrial bioenergetics and susceptibility to mitochondrial inhibition utilizing a microplate based system. Journal of Neuroscience Methods. 198 (1), 36-43 (2011).
- Jackman, M. R., Willis, W. T. Characteristics of mitochondria isolated from type I and type IIb skeletal muscle. American Journal of Physiology – Cell Physiology. 270 (2), 673-678 (1996).
- Ponsot, E., et al. Mitochondrial tissue specificity of substrates utilization in rat cardiac and skeletal muscles. Journal of Cellular Physiology. 203 (3), 479-486 (2005).
- Schönfeld, P., Reiser, G. Why does brain metabolism not favor burning of fatty acids to provide energy-Reflections on disadvantages of the use of free fatty acids as fuel for brain. Journal of Cerebral Blood Flow and Metabolism. 33 (10), 1493-1499 (2013).
- Calderon-Dominguez, M., Mir, J. F., Fucho, R., Weber, M., Serra, D., Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte. 5 (2), 98-118 (2016).
- Divakaruni, A. S., Rogers, G. W., Murphy, A. N. Measuring mitochondrial function in permeabilized cells using the seahorse XF analyzer or a clark-type oxygen electrode. Current Protocols in Toxicology. 2014, 1-16 (2014).
- Iuso, A., Repp, B., Biagosch, C., Terrile, C., Prokisch, H. Assessing mitochondrial bioenergetics in isolated mitochondria from various mouse tissues using Seahorse XF96 analyzer. Methods in Molecular Biology. 1567, 217-230 (2017).
- Rogers, G. W., et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS ONE. 6 (7), 21746 (2011).
- Jordá, A., Zaragozá, R., Portolés, M., Báguena-Cervellera, R., Renau-Piqueras, J. Long-term high-protein diet induces biochemical and ultrastructural changes in rat liver mitochondria. Archives of Biochemistry and Biophysics. 265 (2), 241-248 (1988).
- Jackson, C. B., Gallati, S., Schaller, A. QPCR-based mitochondrial DNA quantification: Influence of template DNA fragmentation on accuracy. Biochemical and Biophysical Research Communications. 423 (3), 441-447 (2012).
- Hirsch, H. M. Tissue autoxidation inhibitors: II. The presence of inhibitor in intact cells; Assay of liver and hepatoma effect on radio-oxidations. Ricerca sul cancro. 16 (11), 1076-1082 (1956).
- Picard, M., et al. Mitochondrial structure and function are disrupted by standard Isolation methods. PLoS ONE. 6 (3), 18317 (2011).
- Tanumihardja, E., Slaats, R. H., Van Der Meer, A. D., Passier, R., Olthuis, W., Van Den Berg, A. Measuring both pH and O2 with a single On-Chip sensor in cultures of human pluripotent stem cell-derived cardiomyocytes to track induced changes in cellular metabolism. ACS Sensors. 6 (1), 267-274 (2021).
- Harms, F., Stolker, R. J., Mik, E. Cutaneous respirometry as novel technique to monitor mitochondrial function: A feasibility study in healthy volunteers. PLoS ONE. 11 (7), 159544 (2016).
- Levitsky, Y., et al. Micro-respirometry of whole cells and isolated mitochondria. RSC Advances. 9 (57), 33257-33267 (2019).

