Controlled Cortical Impact Model of Mouse Brain Injury with Therapeutic Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Cells
Summary
This protocol demonstrates methodologies for a mouse model of open-skull traumatic brain injury and transplantation of cultured human induced pluripotent stem cell-derived cells into the injury site. Behavioral and histologic tests of outcomes from these procedures are also described in brief.
Abstract
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality worldwide. Disease pathology due to TBI progresses from the primary mechanical insult to secondary injury processes, including apoptosis and inflammation. Animal modeling has been valuable in the search to unravel injury mechanisms and evaluate potential neuroprotective therapies. This protocol describes the controlled cortical impact (CCI) model of focal, open-head TBI. Specifically, parameters for producing a mild unilateral cortical injury are described. Behavioral consequences of CCI are analyzed using the adhesive tape removal test of bilateral sensorimotor integration. Regarding experimental therapy for TBI pathology, this protocol also illustrates a process for transplanting cultured cells into the brain. Neural cell cultures derived from human induced pluripotent stem cells (hiPSCs) were chosen for their potential to show superior functional restoration in human TBI patients. Chronic survival of hiPSCs in the host mouse brain tissue is detected using a modified DAB immunohistochemical process.
Introduction
Traumatic brain injury (TBI) is a general term for the acquired injury to the brain due to either indirect mechanical forces (rotational acceleration/deceleration or contra-coup) from blows to the head or direct damage from objects or blast waves. TBI has been estimated to be the cause of roughly 9% of worldwide deaths and observed in an estimated 50 million cases per year1,2. A 2017 report from the Centers for Disease Control and Prevention estimated that in 2013, there were a total of 2.8 million hospital visits and deaths due to TBI in the United States3. Many milder TBIs go unreported every year. Serious TBI can lead to lifelong impairment of cognition, motor function, and overall quality of life. The consequences of mild TBI, especially repetitive sport-related TBI, have been only recently appreciated for their insidious health effects4,5.
Preclinical modeling is a vital component of developing new mechanistic insights and potential restorative therapy for TBI. The controlled cortical impact (CCI) model of TBI is an open-head model of mechanical contusion injury to the cortex. The impact parameters can be modified to produce CCI injuries that range from mild to severe6. CCI injuries are focal rather than diffuse, as seen with other closed head models of TBI. CCI can be performed to induce a unilateral injury, such that the contralateral cortex can serve as an internal comparator. This protocol demonstrates the characteristics of a mild CCI to a portion of the cortex that encompasses primary somatosensory and motor regions. This cortical area was chosen for its involvement in sensorimotor behaviors for which numerous behavior tests can detect injury-induced deficits7. Behavioral improvements due to therapeutic interventions for TBI can be detected, as well.
A hallmark of TBI is widespread neural dysfunction in the injured region. Injured neurons undergo cell death, and neuronal network connectivity is disrupted8,9. TBI disrupts recruitment of endogenous stem cells, which leads to further downstream behavior deficits10,11. Transplantation of neural stem cells and stem cell-derived cells has been explored as a possibility to restore function in the injured brain. In addition to the potential to restore damaged neural circuitry, transplanted cells exert paracrine effects that promote neuronal survival and functional recovery from TBI12. A variety of cell types have been transplanted preclinically to evaluate their restorative potential in models of neurologic disorders13,14,15. The recent popularization of induced pluripotent stem cell technology16 has facilitated the development of numerous human stem cell lines for experimental use. Preclinical testing with hiPSC-derived cells is an important first step to characterizing a given cell line’s potential therapeutic efficacy against human diseases. This laboratory has developed protocols for differentiating hiPSCs to neural phenotypes17 in pursuit of transplantable cells to aid recovery from traumatic brain injury.
Experiments in this protocol use a unilateral CCI to induce TBI to the left somatosensory and motor cortex of adult mice. A mild CCI injury results in a sustained functional deficit in the right forepaw that is used to track the effects of hiPSC-derived neural cell engraftment on functional recovery. Forepaw sensorimotor testing in this protocol was adapted from the methodology established by Bouet and colleagues18 and demonstrated previously by Fleming and colleagues19. This protocol outlines a complete workflow for performing an experimental brain injury, therapeutic transplantation of hiPS cells, and behavioral and histologic analysis of experimental outcome measures.
Protocol
All experiments described in this protocol were reviewed and approved by the Uniformed Services University Animal Care and Use Committee.
1. Craniectomy and controlled cortical impact
- Preparation of the controlled cortical impact device and surgical supplies.
- Load a 1 mL slip-tip syringe with 0.5 mL of sterile saline for wound irrigation. Attach a 25 G needle to the syringe to control irrigation.
- Prepare a dilute solution of CsA in DMSO to final concentration of 1 mg/mL. Load a second 1 mL slip-tip syringe with 0.5 mL of cyclosporine A (CsA) solution for immunosuppression. Attach a 25 G needle or larger to the CsA syringe.
- Attach the controlled cortical impact piston to an arm on a stereotaxic frame and set to an angle of 15°. Attach a 3 mm impactor probe to the piston.
- Set the velocity of impact to 1.5 m/s and the impact dwell time to 0.1 s to produce a mild cortical injury.
- Perform a unilateral craniectomy
- Place the mouse in an anesthesia induction chamber connected to an isoflurane vaporizer with compressed oxygen source. Induce anesthesia with ~3% isoflurane at ~0.7 L/min oxygen. Check for the depth of anesthesia by the lack of response to the toe-pinch.
- Shave the scalp using electric clippers and wipe away any loose fur.
- Place the mouse in a stereotaxic frame with attached anesthetic delivery nose cone.
- Place a warming pad set to 37 °C on the stereotaxic frame under the mouse to maintain body temperature under anesthesia. Fix the head in place with ear bars and a bite bar and orient the head such that the skull frontal bone is horizontal. Maintain anesthesia at ~1.5%-2% isoflurane for the duration of the surgery.
- To perform preoperative care and aseptic surgical preparation, apply antibiotic ophthalmic ointment to the eyes using a sterile cotton swab. Apply an iodine-based solution to the shaved scalp area. Remove this with 70% ethanol. Cover the animal with a fenestrated surgical drape so that the top of the head is visible but the eyes are covered.
- Make a midline incision (1.5-2 cm) on the scalp using a scalpel or scissors. Use sterile cotton swabs to clean the wound and to clear the fascia left of the midline at bregma.
- Use the impactor probe to identify the craniectomy site.
- Set the stereotaxic reference point (X = 0, Y = 0) to bregma. Adjust the probe laterally to 2 mm left of the midline. Outline a 5 mm diameter circle around the probe using a fine-tip surgically safe marker. Raise and rotate the impactor out of position.
- Use the high-speed rotary micromotor kit hand tool to make an open hole in the skull using a round-tip 0.6 mm or 0.8 mm burr drill bit at ~70%-80% maximum speed. Apply light pressure to the skull while drilling along the 5 mm circumferential outline to thin this border.
- Do not apply excess pressure while drilling. This can cause cortical injury due to vibration, compression, or accidental penetration. Allow the speed of the drill bit to do the work.
- Do not drill in any given spot for too long to avoid excess friction heating of the skull. Irrigate the craniectomy occasionally with sterile saline to remove debris and to reduce heating from the rotary tool.
- Pay close attention while drilling over the coronal suture line as these points are vulnerable to hemorrhage.
- Use a pair of fine tweezers to remove the skull flap when the craniectomy outline is sufficiently thinned. Grasp the flap medially, and gently lift and pull laterally with a radial motion.
- Do not to damage the dura mater when lifting the flap; this can cause severe injury and hemorrhaging.
- Perform a mild controlled cortical impact injury
- Clean the impactor probe with a sterile alcohol prep pad. Move the impactor probe back into position over the exposed cortex. Lower the probe until it touches the dura mater surface. Mark this position as Z = 0.
- Withdraw the piston and move to Z = -1.0 mm. Discharge the piston to impact the cortex.
- Quickly raise the piston and move the arm out of position. Apply generous amounts of saline to irrigate the cortex after injury. Rinse the surgery site with saline as needed and suture the scalp incision using simple interrupted stiches with 5.0 silk suture.
- Perform postoperative care on the mouse
- Discontinue anesthesia. Deliver CsA by subcutaneous injection into scruff at 10 mg/kg dose. Place the mouse in a clean and pre-warmed postoperative cage.
- Provide acetaminophen analgesic in the drinking water at 1.0 mg/mL.
NOTE: Provide analgesia according to the appropriate IACUC standard operating procedure and in consideration of experimental outcome variables (e.g., sedation, neuroinflammation). - Provide moistened chow food in a warmed recovery cage to aid in rehydration and recovery.
- Proceed from section 2 to section 4 above when performing craniectomy-only (sham) controls.
2. Stereotaxic transplantation of cell suspension
- Begin cell transplantation procedure roughly 24 h after craniectomy.
- Prepare the cell transplantation equipment and surgical supplies
- Fill a 1 mL slip-tip syringe with sterile saline for wound irrigation. Attach a 25 G needle to the syringe to control irrigation.
- Fill a 1 mL slip-tip syringe with CsA solution for immunosuppression. Attach a 25 G needle or larger to the CsA syringe. Refill the CsA syringe as needed between surgeries.
- Prepare glass needles from 1.0 mm OD borosilicate glass capillary pipettes using standard methods.
- Use fine tweezers to break the needle tips to approximately a 200 µm diameter. Ensure that the cylindrical shaft of the needle is no longer than 2.5 cm.
- Calibrate the syringe pump for use with a 10 µL syringe. Enter a flow rate of 0.2 µL/min to deliver a total volume of 2.0 µL.
- Prepare human induced pluripotent stem cell (iPSC) suspension.
- Perform all cell handling in a cell culture BSL-2 hood using standard sterile handling techniques.
- Prepare cell cultures in advance according to standard conditions defined for the cell type. Refer to Lischka et al.17 as an example.
NOTE: Experiments shown in this demonstration used various neural phenotype cells derived from hiPSCs. - Gently dissociate the cells into a single-cell suspension using a cell detachment solution, or other preferred enzymatic or chemical means.
- Count cells in suspension, then dilute the suspension to 5 x 104 cells/µL in minimal cell culture medium (e.g., DMEM) in a 1.7 mL flip top test tube.
- Observe the following notes for transplantation:
- Maintain the cells in suspension at 37 °C for the duration of procedures.
- Load the syringe only immediately prior to performing intraparenchymal injection (step 4.5 below).
NOTE: Gravity can cause the cell suspension to settle or to cling to the side of the syringe if laid on its side. This leads to irregularities in the number of cells injected. - Allot ~5 x 105 cells (10 µL suspension) per mouse if performing multiple cell transplantation procedures in one day.
- Perform stereotaxic transplantation surgery
- Place the mouse in an anesthesia induction chamber connected to an isoflurane vaporizer with compressed oxygen source. Induce anesthesia with ~3% isoflurane at ~0.7 L/min oxygen.
- Place the mouse in a stereotaxic frame with attached anesthetic nose cone.
- Fix the head in place with ear bars and a bite bar and orient the head such that the skull frontal bone is horizontal. Maintain anesthesia at ~1.5%-2% isoflurane for the duration of the surgery.
- Perform preoperative care and aseptic preparation
- Apply hydrating ophthalmic ointment containing antibiotic to eyes using a cotton swab.
- Lavage the incision site with sterile saline to clean the site and to loosen sutures. Gently apply 70% ethanol with a cotton swab to sterilize the incision site.
- Remove sutures using fine tweezers and ophthalmic scissors. Irrigate surgery site and craniectomy with abundant sterile saline.
- Consider the animal for exclusion if the cortex displays disqualifying characteristics including excessive herniation, discoloration, disrupted vascularization, or hemorrhage.
- Load the cell transplant syringe
- Move cell suspension from the 37 °C incubator to a cell culture biosafety hood. Gently swirl or tap the tube to ensure a homogeneous cell suspension.
- Use a micro pipettor to load ~7.5 µL cell suspension into the Hamilton syringe through the plunger end.
- Hold the syringe at a ~120° angle with the plunger end facing down. Insert the plunger, taking care not to introduce an air bubble between the suspension and plunger tip.
- Attach the gasket assembly to the pipette needle, then attach the needle to the syringe.
- Push the plunger to move cell suspension into the pipette needle. If there is resistance against suspension outflow, use fine tweezers to break the needle tip to enlarge the diameter.
- Attach the syringe to the stereotaxic syringe pump. Advance the plunger to make sure the syringe pump assembly is working properly.
- Move the needle into the coordinates for injection.
- Align the needle tip to bregma. Set the X and Y coordinates to 0. Then move the needle tip over the craniectomy to 2.0 mm lateral and -1.0 mm posterior to bregma. Touch the needle tip to the dura mater surface and set the stereotaxic coordinate to Z = 0.
- Push the plunger to ensure the cell suspension is flowing adequately before introducing the needle into the brain.
- Introduce the needle into the brain to a depth of Z = -1.4 mm. These stereotaxic coordinates place the graft at the gray matter-white matter border of the deep cortex20.
- Start the syringe pump to infuse cell suspension. Set the lab bench timer to 15 min and start the timer. Use a long working distance microscope to monitor cell suspension outflow.
- Irrigate the surgery site with sterile saline during injection to maintain tissue hydration.
- At 15 min, slowly withdraw the transplantation needle. Irrigate the surgery site with saline and close the incision with sutures.
- Perform postoperative care
- Discontinue anesthesia. Deliver CsA by subcutaneous injection into scruff at 10 mg/kg dose. Place the mouse in a clean and pre-warmed postoperative cage.
- Provide acetaminophen analgesic in the drinking water at 1.0 mg/mL.
NOTE: Provide analgesia according to the appropriate IACUC standard operating protocol (SOP) and in consideration of experimental outcome variables. - Provide moistened chow food recovery cage to aid in rehydration and recovery.
- Continue daily CsA injections at 10 mg/kg throughout the survival duration of the mouse.
3. Adhesive tape removal test of sensorimotor integration
- Cut the electrical tape into 3 mm x 5 mm strips using a small razor knife prior to performing the behavior test. Use a smooth glass surface for cutting the adhesive strips.
NOTE: Use yellow and red tape, as mice have difficulty distinguishing between these colors21.- Select a small mirror that fits well inside the clear plastic box.
- Fix the mirror in place at a roughly 45° angle with modeling clay or adhesive tape in order to view animal behavior from below.
- Place the box and mirror assembly on a bench in a quiet dedicated behavior testing room. Arrange the cylinder on the plastic box above the mirror.
- Arrange the handling cloth, tweezers, and adhesive strips on the bench next to the behavior testing box.
- Clean the box and cylinder with 70% ethanol and paper towels. Allow surfaces to dry thoroughly.
- Bring the mice into the behavior testing room. Allow the mice to acclimate to the testing room for at least 30 min prior to behavior testing.
- Remove water supplies from the cages to minimize urination events during testing, which can interfere with efficient test performance.
- Select a small mirror that fits well inside the clear plastic box.
- Perform the adhesive removal test
NOTE: This behavior test is best performed by two to three investigators: one to operate the stopwatches, and one or two to handle the mice and observe the behavior.- Use each tweezer to peel one adhesive strip of each color.
- Choose which strip color corresponds to which forepaw and remain consistent throughout the trial.
- Use the handling cloth to restrain a mouse by the scruff of the neck and back such that the mouse holds the forepaws away from its body and head.
- Use tweezers to place an adhesive strip on the plantar surface of each forepaw. Use delicate and consistent finger pressure to secure the strips to the paws.
- Quickly place the mouse into the plastic cylinder. Start the two stopwatches when the mouse has all four paws on the plastic box.
- Use the two stopwatches to record the latencies for the following four events: left paw notice, left paw removal, right paw notice, right paw removal.
- Record a notice event when the animal makes unambiguous recognition of the adhesive strip by shaking or flinching the paw or biting the strip.
- Record a remove event when the animal effectively removes the strip from the forepaw plantar surface.
NOTE: The remove event is not disqualified by strip readherence or if the strip clings to the lateral surface of the forepaw. - Stop the timer at 120 s if the corresponding strip has not been removed.
- Record the elapsed times for the four events on the data sheet.
- Perform a second trial on each mouse
- Allow a minimum of 5 min to elapse between trials for an individual mouse to reduce stress, which can interfere with efficient performance on the test.
- Clean the apparatus with paper towels between testing mice to remove waste when testing multiple mice from a single cage.
- Clean the apparatus thoroughly with 70% ethanol and paper towels when testing mice from multiple cages.
- Reverse the color-paw placement order for the second trial of the session to randomize any effect of color.
- Calculate the average latency of each event between two trials per daily session for each animal.
- Use each tweezer to peel one adhesive strip of each color.
- Clean the apparatus and handling cloth thoroughly with water, replace the cage water supplies, and return the mice to their holding facility.
- Repeat steps 3.1-3.2 on successive experimental timepoints for a repeated measures trial design.
NOTE: Repeat steps 3.2.1-3.2.5.3 daily for 5 days prior to any data collection to acclimate the animals to the task. Baseline data acquisition prior to surgery is recommended.
4. Diaminobenzidine (DAB) immunohistochemical analysis of graft survival and injury pathology
- Euthanize the animals and perform a transcardial perfusion.
- Prepare solutions of 0.1 M phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA) in PBS. Balance the pH of both solutions to 7.4.
- Place the two solutions on ice in a fume hood. Place a peristaltic pump in the fume hood, and run the pump to fill the tubing with PBS. Set the pump flow rate to ~7 ml/min. Connect a 25 G needle to the outflow tube of the pump.
- Place a drainage tray with a soft substrate in the hood. Raise one end of the tray at a slight angle so that perfusion fluids drain to the lower end.
- Place a mouse in an anesthesia induction chamber. Fill the chamber with 4% isoflurane using compressed 100% oxygen vehicle gas.
- Move the anesthetized mouse from the chamber to an anesthesia nose cone. Decrease the isoflurane to 2%.
- Perform a thoracotomy to expose the heart. Discontinue anesthesia, as the thoracotomy causes euthanasia.
- Carefully place the perfusion pump outflow needle in the left ventricle along the long axis of the heart. Do not pierce the heart septum, as this will cause poor perfusion.
- Use small scissors to cut the right atrium, then immediately turn on the peristaltic pump.
- Continue the PBS flow until the fluid leaving the right atrium runs clear of blood.
- Turn off the pump. Move the pump intake tube to the container of PFA. Restart the pump. Continue perfusing PFA until either the animal is sufficiently rigid or until a 20 mL volume has been pumped through.
- Turn off the pump. Remove the outflow needle from the heart. Move the intake tube from the PFA to the PBS, and thoroughly flush out PFA from the tubing.
- Remove the brain from the skull by careful dissection. Place the brain in a small container filled with 4% paraformaldehyde, and allow the brain to post-fix overnight at 4 °C.
- On the day after post-fixation, replace the P with PBS and 0.1% sodium azide. Store the brains in this solution until preparation for histologic sectioning.
- Collect 40-50 µm sections of formaldehyde-fixed brain tissue via a freezing microtome or room temperature vibratome.
- Pretreat tissues in 0.3% hydrogen peroxide in water to inactivate endogenous peroxidases, which can produce nonspecific staining.
- Perform antigen retrieval using citric acid buffer (10 mM sodium citrate, 0.05% polysorbate-20, pH 6.0) at 60 °C for 30 min.
- Perform antibody labeling by standard methods. Refer to Lundell et al.’s report22 from this laboratory for more details.
- Use a mouse IgG neutralization kit (see Table of Materials) to reduce cross-reactivity with endogenous antibodies. Incubate the tissues in IgG neutralization buffer for at least 1 h at room temperature (RT), following the manufacturer’s directions.
- Use a mouse anti-human nuclear antigen primary antibody (hNA; 1:500 dilution) when transplanting human iPSC-derived cells to locate the transplanted cells. Incubate tissues with the primary antibody at 4 °C for 48-72 h using gentle agitation.
- After rinsing away primary antibody solution, incubate tissues with HRP-conjugated anti-mouse secondary antibody at 1:250 dilution for 2 h at RT.
- Perform DAB chromogen reaction by standard methods to reveal immunolabeling. Allow DAB reaction to develop for 5 to 10 min at RT.
- Attach stained tissues to slides and apply cover slips using standard methods.
- Quantify numbers of labeled cells using unbiased stereology as described previously23,24. Perform analysis using 20 µm optical sections and a between section interval of three.
Representative Results
Craniectomy surgery facilitates experimental brain injury and therapeutic cell transplantation: the controlled cortical impact model of brain injury and subsequent cell transplantation therapy require careful removal of the overlying skull. The craniectomy may be performed on any dorsal surface of the skull to permit manipulations to the brain region of interest. The diagram in Figure 1 depicts a 5 mm diameter craniectomy schematic to uncover primary somatosensory and motor cortices (Figure 1A). At 24 h after craniectomy, a second surgery was performed to inject human iPSC-derived neural cell suspension into deep layers of the cortex (Figure 1B). Some cerebral edema is normal on the first day following craniectomy, and particularly after CCI. However, cerebral vasculature sparing during all phases of this procedure is crucial for survival of the cortex. Figure 2 illustrates the cell transplantation procedure in a mouse with minimal cerebral herniation, minimal bleeding, and extensive cortical vascularization. These features are good prognostic indicators of a successful surgery.
Adhesive tape removal testing reveals sensorimotor deficits after unilateral brain injury: the parameters of the brain injury model described above were predicted to affect forelimb sensory and motor function. The adhesive tape removal test was chosen to evaluate the severity of forelimb functional deficits, and the potential therapeutic benefits of cell transplantation. Mice were trained on the testing procedure for 5 days, then allowed to rest for two days prior to baseline behavior testing. Surgeries were performed on the day following baseline testing. Behavior tests in this study were performed on postoperative days 1, 3, 5, 7, 10, 14, 21, 28, 35, and 42. Figure 3 shows results from a pilot experiment in which forelimb function in mice with craniectomy alone (sham) and with CCI injury were compared to forelimb function in naïve mice (n = 11 naïve, 12 sham, 11 CCI). Mice that underwent surgery exhibited transient increased latencies to notice adhesive stimuli for 1-3 days immediately after surgery (Figure 3A,B). Mice showed transient postoperative deficits in adhesive removal from the ipsilateral forepaw as well (Figure 3C). However, mice that underwent CCI exhibited significant deficits in motor performance in the forepaw contralateral to injury compared to naïve mice out to postoperative day 28 (Figure 3D). These data also describe the unexpected severity of sensorimotor loss in craniotomized mice without CCI, indicating that surgical craniectomy to this area also induces TBI-related neurofunctional deficits.
Immunodetection of human induced pluripotent stem cell (iPSC)-derived cell grafts in mouse brain sections: experiments were performed to determine whether human iPSC-derived neural cells would survive long-term transplantation in the mouse brain. Human neural stem cells (NSCs) derived from iPSCs were differentiated into either immature neurons or astrocytes in vitro using established methods17. Transplants of each of the three neural cell phenotypes were tested in our CCI model of traumatic brain injury using the procedure described above and depicted in Figure 2. The mice were euthanized for histologic analysis at 7 days after transplantation. Mouse brain sections were immunostained for the human nuclear antigen (hNA). Human cell grafts could be clearly distinguished from host tissue in sham surgery and CCI brains (Figure 4). Astrocyte grafts (n = 3 sham, 2 CCI) showed poor survival compared to NSCs (n = 12 sham, 15 CCI) and neurons (n = 11 sham, 10 CCI), and were not considered for future experiments.
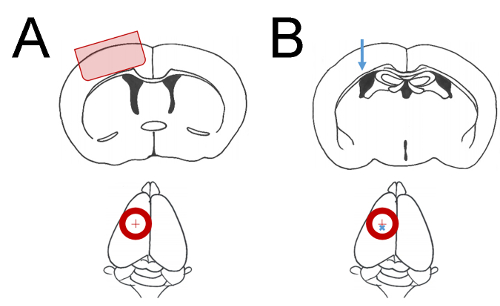
Figure 1: Coordinate parameters of surgical manipulations. Cartoon depictions of mouse brain regions of interest. Red circles indicate a ~5 mm diameter craniectomy. A red cross indicates the craniectomy central point 2 mm lateral to bregma. (A) The shaded region of cerebral cortex in the upper diagram is affected by mild CCI when a craniectomy is performed as shown in lower diagram. (B) The blue arrow in upper diagram indicates the approximate location of cell injections at 1.4 mm depth from cortical surface. The blue cross in the lower diagram indicates the placement of cell injection 2 mm lateral and 1 mm posterior to bregma. Please click here to view a larger version of this figure.
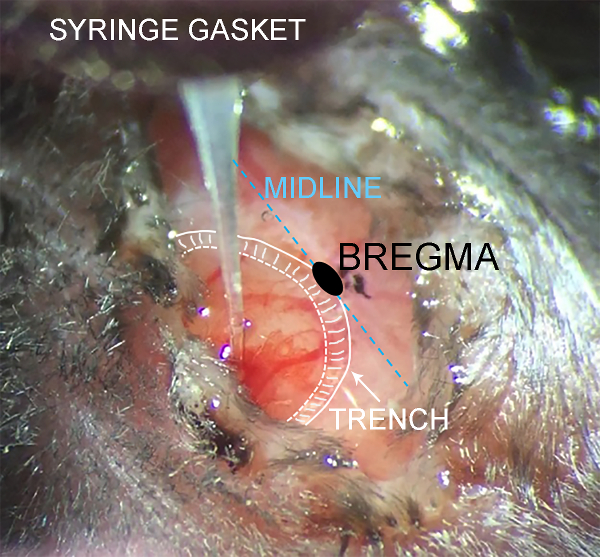
Figure 2: Intraoperative monitoring of cell suspension injection. Photograph taken through a long working distance microscope during intraparenchymal cell injection. Anatomic features are annotated for clarity. The scalp partially obscures the surgery site to minimize dehydration during the procedure. Minor bleeding may occur during needle penetration as shown, which is not cause for concern if large cortical vessels remain intact. Please click here to view a larger version of this figure.
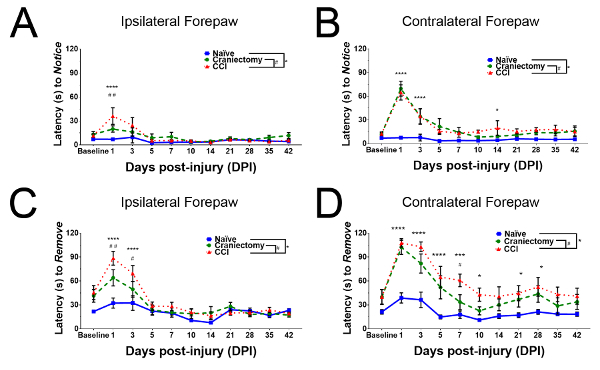
Figure 3: Behavioral evaluation of sensorimotor integration after brain injury. Mice that underwent craniectomy and CCI were compared to naïve controls and to mice that underwent only sham surgery (n = 11 naïve, 12 sham, 11 CCI). Data are presented as group mean latencies, with error bars indicating SEM. (A) Mice that underwent CCI exhibited increased latency to recognize adhesive stimuli applied to the ipsilateral forepaw on the first postoperative day. (B) Mice that underwent craniectomy or CCI exhibited substantially increased latency to notice adhesive stimuli applied to the contralateral forepaw on postoperative days 1 and 3. (C) Mice that underwent craniectomy or CCI exhibited substantially increased latency to remove adhesive stimuli from the ipsilateral forepaw on postoperative days 1 and 3. (D) Mice that underwent craniectomy or CCI exhibited substantially increased latency to remove adhesive stimuli from the contralateral forepaw on postoperative days 1-5. Motor deficits in mice with CCI persisted strongly for 28 days after injury. Please click here to view a larger version of this figure.
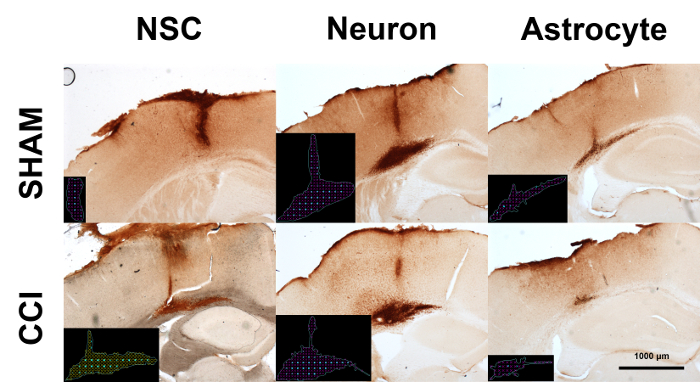
Figure 4: DAB immunohistochemistry for human cell grafts in mouse brains. Human iPSCs were differentiated into neural stem cells (NSCs), neurons, or astrocytes in vitro. Cell cultures were transplanted into mouse brains with or without CCI. Mice were euthanized for histologic analysis seven days after cell transplantation. Micrographs depict representative results of human nuclear antigen staining. Black insets depict markers for stereologic quantification of cell numbers (cyan) and graft volume (red). Please click here to view a larger version of this figure.
Discussion
Mild CCI as a model system for testing experimental regenerative therapy
The CCI model is a valuable tool for investigating mechanisms of tissue dysfunction after mechanical injury to the cortex. The tunability of the injury parameters is an attractive feature of this model. Altering the Z depth of impact, the velocity, or dwell time can increase or decrease severity of the injury as desired by the investigator10,25. The mild CCI model of contusive brain injury, when performed correctly, should cause modest cortical cell death and minimal cavitation. Craniectomy and skull flap removal must be performed with great care. Excessive downward force applied while drilling the craniectomy trench can cause cortical injury due to heating and vibration. Mechanical disruption of the dura mater during skull flap removal almost uniformly predicts severe cortical injury. Disruption of major cortical blood vessels is likely to result in excessive lesioning of the cortex and is grounds for excluding the animal from the experiment. Unfortunately, the signs of an exacerbated injury can be subtle on the day after surgery. Neither edema nor small cortical vessel rupture are necessarily negative indicators. Hematomas and abnormal coloration due to ischemia are clearer indicators of surgical complication. Documenting intraoperative events and correlating complications with histopathologic outcomes are crucial to refining good craniectomy technique.
It must be noted that mTBI modeling in animals comes with certain caveats. There are numerous preclinical models of mTBI other than the model presented here. Experimental TBI can be induced through mechanical forces, blast waves through air, or a combination of these forces26,27,28. Mild to severe injuries are judged by a combination of histopathological and behavioral outcomes (reviewed in Petraglia29 and Siebold30). Behavior deficits in rodents can resolve within days to weeks31 whereas human mTBI patients’ deficits can persist for months in the form of post-concussive syndrome32. Although no single model is a complete analog for clinical mTBI, preclinical testing reveals physiologic mechanisms that cannot be assessed in the human condition.
The most important steps in the cell transplantation procedure are the handling and injection of the cell suspension. Rough handling causes cell lysis, leading to the release of sticky genomic DNA and aggregation of the surviving suspended cells. The needle tip diameter must be wide enough to permit smooth outflow of the suspension; restricted flow causes discontinuous delivery as the suspension sediments inside the needle. When following this protocol and avoiding the pitfalls discussed here, robust grafts were visible after 7-day survival times (Figure 4). Long term graft survival in a preclinical model is key to determining potential therapeutic efficacy of this approach.
Application of the adhesive tape removal test to contusive brain injury modeling
The adhesive removal test reveals positive neurologic deficits in terms of increased latency to remove the adhesive stimuli. The main potential drawback to this test is the likelihood of inhibited performance due to factors not related to injury. Handling stress can reduce mouse exploratory behavior33, so it is crucial that the animals undergo extensive restraint acclimation prior to baseline data collection. It is important to remove water supplies at least 30 min prior to testing in order to mitigate urination-related freezing events. Finally, the testing apparatus must be cleaned often as animals can become distracted into investigatory sniffing of odor cues from unfamiliar animals.
Results presented here show significant forepaw motor deficits contralateral to mild CCI up to 28 days after injury. By contrast, concurrent testing using the cylinder test34 and accelerating rotarod3 showed injury-induced functional deficits that resolved within 5–10 days (data not shown). Adhesive tape removal has been used in a variety of experiments evaluating unilateral deficits in sensorimotor integrative behavior7,35. The test was also recently used to assess motor function recovery following regenerative intervention for cervical spinal cord injury36. The dynamic range of performance on this test can be tuned for latency or species variation by selecting adhesives of different strength. Here, it is suggested that 3M electrical tape is an optimal stimulus for mice based on availability, durability, and substantially increased latency to remove stimuli following mild CCI. Although the preliminary experiments shown here do not combine cell transplantation and behavior testing, ongoing experiments in our laboratory will assess transplant survival and concurrent effects on sensorimotor behavioral recovery from brain injury at 56 days after transplantation.
Refinement to DAB immunodetection of human cell grafts
DAB immunohistochemistry was chosen in order to produce strong, persistent labeling of human cells in mouse tissue for subsequent stereologic quantification. Pretreatment with hydrogen peroxide is crucial for reducing nonspecific staining in brain tissue due to endogenous peroxidases in erythrocytes. Nonspecific staining in these experiments was further reduced using a mouse IgG neutralization kit. This kit uses proprietary chemicals to reduce antigenicity of endogenous mouse IgGs, which can extravasate into brain tissue after TBI37. Early attempts employed a combination of mouse anti-hNA primary antibody, biotin-conjugated secondary antibodies, and streptavidin-HRP conjugate tertiary labeling using the Vector Laboratories ABC kit. The ABC conjugate exhibited extensive nonspecific DAB staining in the cortex ipsilateral to the craniectomy (data not shown). Subsequent staining trials employed secondary antibodies directly conjugated with HRP. This modified protocol produces high-resolution nuclear staining with greatly reduced background for all hiPS-derived cell types and surgery conditions (Figure 4). Unpublished experiments from this laboratory using this modified DAB staining technique detect hNA-positive cells in mouse brains 56 days after mild CCI. Overall, a binary antibody labeling procedure saved time and produced clearer immunostaining compared to the traditional tertiary labeling ABC kit procedure. This protocol could be useful for other preclinical studies of human cells transplanted into the mouse central nervous system.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the Center for Neuroscience and Regenerative Medicine (CNRM, grant number G170244014). We appreciate the assistance of Mahima Dewan and Clara Selbrede in adhesive removal pilot studies. Kryslaine Radomski performed preliminary brain injury and cell transplantation surgeries. Amanda Fu and Laura Tucker of the USU CNRM Preclinical Studies core laboratory provided valuable advice on animal surgeries and behavior testing, respectively.
Materials
| 1 ml syringes | Becton Dickinson (BD) | 309659 | |
| 1.7 ml flip top test tubes | Denville | C2170 | |
| 10 microliter syringe | Hamilton | 7635-01 | |
| 25G Precision Glide syringe needles | Becton Dickinson (BD) | 305122 | |
| 70% ethanol | Product of choice; varies by region | ||
| acetaminophen oral suspension | Tylenol (Children's) | Dilute to 1 mg/ml in water | |
| anesthetic vaporizer | Vetland | 521-11-22 | |
| animal handling cloth | Purchase from department store | ||
| Betadine | Purdue Products | NDC-67618-151-32 | |
| compressed oxygen | Product of choice; varies by region | ||
| cyclosporine A | Sigma-Aldrich | 30024-100mg | |
| DAB staining kit | Vector Laboratories | SK-4100 | |
| dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D8418-500ml | |
| DMEM | Invitrogen (ThermoFisher) | A14430-01 | |
| donkey anti-mouse IgG antibody, HRP conjugated | Jackson ImmunoResearch | 715-035-151 | |
| electrical tape | 3M Corporation | Purchase from department store | |
| fine tweezers | Fine Science Tools | 11254-20 | |
| forceps | Fine Science Tools | 91106-12 | |
| glass capillary pipettes, 1 mm OD, 0.58 mm ID | World Precision Instruments | 1B100F-3 | |
| High Speed Rotary Micromotor Kit | Foredom Electric Co. | K.1070 – K.107018 | |
| Ideal Micro Drill Burr Set Of 5 | Cell Point Scientific | 60-1000 | |
| Impact One Stereotaxic Impactor for CCI | Leica Biosystems | 39463920 | |
| isoflurane | Baxter | NDC-10019-360-60 | |
| lab bench timers | Fisher Scientific | 14-649-17 | |
| Micropipette puller | MicroData Instruments, Inc. | PMP-102 | Any puller will suffice |
| Microscope cover slips | Fisherbrand | 12-545-E | |
| Microscope slide mounting medium | Product of choice | ||
| mirror | Purchase from department store | ||
| mouse anti-human nuclear antigen antibody | Millipore | MAB1281 | |
| Mouse on Mouse blocking kit | Vector Laboratories | BMK-2202 | |
| needle holder hemostat | Fine Science Tools | 12002-12 | |
| ophthalmic ointment | Falcon Pharmaceuticals | NDC-61314-631-36 | |
| ophthalmic spring scissors | Fine Science Tools | 15018-10 | |
| plastic box | Purchase from department store | ||
| plastic cylinder | Purchase from department store | ||
| QSI motorized syringe pump | Stoelting | 53311 | |
| Removable needle compression fitting | Hamilton | 55750-01 | |
| small rodent stereotaxic frame | Stoelting | 51925 | |
| small scissors | Fine Science Tools | 14060-09 | |
| StemPro Accutase | Invitrogen (ThermoFisher) | A1110501 | |
| Sterile alcohol prep pads | Fisherbrand | 06-669-62 | |
| sterile cotton swabs/Kendall Q-tips | Tyco Healthcare | 540500 | |
| Sterile saline | Hospira | NDC-0409-1966-07 | |
| Stopwatches (2) | Fisher Scientific | 06-662-56 | |
| Superfrost Plus Gold microscope slides | Fisherbrand | 15-188-48 | |
| sutures – 5.0 silk with curved needle | Oasis | MV-682 |
References
- Maas, A. I. R., et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. The Lancet Neurology. 16, 987-1048 (2017).
- Murray, C. J., et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 380, 2197-2223 (2012).
- Taylor, C. A., Bell, J. M., Breiding, M. J., Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths – United States, 2007 and 2013. Morbidity and mortality weekly report: Surveillance summaries. 66, 1-16 (2017).
- Fehily, B., Fitzgerald, M. Repeated Mild Traumatic Brain Injury: Potential Mechanisms of Damage. Cell Transplantation. 26, 1131-1155 (2017).
- Kulbe, J. R., Hall, E. D. Chronic traumatic encephalopathy-integration of canonical traumatic brain injury secondary injury mechanisms with tau pathology. Progress in Neurobiology. 158, 15-44 (2017).
- Romine, J., Gao, X., Chen, J. Controlled cortical impact model for traumatic brain injury. Journal of Visualized Experiments. , e51781 (2014).
- Schallert, T., Fleming, S. M., Leasure, J. L., Tillerson, J. L., Bland, S. T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 39, 777-787 (2000).
- Mishra, A. M., et al. Decreased resting functional connectivity after traumatic brain injury in the rat. PloS ONE. 9, 95280 (2014).
- Sours, C., et al. Default mode network interference in mild traumatic brain injury – a pilot resting state study. Brain Research. 1537, 201-215 (2013).
- Radomski, K. L., Zhou, Q., Yi, K. J., Doughty, M. L. Cortical contusion injury disrupts olfactory bulb neurogenesis in adult mice. BMC Neuroscience. 14, 142 (2013).
- Wang, X., Gao, X., Michalski, S., Zhao, S., Chen, J. Traumatic Brain Injury Severity Affects Neurogenesis in Adult Mouse Hippocampus. Journal of Neurotrauma. 33, 721-733 (2016).
- Aertker, B. M., Bedi, S., Cox, C. S. Strategies for CNS repair following TBI. Experimental Neurology. 275 (3), 411-426 (2016).
- Kikuchi, T., et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 548, 592-596 (2017).
- Kondo, T., et al. Focal transplantation of human iPSC-derived glial-rich neural progenitors improves lifespan of ALS mice. Stem Cell Reports. 3, 242-249 (2014).
- Tong, L. M., et al. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Abeta accumulation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 34, 9506-9515 (2014).
- Takahashi, K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131, 861-872 (2007).
- Lischka, F. W., et al. Neonatal mouse cortical but not isogenic human astrocyte feeder layers enhance the functional maturation of induced pluripotent stem cell-derived neurons in culture. Glia. 66, 725-748 (2018).
- Bouet, V., et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nature Protocols. 4, 1560-1564 (2009).
- Fleming, S. M., Ekhator, O. R., Ghisays, V. Assessment of sensorimotor function in mouse models of Parkinson’s disease. Journal of Visualized Experiments. , e50303 (2013).
- Paxinos, G., Franklin, K. B. J. . The mouse brain in stereotaxic coordinates. Compact 2nd edn. , (2004).
- Jacobs, G. H., Williams, G. A., Cahill, H., Nathans, J. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science. 315, 1723-1725 (2007).
- Lundell, T. G., Zhou, Q., Doughty, M. L. Neurogenin1 expression in cell lineages of the cerebellar cortex in embryonic and postnatal mice. Developmental Dynamics: An Official Publication of the American Association of Anatomists. 238, 3310-3325 (2009).
- Kempermann, G., Kuhn, H. G., Gage, F. H. More hippocampal neurons in adult mice living in an enriched environment. Nature. 386, 493-495 (1997).
- Piltti, K. M., et al. Transplantation dose alters the dynamics of human neural stem cell engraftment, proliferation and migration after spinal cord injury. Stem Cell Research. 15, 341-353 (2015).
- Yu, S., et al. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Research. 1287, 157-163 (2009).
- Kabadi, S. V., Hilton, G. D., Stoica, B. A., Zapple, D. N., Faden, A. I. Fluid-percussion-induced traumatic brain injury model in rats. Nature Protocols. 5, 1552-1563 (2010).
- Namjoshi, D. R., et al. Defining the biomechanical and biological threshold of murine mild traumatic brain injury using CHIMERA (Closed Head Impact Model of Engineered Rotational Acceleration). Experimental Neurology. 292, 80-91 (2017).
- Shetty, A. K., Mishra, V., Kodali, M., Hattiangady, B. Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Frontiers in Cellular Neuroscience. 8, 232 (2014).
- Petraglia, A. L., Dashnaw, M. L., Turner, R. C., Bailes, J. E. Models of mild traumatic brain injury: translation of physiological and anatomic injury. Neurosurgery. 75, 34-49 (2014).
- Siebold, L., Obenaus, A., Goyal, R. Criteria to define mild, moderate, and severe traumatic brain injury in the mouse controlled cortical impact model. Experimental Neurology. 310, 48-57 (2018).
- Tucker, L. B., Fu, A. H., McCabe, J. T. Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research. Journal of Neurotrauma. 33, 880-894 (2016).
- Rose, S. C., Fischer, A. N., Heyer, G. L. How long is too long? The lack of consensus regarding the post-concussion syndrome diagnosis. Brain Injury. 29, 798-803 (2015).
- Hurst, J. L., West, R. S. Taming anxiety in laboratory mice. Nature Methods. 7, 825-826 (2010).
- Li, X., et al. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Experimental Neurology. 187, 94-104 (2004).
- Andersen, A. B., Finger, S., Andersen, C. S., Hoagland, N. Sensorimotor cortical lesion effects and treatment with nimodipine. Physiology & Behavior. 47, 1045-1052 (1990).
- Al-Ali, H., et al. The mTOR Substrate S6 Kinase 1 (S6K1) Is a Negative Regulator of Axon Regeneration and a Potential Drug Target for Central Nervous System Injury. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 37, 7079-7095 (2017).
- Pleasant, J. M., et al. Rate of neurodegeneration in the mouse controlled cortical impact model is influenced by impactor tip shape: implications for mechanistic and therapeutic studies. Journal of Neurotrauma. 28, 2245-2262 (2011).

