Efficient Transcriptionally Controlled Plasmid Expression System for Investigation of the Stability of mRNA Transcripts in Primary Alveolar Epithelial Cells
Summary
Here, we present a tool that can be used to study the posttranscriptional modulation of a transcript in primary alveolar epithelial cells by using an inducible expression system coupled to a pipette electroporation technique.
Abstract
Studying posttranscriptional regulation is fundamental to understanding the modulation of a given messenger RNA (mRNA) and its impact on cell homeostasis and metabolism. Indeed, fluctuations in transcript expression could modify the translation efficiency and ultimately the cellular activity of a transcript. Several experimental approaches have been developed to investigate the half-life of mRNA although some of these methods have limitations that prevent the proper study of posttranscriptional modulation. A promoter induction system can express a gene of interest under the control of a synthetic tetracycline-regulated promoter. This method allows the half-life estimation of a given mRNA under any experimental condition without disturbing cell homeostasis. One major drawback of this method is the necessity to transfect cells, which limits the use of this technique in isolated primary cells that are highly resistant to conventional transfection techniques. Alveolar epithelial cells in primary culture have been used extensively to study the cellular and molecular biology of the alveolar epithelium. The unique characteristics and phenotype of primary alveolar cells make it essential to study the posttranscriptional modulations of genes of interest in these cells. Therefore, our aim was to develop a novel tool to investigate the posttranscriptional modulations of mRNAs of interest in alveolar epithelial cells in primary culture. We designed a fast and efficient transient transfection protocol to insert a transcriptionally controlled plasmid expression system into primary alveolar epithelial cells. This cloning strategy, using a viral epitope to tag the construct, allows for the easy discrimination of construct expression from that of endogenous mRNAs. Using a modified ΔΔ quantification cycle (Cq) method, the expression of the transcript can then be quantified at different time intervals to measure its half-life. Our data demonstrate the efficiency of this novel approach in studying posttranscriptional regulation in various pathophysiological conditions in primary alveolar epithelial cells.
Introduction
Several techniques have been developed to determine the half-life of mRNAs. The pulse-chase decay technique, which utilizes labeled mRNAs, allows for the simultaneous evaluation of a large pool of mRNAs with minimal cellular disturbance. However, this approach does not allow a direct estimation of the half-life of a single gene transcript and cannot be implemented to study the posttranscriptional modulation of an mRNA following stimulation with growth factors, ROS, alarmins, or inflammation1.
The use of transcription inhibitors, such as actinomycin D and α-amanitin, is a relatively simple method for measuring mRNA degradation kinetics over time. One main advantage of this approach over that of previous techniques, (i.e., pulse-chase) relies on the ability to directly estimate the half-life of a given transcript and compare how different treatments could affect its degradation kinetics. However, the significant deleterious impact of transcription inhibitors on cell physiology represents a major drawback of the approach2. Indeed, the inhibition of the whole cell transcriptome with these drugs has the negative side effect of perturbing the synthesis of key elements involved in mRNA stability, such as microRNAs (miRNAs), as well as the expression and synthesis of RNA-binding proteins, which are important for mRNA degradation and stability. The severe perturbation of gene transcription by these drugs could therefore artefactually modify the degradation curves of transcripts.
The promoter induction system represents a third approach to measure the half-life of a specific mRNA. This method measures the degradation of a specific mRNA in a similar way as methods that use transcription inhibitors. Two types of induction systems are frequently used: the serum-induced c-fos promoter3 and the Tet-Off inducible system4. With the c-fos system, the use of transcription inhibitors that can be toxic to the cell is not needed. However, this method requires cell cycle synchronization, which prevents the evaluation of the actual stability of a transcript during interphase5. In contrast, the Tet-Off system allows the strong expression of the gene of interest (GOI) under the control of a synthetic tetracycline-regulated promoter. This system requires the presence of two elements that must be cotransfected into the cell to be functional. The first plasmid (pTet-Off) expresses the regulatory protein tTA-Adv, a hybrid synthetic transcription factor composed of the prokaryotic repressor TetR (from Escherichia coli) fused to three transcription transactivation domains from the viral protein HSV VP16. The GOI is cloned into the pTRE-Tight plasmid under the control of a synthetic promoter (PTight), comprising the minimal sequence of the cytomegalovirus (CMV) promoter fused to seven repeats of the tetO operator sequence. The transcription of the gene downstream of PTight is dependent on the interaction of TetR with tetO. In the presence of tetracycline or its derivative, doxycycline, the TetR repressor loses its affinity for the tetO operator, leading to a cessation of transcription4. The characteristics of the Tet-Off system make it an ideal model for the study of specific mRNA expression in eukaryotic cells while avoiding potential pleiotropic effects that are secondary to the absence of prokaryotic regulatory sequences in eukaryotic cell6. Usually, doubly stable Tet-Off cell lines (HEK 293, HeLa, and PC12) are used with this system to integrate copies of the regulator and response plasmids for convenient access to controllable gene expression7,8,9.
Several models of alveolar epithelial cells in culture have been used to study the cellular and molecular biology of the alveolar epithelium. For years, researchers have extensively utilized human or rodent primary cells10,11 as well as immortalized cell lines such as human A549 or rat RLE-6TN cells12,13. Although they are generally less proliferative and more difficult to culture and to transfect, alveolar epithelial cells in primary culture remain the gold standard for the study of the function and dysfunction of the alveolar epithelium in physiological and pathological conditions. Indeed, immortalized cell lines such as A549 cells do not exhibit the complex characteristics and phenotypes of primary cells, whereas alveolar epithelial cells in primary culture recapitulate the main properties of the alveolar epithelium, in particular the ability to form a polarized and tight barrier14,15. Unfortunately, these cells are very resistant to conventional transfection techniques, such as those utilizing liposomes, making the use of a promoter-induced system such as Tet-Off very difficult.
The posttranscriptional modulation of mRNAs is one of the most effective methods for rapidly modulating the gene expression of a transcript16. The mRNA 3' untranslated region (3' UTR) plays an important role in this mechanism. It has been shown that, unlike the 5' UTR, there is an exponential correlation between the length of the 3' UTR and the cellular and morphological complexities of an organism. This correlation suggests that the 3' UTR, like the mRNA coding regions, has been subjected to natural selection to allow for increasingly complex posttranscriptional modulation throughout evolution17. The 3' UTR contains several binding sites for proteins and miRNAs that affect the stability and translation of the transcript.
In the present work, we developed a tool to investigate the role of highly conserved domains in the 3' UTR of a GOI for the control of transcript stability. We focused on the epithelial sodium channel, alpha subunit (αENaC), which plays a key role in alveolar epithelial physiology18. Alveolar epithelial cells in primary culture were successfully transiently transfected with the two components of the Tet-Off system, which allows for the study of the role of the 3' UTR in mRNA stability with a system that minimally affects cell physiology and metabolism in comparison to the use of transcription inhibitors with other protocols. A cloning strategy was developed to differentiate the expression of the GOI from that of the endogenous gene using a nonendogenously expressed epitope (V5). The response and regulatory plasmids were then transferred into alveolar epithelial cells using a pipette electroporation technique. Subsequently, the expression of the transcript was measured by incubating the cells with doxycycline at different time intervals. The half-life of the transcript was evaluated by RT-qPCR with a modified Cq method using the transfected tTA-Ad mRNA product for normalization. Through our protocol, we offer a convenient way for studying the posttranscriptional modulation of a transcript under different conditions and defining the involvement of the untranslated regions in more detail.
Protocol
All animal procedures were conducted according to the guidelines of the Canadian Council on Animal Care and were approved by the Institutional Animal Care Committee of the Research Center of Centre Hospitalier de l'Université de Montréal (CRCHUM).
1. Design and generation of the response plasmid expressing the gene of interest (GOI)
- Use an inducible tetracycline-off vector, such as pTRE-Tight.
- Analyze the sequence of the GOI and the multiple cloning site (MCS) of the vector to identify the restriction sites in the MCS that are not present internally in the GOI.
- Isolate primary alveolar epithelial cells from male Sprague-Dawley rat lungs as described previously19,20.
- Purify the total RNA from the alveolar epithelial cells by RNA extraction using a standard method, such as phenol/chloroform extraction or the use of silica-based RNA spin columns.
- Reverse-transcribe the mRNA into complementary DNA (cDNA) using oligo(dT) and high-fidelity reverse transcriptase.
- Use high-fidelity Taq polymerase and standard overlap PCR techniques to flank the GOI with two selected restriction enzyme recognition sites using designed primers.
- Have the forward primer contain a Kozak consensus ribosome binding site21 to improve expression levels to study protein expression in parallel with mRNA stability. A sequence encoding the V5 epitope upstream of the GOI must be included to distinguish the expression of the transfected GOI from endogenous expression (Table 1).
- Have the reverse primer contain a polyadenylation signal after the stop codon.
- Mutants can be generated by sequential deletion to study the effects of different 3' UTR regions on the stability of the mRNA of the GOI using reverse primers encoding a polyadenylation site that gradually deletes the 3' end of the GOI 3' UTR (Figure 6). Alternatively, PCR-directed mutagenesis can be used to target a specific region of interest in the 3' UTR22.
- Digest the inducible vector and the insert with the previously chosen restriction enzymes at the appropriate incubation temperature for 1 h, followed by treatment with phosphatase during the vector reaction for 30 min to avoid self-ligation.
- Separate the digested vector and insert segments by electrophoresis in a 1-1.5% agarose gel (concentration depending on the size of the insert).
- Using a blade and a UV light, collect the DNA fragments containing the desired insert and vector to be ligated.
NOTE: The protocol can be paused here. - Purify the collected segments from the agarose gel using a silica-based PCR purification kit and measure the concentration by spectrophotometry at 260 nm.
- Ligate the GOI and the inducible vector with T4 DNA ligase using a vector:insert molar ratio of 1:3 to increase the probability of ligation. Incubate the reaction at room temperature (RT) for 3 h.
- Transform the ligation reaction into competent E. coli (DH5α).
- Add 1-10 ng of vector and gene to a tube containing 100 µL of competent cells. Incubate the cells on ice for 30 min and then heat-shock cells at 42 °C for 45 s. Place the tube on ice for 2 min and add 900 µL of RT LB medium. Incubate the cells for 1 h at 37 °C with shaking at 225 rpm.
- Spread 100 µL of the reaction on an LB agar plate with a suitable antibiotic (e.g., 100 µg/mL ampicillin for the pTRE-Tight vector) to select the transformed bacteria. Incubate the plate overnight at 37 °C.
- Select individual colonies using an inoculation loop or a 20 µL tip and incubate overnight in 5 mL LB medium containing the suitable antibiotic at 37 °C with shaking. The transformed bacteria may be stored in glycerol stocks at -80 °C at a ratio of 400:600 of LB medium to glycerol.
- Extract the plasmid DNA using silica-based plasmid columns and measure the concentration by spectrophotometry at 260 nm. Confirm the insertion of the GOI by restriction analysis and its orientation and the absence of mutations potentially introduced during RT-PCR by sequencing.
2. Transfection of the response plasmid expressing the gene of interest (GOI) into primary alveolar epithelial cells
- Isolate type II alveolar epithelial cells from rat lungs.
- Seed the cells at a density of 1 x 106 cells/cm2 in 100 mm Petri dishes with complete minimum essential medium (complete MEM). Complete MEM is MEM supplemented with 10% FBS, 0.08 mg/L tobramycin, Septra (3 µg/mL trimethoprim and 17 µg/mL sulfamethoxazole), 0.2% NaHCO3, 0.01 M HEPES (pH = 7.3), and 2 mM L-glutamine. Culture the cells for 24 h at 37 °C in 5% CO2 in a humidified incubator.
- On the next day, place 500 µL of complete MEM without antibiotic in each well of a new 12 well plate and prewarm the plate at 37 °C for 30 min. During this step, it is important to use fetal bovine serum free of contaminating tetracyclines or with a level too low to interfere with inducibility.
- Prepare 1.5 mL tubes containing the plasmid with the inducible GOI (GOI plasmid) and the regulatory vector (e.g., pTet-Off) by adding 1 µg of GOI plasmid and 1 µg of regulatory vector per well at RT. For coexpression experiments with RNA-binding proteins (RBP), 1 µg of a constitutive vector (e.g., pcDNA3) expressing the RPB of interest is added to the DNA mix (Figure 7).
- Aspirate the medium and gently rinse the cells with PBS (without calcium and magnesium) prewarmed at 37 °C.
- Add 5 mL of 0.05% trypsin prewarmed at 37 °C and incubate the cells until the cells are detached (2-4 min). Neutralize the trypsin by adding 10 mL of complete MEM without antibiotic.
- Collect the cell suspension in a 50 mL tube, wash the Petri dish with 4 mL of medium to collect as many remaining cells as possible, and then centrifuge the cell suspension at 300 x g for 5 min.
- Gently aspirate and discard the supernatant and resuspend the pellet in 1 mL of PBS. Count and calculate the number of cells using a hemocytometer.
- Centrifuge the cells at 300 x g for 5 min. Gently aspirate the supernatant and resuspend the pellet in resuspension buffer at a concentration of 4 x 107 cells/mL. Add the cells from the 1.5 mL tube prepared in step 2.4 at a concentration of 400,000 cells per well and gently mix them by pipetting up and down.
- Place the tube in the electroporation device and fill it with 3.5 mL of electrolytic buffer.
- Insert a gold-plated electrode tip into a pipette by completely pressing the piston. Gently mix the contents of the 1.5 mL tube and carefully aspirate the cells with the pipette. Be careful to prevent air bubbles from entering the tip, as this will cause electric arcing during electroporation and lead to decreased transfection efficiency.
- Insert the pipette in the electroporation station until there is a clicking sound.
- Select the appropriate electroporation protocol for alveolar epithelial cells, corresponding to a pulse voltage of 1,450 V and 2 pulses with a width of 20 ms, and press Start on the touchscreen.
- Immediately after transfection, remove the pipette and transfer the cells to a well previously filled with complete MEM without antibiotic that has been prewarmed to 37 °C.
- Repeat steps 2.11-2.14 for the remaining samples.
- Gently shake the plate to spread the cells evenly over the well surface. Incubate the cells at 37 °C in 5% CO2 in a humidified incubator. After 2 days, replace the medium with complete MEM with antibiotics.
- Confirm the success of the transfection by observing the expression of eGFP under a fluorescence microscope or by flow cytometry using a control vector (Figure 1).
NOTE: This step is optional and requires an additional transfection step using a different plasmid expressing eGFP, such as pcDNA3-EGFP.
3. Induction of the transcription inhibition of the GOI
NOTE: The cells can be pretreated with the desired treatments before doxycycline induction to assess their impact on mRNA stability (Figure 5).
- Prepare a doxycycline stock solution of 1 mg/mL in deionized water. Store the stock solution at -20 °C protected from light. Doxycycline, a tetracycline derivative, is used instead of tetracycline because it has a longer half-life (2x) than tetracycline. Moreover, a lower concentration of doxycycline is required for the complete inactivation of the tet operon23.
NOTE: Doxycycline could affect the mRNA expression of the endogenous GOI. To verify this, the effect of a 24 h treatment with doxycycline on alveolar cells should be tested to confirm the absence of any changes in GOI expression (Figure 2). - Prepare a fresh 1 µg/mL doxycycline solution in complete MEM 72 h posttransfection and warm it to 37 °C.
- Replace the medium with 1 mL of complete MEM containing 1 µg/mL doxycycline per well to inhibit the transcription of the GOI.
- Incubate multiple wells at 37 °C in 5% CO2 for different amounts of time from 15 min-6 h to assess the mRNA half-life of the GOI.
- At the end of the treatment, wash the cells with ice-cold PBS and lyse them with a commercially available phenol-chloroform RNA extraction kit by adding 500 µL of buffer per well and shaking the plate to homogenize the cells.
- Isolate the RNA according to the manufacturer's protocol. Determine the RNA yield and purity by spectrophotometry at 230, 260, and 280 nm. RNA samples with 260:230 and 260:280 ratios of 1.8 and 2.0, respectively, are considered pure.
NOTE: The protocol can be paused here.
4. Determining the mRNA stability of the GOI
- Treat 1 µg of total RNA with RNAse-free DNAse I (amplification grade) to remove any trace of plasmid DNA that could interfere with subsequent DNA amplification.
- In a 0.2 mL PCR tube, combine 1 µg of total RNA, 1 µL of 10x DNase I reaction buffer, 1 µL of DNAse I (1 U/µL), and RNAse-free water to obtain a total volume of 10 µL.
- Incubate the reaction at RT for 20 min.
- Deactivate DNAse I by adding 1 µL of 25 mM EDTA to the 10 µL reaction mix and incubating the reaction at 70 °C for 10 min.
- Reverse-transcribe the DNA-depleted total RNA into cDNA using a commercially available cDNA synthesis kit with a blend of oligo(dT) and random hexamer primers to improve the reverse transcription efficiency.
- Briefly, add 4 µL of 5x reaction mix, 1 µL of reverse transcriptase, and 4 µL of RNAse-free water to 11 µL of the DNA-depleted total RNA mix to obtain a total reaction volume of 20 µL. Mix the reaction well by pipetting it up and down.
- Incubate the reaction for 5 min at 25 °C, followed by 20 min at 46 °C, and then inactivate the reaction by incubating it at 95 °C for 1 min. Each reaction will yield 50 ng/µL of cDNA product.
- Dilute the cDNA reaction to a concentration of 5 ng/µL by adding 180 µL of molecular biology-grade water to the 20 µL reaction mix. Store the cDNA products at -80 °C or proceed immediately to performing real-time quantitative PCR (qPCR).
NOTE: The protocol can be paused here.
- Design forward and reverse qPCR primers specific to the GOI.
- Due to the endogenous expression of the GOI in the cells, the primers must be designed to amplify a 100-150 bp amplicon of the V5 epitope coupled to the GOI (Table 1).
- Internal reference gene primers must also be used as normalization controls. Usually, housekeeping genes, such as the beta-actin and hypoxanthine phosphoribosyltransferase 1 genes, are used as reference genes. However, these cannot be used with this induction system due to the variation of the transfection efficiency. Instead, the expression of the tTA-Ad transcript is assessed for the purposes of normalization, because its expression is constitutive in cells due to the activity of the cytomegalovirus promoter. Any variation in its expression measured by qPCR will be representative of the transfection efficiency (primers: forward 5'-GCC TGA CGA CAA GGA AAC TC-3' and reverse 5'-AGT GGG TAT GAT GCC TGT CC-3; 129 bp amplicon) and will allow the normalization of the expression of the transfected clones (Figure 3).
- Prepare each qPCR reaction in triplicate using a SYBR Green dye master mix.
- Dilute the 5 ng/µL cDNA mix to a concentration of 1.25 ng/µL using molecular biology-grade water.
- Combine 5 µL of SYBR Green dye master mix (2x), 0.1 µL of molecular biology-grade water, 0.45 µL of 7.5 µM forward primer, 0.45 µL of 7.5 µM reverse primer, and 4 µL of 1.25 ng/µL cDNA to obtain a total reaction volume of 10 µL. Mix well by pipetting up and down in a 96 well PCR plate. Use optical adhesive film to ensure that the plate cover is sealed and to prevent contamination and evaporation.
- Spin down the reaction mix briefly by centrifugation and place the plate in a qPCR thermocycler.
- Amplify the V5-tagged GOI and tTA-Ad amplicons by using the following qPCR conditions: 95 °C for 10 min as a denaturation step, followed by 40 cycles of 95 °C for 10 s, 58 °C for 15 s, and 72 °C for 20 s. A high-resolution melting curve must be generated after the amplification cycles are performed to assess the specific melting temperatures of the desired amplicons and to ensure the absence of noise amplicon peaks.
- Include negative controls for the qRT-PCR by performing qPCR with RNA as a template without reverse transcriptase to serve as a control for potential plasmid DNA contamination and qPCR with no cDNA added to the qPCR mix to ensure the lack of primer dimers or contaminants.
- The optimal cDNA concentration, primer efficiency, and concentration must be optimized according to the GOI by a standard curve assay. To do so, perform a serial dilution using cDNA from untreated cells (cultured without doxycycline). The standard curve is generated by plotting the Cq values against the log of the cDNA dilution factor, and the amplification efficiency (E) is calculated according to the slope of the standard curve using the following formula:
E = 10-1/slope
NOTE: The amplification efficiency should be approximately 0.9 to 1.05. Otherwise, the primers must be redesigned.
- Analyze the qPCR data using the comparative Cq method by normalizing the expression values of the GOI to the expression of tTA-Ad to obtain the relative expression levels of the GOI and to report its expression as a percentage of the mRNA expression of the GOI in cells from the same animal at the starting point (t = 0) (Figure 4).
- The half-life is determined from the rate constant (K) of the GOI mRNA degradation curve using the following equation:
t1/2 = ln 2/K.
Representative Results
This protocol was successfully used to generate a Tet-Off transcriptionally controlled plasmid expression system to evaluate the importance of different portions of the αENaC 3' UTR in the modulation of transcript stability in primary alveolar epithelial cells.
The first step in the implementation of this system was to establish a fast, easy, and efficient transfection technique for alveolar epithelial cells in primary culture, which are difficult to transfect. As shown in Figure 1, the pipette electroporation technique allowed for a 25-30% transfection efficiency rate, as shown by the ratios of eGFP cells detected by fluorescence microscopy and flow cytometry.
Before applying this induction system, which is controlled by tetracycline and its derivatives, the impact of this drug on the expression of our GOI was verified. The alveolar epithelial cells were treated with 1.0 µg/mL doxycycline to assess its impact on endogenous αENaC mRNA expression. Treatments carried out over a period of 1-24 h had no significant impact on the expression of the endogenous transcript, as shown in Figure 2.
Our transcriptionally controlled plasmid expression system uses a qPCR normalization technique that differs from the standard technique that uses housekeeping genes. In the case of the expression of V5-αENaC, the signal was normalized according to the tTA-Adv signal to determine the efficiency of transfection using the Cq method. Figure 3 shows the use of the tTA-Adv transcript to normalize mRNA expression.
Previously published results suggest that the use of actinomycin D is inappropriate for the estimation of αENaC transcript stability, as it indicated an abnormally high mRNA half-life (approximately 12 h)24. The half-life of αENaC mRNA in the presence of the Tet-Off system (99 min) was up to 7x shorter than that found in the presence of actinomycin D, as shown in Figure 4. This confirms that actinomycin D leads to an artifactual αENaC mRNA stabilization.
This Tet-Off system was also used successfully to test whether different cell stressors known to affect αENaC gene expression could modulate αENaC mRNA stability. As shown in Figure 5, cycloheximide treatment significantly decreased the stability (36 min) of the transcript, while lipopolysaccharides (LPS, used to mimic an infectious stimuli) did not. Finally, the pro-inflammatory cytokine TNF-α caused a drastic drop in V5-αENaC mRNA stability (with a half-life of 16 min).
In the present work, the cloning strategy was intended to elucidate the contribution of the 3' UTR to the modulation of αENaC transcript. Several sequential deletion mutants were generated and tested to map the contribution of different domains of the αENaC 3' UTR to transcript stability. Figure 6 shows the significant changes in the modulation of the stability of V5-αENaC mRNA depending on the deleted and included regions of the 3' UTR.
Finally, the modulation of the stability of αENaC mRNA by RNA-binding proteins (RBP) was studied with this model. The transcription of αENaC mRNA with the V5 epitope from the pTRE-Tight vector is under the exclusive control of tTA-Adv. Dhx36, Tial1, and hnRNPK are three RBPs that are linked to the 3' UTR of αENaC24. Therefore, any modulation of the expression of V5-αENaC following cotransfection with Dhx36, Tial1, or hnRNPK, would be the consequence of the modulation of the mRNA stability and not of transcription modulation. Figure 7 shows that the overexpression of Dhx36 or Tial1, unlike that of hnRNPK, decreased the stability of V5-αENaC mRNA compared to transfection with the empty pcDNA3 plasmid, which shows the specific modulation of some RBPs.
Collectively, these results confirmed that the Tet-Off transcriptionally controlled plasmid expression system that we developed represents an appropriate tool for evaluating the actual half-life of a transcript and the mechanisms involved in its modulation. This tool will be useful in acquiring novel insights into the posttranscriptional regulation of key GOIs involved in the function of the alveolar epithelium in physiological and pathological conditions.
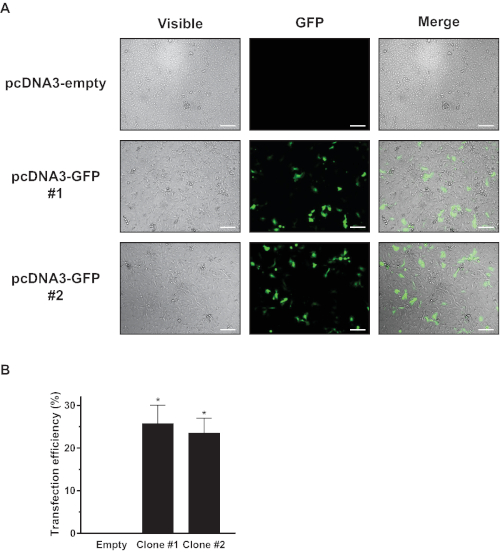
Figure 1: Efficiency of the transfection of alveolar epithelial cells in primary culture by pipette electroporation. Primary alveolar epithelial cells were transiently transfected with 2 µg of a pcDNA3 plasmid (empty, clones #1 and #2) that expressed or did not express GFP protein. Transfection efficiency was assessed 48 h following transfection by (A) fluorescence microscopy or (B) flow cytometry. One-way ANOVA and Bonferroni post hoc test; *p < 0.001 vs. empty. Cells from at least four different rats (n ≥ 4) were used for each experimental condition. Scale bar = 200 µm. Please click here to view a larger version of this figure.
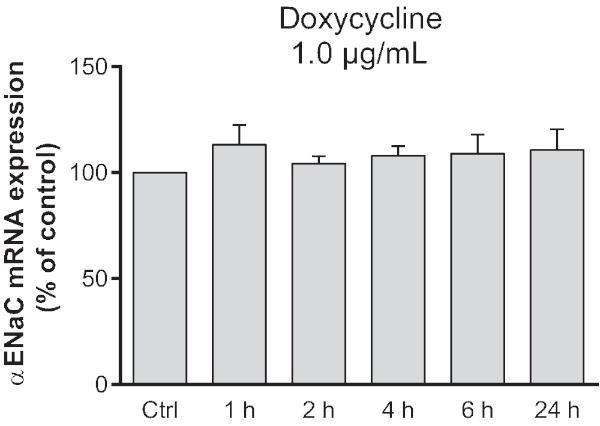
Figure 2: Modulation of endogenous αENaC mRNA by doxycycline in alveolar epithelial cells. Alveolar epithelial cells were treated with 1.0 µg/mL doxycycline for a period of 1-24 h. The expression of αENaC mRNA was quantified by quantitative RT-PCR and presented as the expression of αENaC mRNA ± SEM compared to that in untreated cells (Ctrl; t = 0) after normalization according to β-actin expression (one-way ANOVA, n = 4). Doxycycline did not modulate endogenous αENaC mRNA over time. Previously published as Figure S4 in Migneault et al.24. Please click here to view a larger version of this figure.
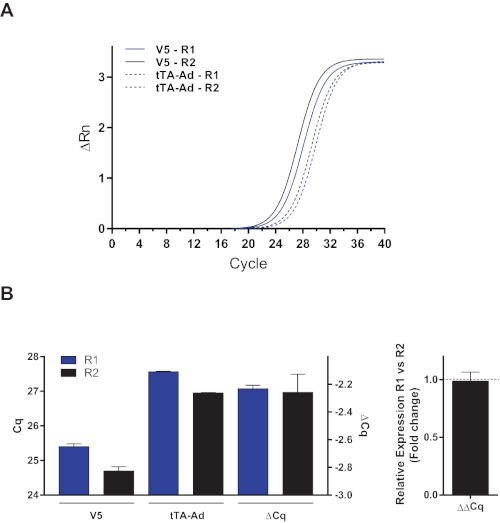
Figure 3: Normalization of GOI mRNA expression with a modified Cq method according to the expression of the regulatory mRNA tTA-Ad. Alveolar epithelial cells were transiently cotransfected with the pTet-Off plasmid and the pTRE-Tight plasmid encoding αENaC cDNA bearing a V5 epitope upstream of its open reading frame and a complete 3' UTR sequence. The expression of V5-αENaC and tTA-Ad mRNAs was measured by quantitative RT-PCR in untreated cells. (A) The delta-normalized SYBR Green fluorescent signals (ΔRn) of V5-αENaC and tTA-Ad from two separate transfections (R1 and R2) are depicted. (B) Left graph: mRNA expression of V5-αENaC and tTA-Ad in each experiment (R1 and R2) are expressed as the cycle quantification value (Cq). The difference between V5-αENaC and tTA-Ad mRNA expression (ΔCq) is presented. Right graph: Using tTA-Ad mRNA in place of the housekeeping gene allows the efficient normalization of the expression of the GOI, which showed a relative expression of 0.98 in R1 compared to that in R2. Please click here to view a larger version of this figure.
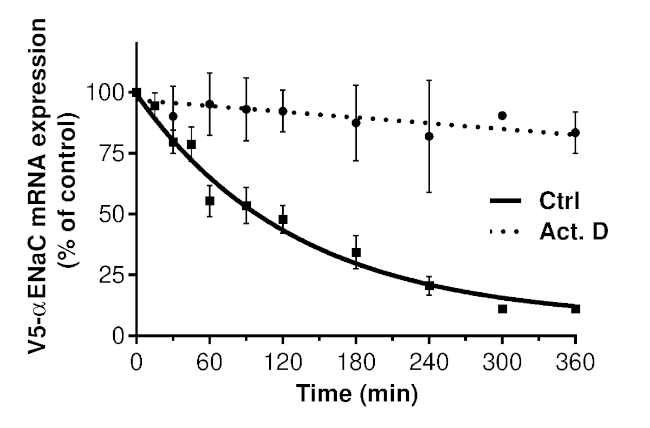
Figure 4: Degradation kinetics of V5-αENaC mRNA when using the transcriptionally controlled plasmid expression system in the presence and absence of actinomycin D. Primary alveolar epithelial cells were transiently cotransfected with the pTet-Off plasmid and the pTRE-tight plasmid encoding αENaC cDNA bearing a V5 epitope upstream of its open reading frame and complete 3' UTR sequences. Cells pretreated or untreated with actinomycin D (5.0 µg/mL) for 30 min were incubated thereafter with doxycycline (1.0 µg/mL) for 15 min-6 h. Expression of V5-αENaC mRNA was measured by quantitative RT-PCR and presented as the percentage ± SEM of V5-αENaC mRNA expression in untreated cells (t = 0) after normalization according to the expression of tTA-Ad. The V5-αENaC mRNA half-life was estimated by one-phase decay nonlinear regression for each cell preparation. Multiple regression analysis revealed a statistically significant difference in V5-αENaC mRNA stability in cells treated with actinomycin D compared with that in untreated cells (p < 0.0001). Cells from at least four different rats (n ≥ 4) were used for each experimental condition. Previously published as Figure 1 in Migneault et al.24. Please click here to view a larger version of this figure.
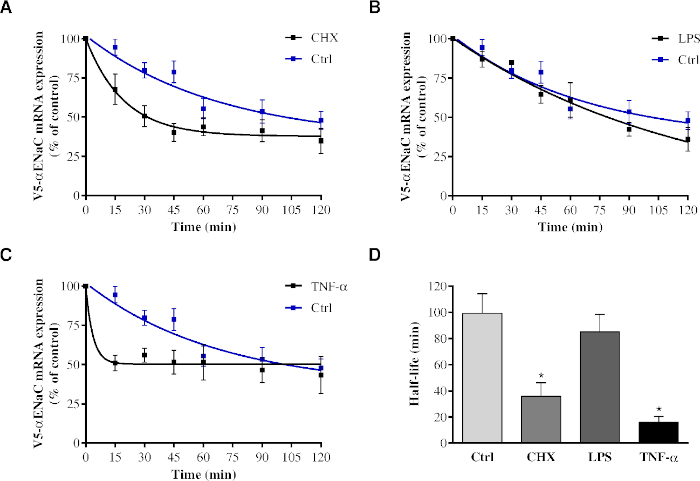
Figure 5: Modulation of V5-αENaC mRNA stability by different cellular and inflammatory stresses. Primary alveolar epithelial cells were transiently cotransfected with the pTet-Off plasmid and the pTRE-tight plasmid encoding αENaC cDNA bearing a V5 epitope upstream of its open reading frame and complete 3' UTR sequences. The cells were pretreated for 30 min with 1.0 µM cycloheximide (CHX) (A) or 15 µg/mL LPS (B) or for 5 h with 100 ng/mL TNF-α (C), followed by treatment with 1.0 µg/mL doxycycline for a period of 15-120 min. Expression of V5-αENaC mRNA was measured by quantitative RT-PCR and presented as the percentage ± SEM of V5-αENaC mRNA expression in untreated cells (t = 0) after normalization according to the expression of tTA-Ad. Cells from at least three different rats (n ≥ 3) were used for each experimental condition. (D) The half-life (t1/2) of V5-αENaC mRNA in treated cells was compared to the half-life of mRNA in cells (Ctrl). The half-lives were measured according to the rate constant (K) of the V5-αENaC mRNA degradation curve using the equation t1/2 = ln 2/K and then expressed as min ± SEM (one-way ANOVA test and Bonferroni post hoc test; *p < 0.01 vs. control; n ≥ 3). Adapted from Figure 36 previously published in Migneault, F.25. Please click here to view a larger version of this figure.
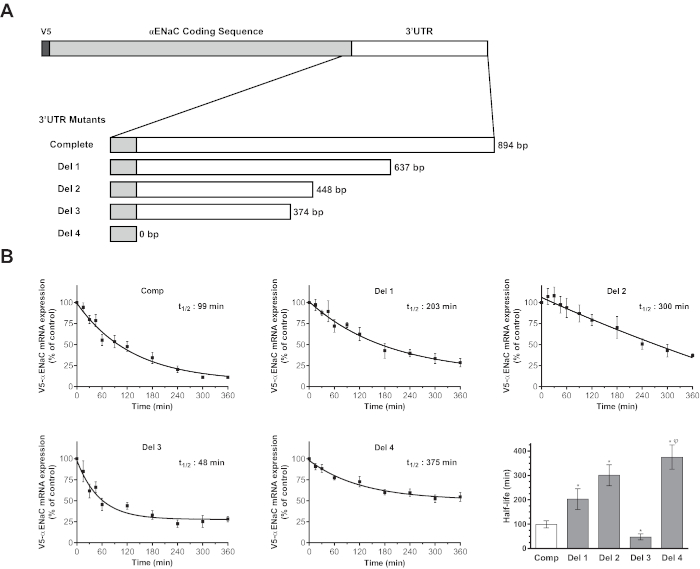
Figure 6: Use of the sequential deletion cloning strategy to reveal the role of different regions of the αENaC 3' UTR in the modulation of mRNA stability. (A) Schematic map of the V5-αENaC transcript with the complete 3' UTR inserted in the pTRE-tight expression vector. The open reading frame is depicted as a gray box, while the 3' UTR is shown as a white box. The 3' UTR portion of αENaC mRNA is depicted for the clone bearing a complete 3' UTR and for the different deletion mutants (Del 1 to Del 4). (B) Primary alveolar epithelial cells were transiently cotransfected with pTRE-tight plasmids encoding different αENaC 3' UTR deletion mutants along with the pTet-Off plasmid, which expresses tTA-Ad, to allow the specific expression of the construct and its inhibition by doxycycline. Seventy-two h after transfection, cells were treated with doxycycline (1.0 µg/mL) for 15 min to 6 h. Expression of V5-αENaC mRNA was measured by quantitative RT-PCR and presented as the percentage ± SEM of V5-αENaC mRNA expression in untreated cells (t = 0) after normalization according to the expression of tTA-Ad. The V5-αENaC mRNA half-life for each construct was estimated from the rate constant (K) of the V5-αENaC mRNA degradation curve using the following equation: t1/2 = ln 2/K. The V5-αENaC mRNA decay is shown for the clone with the complete 3' UTR (Comp) and for the Del 1 to Del 4 3' UTR deletion mutants. The half-life (t1/2) of mRNA decay is given for each clone. In the lower right quadrant, the graph shows a comparison of the different t1/2 ± SEM values estimated for each clone. p < 0.05 between the different groups according to the Kruskal-Wallis test. *p < 0.05 according to Dunn's post hoc test compared to the complete 3' UTR. Φp < 0.05 according to Dunn's post hoc test compared to Del 3. Cells from at least five different animals (n ≥ 5) were used for each experimental condition. Adapted from Figures 2 and 3 previously published in Migneault et al.24. Please click here to view a larger version of this figure.
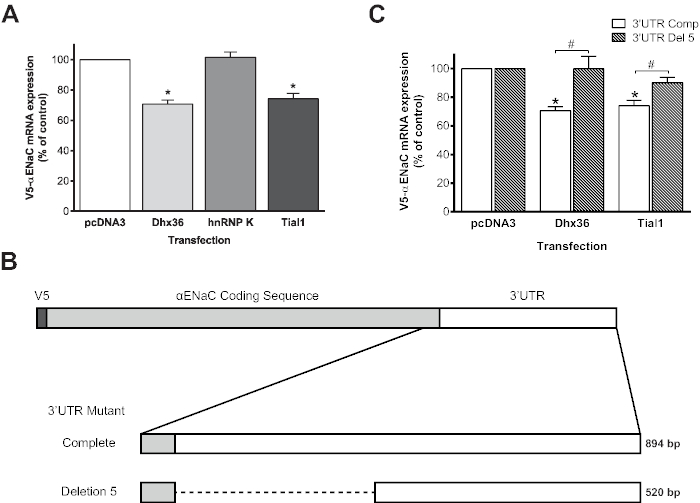
Figure 7: Posttranscriptional modulation of V5-αENaC mRNA by the RNA-binding proteins hnRNPK, Dhx36, and Tial1. (A) Primary alveolar epithelial cells were cotransfected with the pTRE-tight plasmid encoding V5-αENaC mRNA along with an expression vector for the Dhx36, hnRNPK, or Tial1 RBPs and the pTet-Off plasmid. V5-αENaC mRNA expression was quantified by RT-qPCR 72 h posttransfection and expressed as the percentage ± SEM of V5-αENaC mRNA expression compared to that in cells transfected with an empty vector (pcDNA3) after normalization according to the expression tTA-Ad. Overexpression of Dhx36 and Tial1 significantly inhibited V5-αENaC mRNA expression, whereas overexpression of hnRNPK had no effect. *p < 0.05 according to the Kruskal-Wallis test and Dunn's post hoc test compared to empty vector; n ≥ 3 samples from different animals were tested in duplicate for each experimental condition. (B) The proximal portion of the αENaC 3' UTR was deleted by cloning the distal region of the 3' UTR next to the αENaC stop codon in the pTRE-tight plasmid (V5-αENaC-Del5). (C) Primary alveolar epithelial cells were cotransfected with V5-αENaC or V5-αENaC-Del5 in the pTRE-tight vector along with the pTet-Off plasmid and the expression vector for Dhx36 or Tial1 RBP overexpression. V5-αENaC mRNA expression was quantified by RT-qPCR 72 h posttransfection and expressed as the percentage ± SEM of V5-αENaC mRNA expression compared with that in cells transfected with an empty vector (pcDNA3) after normalization according to the expression of tTA-Ad. Overexpression of Dhx36 and Tial1 had no effect on V5-αENaC-Del5 mRNA expression. *p < 0.05 according to the Kruskal-Wallis test and Dunn's post hoc tests upon comparison of the experimental vectors to the empty vector; #p < 0.05 according to the Mann-Whitney U-test upon comparison of the experimental vectors to the complete 3' UTR mutant; n ≥ 6 for each experimental condition. Adapted from Figures 5 and 7 previously published in Migneault et al.24. Please click here to view a larger version of this figure.
| A | |||
| Template | 3' UTR | Sense | Sequence |
| αENaC cDNA | Complete | F | 5'-ATCGCAGCTAGCACCATGGGTG GTAAGCCTATCCCTAACCCT CTC-3' |
| R | 5'-GCACTAATCGATTTTATTGAGTAC CTGCCTACCCGTC-3' |
||
| Del 1 | R | 5'- GCACTAATCGATTTTATTTGTTCT GAGGGACAGTGAAAG-3' |
|
| Del 2 | R | 5'- GCACTAATCGATTTTATTAACTAA CAAGGGGGCTTTTGGG-3' |
|
| Del 3 | R | 5'-GCACTAATCGATTTTATTGTGTCC CTGAAGGCAGTGAGGC-3' |
|
| Del 4 | R | 5'-GCACTAATCGATTTTATTTCAGAG CGCCGCCAGGGCACAG-3' |
|
| Del 5 | R | 5'-ATCGCAGAATTCTCAGAGCGCC GCCAGGGCACAG-3' |
|
| F | 5'-ATCGCAGAATTCTGATGTCTGCT CCTCTCCTTG-3' |
||
| B | |||
| Target | Sense | Sequence | |
| V5-αENaC | F | 5'-CCTAACCCTCTCCTCGGTCT-3' | |
| R | 5'-TTGAATTGGTTGCCCTTCAT-3' | ||
| tTA-Adv | F | 5'-GCCTGACGACAAGGAAACTC-3' | |
| R | 5'-AGTGGGTATGATGCCTGTCC-3' | ||
Table 1: Primers used in this study. (A) Primers used for cloning and generation of the V5-αENaC mutants in the inducible pTRE-tight vector. (B) Primers used for the measurement of mRNA expression by quantitative RT-PCR.
Discussion
The low transfection rate of alveolar epithelial cells in primary culture has been a serious limitation for the use of the Tet-Off system to assess mRNA stability in these cells. However, this limitation was overcome by pipette electroporation, allowing a 25-30% transfection efficiency (Figure 1 and Figure 3)26.
The measurement of transcript stability is fundamental to understanding the modulation of a given mRNA and its impact on cell homeostasis and metabolism. Variation in the bioavailability of a GOI may change the translation efficiency and have a direct impact on expression of the GOI in the cell. For these reasons, several techniques have been developed to determine the half-life of mRNAs. As discussed above, each of these techniques has limitations and constraints that prevent the proper study of a transcript of interest. Compared to other techniques, the TET-off model developed here has the advantage of allowing the half-life estimation of a given mRNA under any experimental condition. However, it does not allow us to directly study the stability of an endogenous transcript. To overcome this limitation as much as possible, it is suggested to include the 5' UTR and 3' UTR sequences of the transcript in the plasmid so that the construct is as similar as possible to the endogenous transcripts. The addition of the V5 epitope allows the specific amplification of the target mRNA by RT-qPCR versus that of the endogenous transcript. Regarding the cloning strategy, the choice of the sequence inserted upstream of the gene of interest is crucial to prevent the amplification of nonspecific signals. The addition of the V5 epitope sequence 5' of the ORF is necessary for the specific qPCR amplification of the construct due to its short length and the lack of its expression in alveolar epithelial cells. This strategy also provides the opportunity to study the modulation of protein translation using the same inducible system. Despite the small size of the V5 epitope (42 nt), it is still possible that this may affect the stability of transcription. For this reason, it is suggested to also test other epitopes, such as human influenza hemagglutinin (HA) or any other sequence not found in the transcriptome of alveolar epithelial cells.
One limitation of the system is the use of doxycycline to inhibit the transcription of a GOI. Several reports show that doxycycline could have a significant impact on cell metabolism. The use of this antibiotic in 16HBE14 bronchial cells reduces proliferation and increases mortality due to apoptosis27. In addition, doxycycline has already been shown to be involved in the modulation of the LPS inflammatory response28. Therefore, this antibiotic could have off-target effects that may affect the mRNA stability of a given GOI. For these reasons, we assessed the impact of 1 µg/mL doxycycline over a 24 h period on nontransfected cells and found that it did not induce any change in endogenous αENaC mRNA expression (Figure 2). This concentration was chosen because it is widely used to completely inhibit the transcription of the Tet-Off system and is recommended to minimize cytotoxic effects29. We suggest using this concentration in alveolar epithelial cells and recommend validating the optimal concentration for the study of other genes or the use of other cell types with the Tet-Off system.
Our system utilizes the constitutive expression of the GOI until its transcription is inhibited by doxycycline. It has been suggested that excessive mRNA concentrations may be toxic to the cell and affect its metabolism. This could lead to a change in the stability of the GOI transcript, causing its rapid degradation because of nonphysiological overexpression30. In the case of our Tet-Off model, such toxicity was not observed, because the transfected cells showed a morphology similar to those of healthy nontransfected cells (data not shown). In accordance with Tani et al.31, who demonstrated the validity of the Tet-Off system in determining the half-life of a mRNA, our results showed that the Tet-Off system is not toxic in alveolar epithelial cells and yields a half-life for αENaC mRNA that does not reflect the overstabilization that was reported in the presence of transcriptional inhibitors such as actinomycin D (Figure 4).
The development of this system to measure the half-life of mRNA challenges the results observed when the stability of transcripts were measured in the presence of transcription inhibitors such as actinomycin D. This method shows that a transcript half-life may be much shorter than previously measured.
Our model is highly appropriate for studying the posttranscriptional modulation of a specific transcript under different conditions, including pro-inflammatory conditions or RBP overexpression, as well as after treatment with silencing RNA. In addition, it allows the characterization of untranslated regions essential to the posttranscriptional modulation of mRNAs in pathophysiological conditions that affect alveolar epithelial cells.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Francis Migneault was supported by a fellowship provided by the Quebec Respiratory Health Network and the Canadian Institutes of Health Research (CIHR) lung training program, a studentship from FRSQ and a studentship from the Faculté des Études Supérieures et Postdoctorales, Université de Montréal. This work was supported by the Gosselin-Lamarre Chair in clinical research and the Canadian Institutes of Health Research [YBMOP-79544].
Materials
| Actinomycin D | Sigma-Aldrich | A9415 | |
| Ampicillin | Sigma-Aldrich | A1593 | |
| Bright-LineHemacytometer | Sigma-Aldrich | Z359629 | |
| Chloroform – Molecular biology grade | Sigma-Aldrich | C2432 | |
| ClaI | New England Biolabs | R0197S | |
| Cycloheximide | Sigma-Aldrich | C7698 | |
| DM IL LED Inverted Microscope with Phase Contrast | Leica | – | |
| DNase I, Amplification Grade | Invitrogen | 18068015 | |
| Doxycycline hyclate | Sigma-Aldrich | D9891-1G | |
| Dulbecco’s Phosphate-buffered Saline (D-PBS), without calcium and magnesium | Wisent Bioproducts | 311-425-CL | |
| Ethanol – Molecular biology grade | Fisher Scientific | BP2818100 | |
| Excella E25 ConsoleIncubatorShaker | Eppendorf | 1220G76 | |
| Glycerol | Sigma-Aldrich | G5516 | |
| HEPES pH 7.3 | Sigma-Aldrich | H3784 | |
| Heracell 240i | ThermoFisher Scientific | 51026420 | |
| iScript cDNA Synthesis Kit | Bio-Rad Laboratories | 1708890 | |
| Isopropanol – Molecular biology grade | Sigma-Aldrich | I9516 | |
| LB Broth (Lennox) | Sigma-Aldrich | L3022 | |
| LB Broth with agar (Lennox) | Sigma-Aldrich | L2897 | |
| L-glutamine | Sigma-Aldrich | G7513 | |
| Lipopolysaccharides fromPseudomonas aeruginosa10 | Sigma-Aldrich | L9143 | |
| MEM, powder | Gibco | 61100103 | |
| MicroAmp Optical 96-Well Reaction Plate | Applied Biosystems | N8010560 | |
| MicroAmp Optical Adhesive Film | Applied Biosystems | 4360954 | |
| MSC-Advantage Class II Biological Safety Cabinets | ThermoFisher Scientific | 51025413 | |
| Mupid-exU electrophoresis system | Takara Bio | AD140 | |
| NanoDrop 2000c | ThermoFisher Scientific | ND-2000 | |
| Neon Transfection System 10 µL Kit | Invitrogen | MPK1025 | |
| Neon Transfection System Starter Pack | Invitrogen | MPK5000S | |
| NheI | New England Biolabs | R0131S | |
| One Shot OmniMAX 2 T1RChemically CompetentE. coli | Invitrogen | C854003 | |
| pcDNA3 vector | ThermoFisher Scientific | V790-20 | |
| pcDNA3-EGFP plasmid | Addgene | 13031 | |
| PlatinumTaqDNA Polymerase High Fidelity | Invitrogen | 11304011 | |
| pTet-Off Advanced vector | Takara Bio | 631070 | |
| pTRE-Tight vector | Takara Bio | 631059 | |
| Purified alveolar epithelial cells | n.a. | n.a. | |
| QIAEX II Gel Extraction Kit | QIAGEN | 20021 | |
| QIAGEN Plasmid Maxi Kit | QIAGEN | 12162 | |
| QIAprep Spin Miniprep Kit | QIAGEN | 27104 | |
| QuantStudio 6 and 7 Flex Real-Time PCR System Software | Applied Biosystems | n.a. | |
| QuantStudio 6 Flex Real-Time PCR System, 96-well Fast | Applied Biosystems | 4485697 | |
| Recombinant Rat TNF-alpha Protein | R&D Systems | 510-RT-010 | |
| Septra | Sigma-Aldrich | A2487 | |
| Shrimp Alkaline Phosphatase (rSAP) | New England Biolabs | M0371S | |
| Sodium bicarbonate | Sigma-Aldrich | S5761 | |
| SsoAdvanced Universal SYBR Green Supermix | Bio-Rad Laboratories | 1725270 | |
| SuperScript IV Reverse Transcriptase | Invitrogen | 18090010 | |
| T4 DNA Ligase | ThermoFisher Scientific | EL0011 | |
| Tet System Approved FBS | Takara Bio | 631367 | |
| Tobramycin | Sigma-Aldrich | T4014 | |
| TRIzol Reagent | Invitrogen | 15596018 | |
| Trypsin-EDTA (0.05%), phenol red | Gibco | 25300054 | |
| UltraPure Agarose | Invitrogen | 16500500 | |
| Water, Molecular biology Grade | Wisent Bioproducts | 809-115-EL |
References
- Munchel, S. E., Shultzaberger, R. K., Takizawa, N., Weis, K. Dynamic profiling of mRNA turnover reveals gene-specific and system-wide regulation of mRNA decay. Molecular Biology of the Cell. 22 (15), 2787-2795 (2011).
- Ljungman, M. The transcription stress response. Cell Cycle. 6 (18), 2252-2257 (2007).
- Ross, J. mRNA stability in mammalian cells. Microbiological Reviews. 59 (3), 423-450 (1995).
- Gossen, M., Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proceedings of the National Academy of Sciences. 89 (12), 5547-5551 (1992).
- Meyer, D. J., Stephenson, E. W., Johnson, L., Cochran, B. H., Schwartz, J. The serum response element can mediate induction of c-fos by growth hormone. Proceedings of the National Academy of Sciences. 90 (14), 6721-6725 (1993).
- Harkin, D. P., et al. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 97 (5), 575-586 (1999).
- Formisano, L., et al. The two isoforms of the Na+/Ca2+ exchanger, NCX1 and NCX3, constitute novel additional targets for the prosurvival action of Akt/protein kinase B pathway. Molecular Pharmacology. 73 (3), 727-737 (2008).
- Yin, D. X., Schimke, R. T. BCL-2 expression delays drug-induced apoptosis but does not increase clonogenic survival after drug treatment in HeLa cells. 암 연구학. 55 (21), 4922-4928 (1995).
- Johnstone, R. W., et al. Functional analysis of the leukemia protein ELL: evidence for a role in the regulation of cell growth and survival. Molecular and Cellular Biology. 21 (5), 1672-1681 (2001).
- Olotu, C., et al. Streptococcus pneumoniae inhibits purinergic signaling and promotes purinergic receptor P2Y2 internalization in alveolar epithelial cells. Journal of Biological Chemistry. 294 (34), 12795-12806 (2019).
- Goldmann, T., et al. Human alveolar epithelial cells type II are capable of TGFbeta-dependent epithelial-mesenchymal-transition and collagen-synthesis. Respiratory Research. 19 (1), 138 (2018).
- Huang, C., et al. Ghrelin ameliorates the human alveolar epithelial A549 cell apoptosis induced by lipopolysaccharide. Biochemical and Biophysical Research Communications. 474 (1), 83-90 (2016).
- Gao, R., et al. Emodin suppresses TGF-beta1-induced epithelial-mesenchymal transition in alveolar epithelial cells through Notch signaling pathway. Toxicology and Applied Pharmacology. 318, 1-7 (2017).
- Cooper, J. R., et al. Long Term Culture of the A549 Cancer Cell Line Promotes Multilamellar Body Formation and Differentiation towards an Alveolar Type II Pneumocyte Phenotype. PLoS One. 11 (10), e0164438 (2016).
- Hirakata, Y., et al. Monolayer culture systems with respiratory epithelial cells for evaluation of bacterial invasiveness. Tohoku Journal of Experimental Medicine. 220 (1), 15-19 (2010).
- Grzybowska, E. A., Wilczynska, A., Siedlecki, J. A. Regulatory functions of 3’UTRs. Biochemical and Biophysical Research Communications. 288 (2), 291-295 (2001).
- Chen, C. Y., Chen, S. T., Juan, H. F., Huang, H. C. Lengthening of 3’UTR increases with morphological complexity in animal evolution. Bioinformatics. 28 (24), 3178-3181 (2012).
- Eaton, D. C., Helms, M. N., Koval, M., Bao, H. F., Jain, L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annual Review of Physiology. 71, 403-423 (2009).
- Boncoeur, E., et al. Modulation of epithelial sodium channel activity by lipopolysaccharide in alveolar type II cells: involvement of purinergic signaling. American Journal of Physiology-Lung Cellular and Molecular Physiology. 298 (3), L417-L426 (2010).
- Gonzalez, R. F., Dobbs, L. G. Isolation and culture of alveolar epithelial Type I and Type II cells from rat lungs. Methods in Molecular Biology. 945, 145-159 (2013).
- Kozak, M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. Journal of Molecular Biology. 196 (4), 947-950 (1987).
- Ke, S. H., Madison, E. L. Rapid and efficient site-directed mutagenesis by single-tube ‘megaprimer’ PCR method. Nucleic Acids Research. 25 (16), 3371-3372 (1997).
- Gossen, M., Bujard, H., Nelson, M., Hillen, W., Greenwald, R. A. . Tetracyclines in Biology, Chemistry and Medicine. , (2001).
- Migneault, F., et al. Post-Transcriptional Modulation of aENaC mRNA in Alveolar Epithelial Cells: Involvement of its 3′ Untranslated Region. Cellular Physiology and Biochemistry. 52 (5), 984-1002 (2019).
- Migneault, F. . Modulation de la stabilité de l’ARNm alphaENaC dans les cellules épithéliales alvéolaires: détermination du rôle des séquences 3′ non traduites. , (2015).
- Grzesik, B. A., et al. Efficient gene delivery to primary alveolar epithelial cells by nucleofection. American Journal of Physiology-Lung Cellular and Molecular Physiology. 305 (11), L786-L794 (2013).
- Sourdeval, M., Lemaire, C., Brenner, C., Boisvieux-Ulrich, E., Marano, F. Mechanisms of doxycycline-induced cytotoxicity on human bronchial epithelial cells. Frontiers in Bioscience. 11, 3036-3048 (2006).
- Moon, A., Gil, S., Gill, S. E., Chen, P., Matute-Bello, G. Doxycycline impairs neutrophil migration to the airspaces of the lung in mice exposed to intratracheal lipopolysaccharide. Journal of Inflammation-London. 9 (1), 31 (2012).
- Hovel, H., Frieling, K. H. The use of doxycycline, mezlocillin and clotrimazole in cell culture media as contamination prophylaxis. Developments in Biological Standardization. 66, 23-28 (1987).
- Houseley, J., Tollervey, D. The many pathways of RNA degradation. Cell. 136 (4), 763-776 (2009).
- Tani, H., et al. Genome-wide determination of RNA stability reveals hundreds of short-lived noncoding transcripts in mammals. Genome Research. 22 (5), 947-956 (2012).

