The Organotypic Hippocampal Slice Culture Model for Examining Neuronal Injury
Summary
The organoptypic hippocampal slice culture model is an in vitro model used to examine neuronal injury in a variety of paradigms. In this article, we describe the methods for generating slice cultures and quantifying neuronal injury.
Abstract
Organotypic hippocampal slice culture is an in vitro method to examine mechanisms of neuronal injury in which the basic architecture and composition of the hippocampus is relatively preserved 1. The organotypic culture system allows for the examination of neuronal, astrocytic and microglial effects, but as an ex vivo preparation, does not address effects of blood flow, or recruitment of peripheral inflammatory cells. To that end, this culture method is frequently used to examine excitotoxic and hypoxic injury to pyramidal neurons of the hippocampus, but has also been used to examine the inflammatory response. Herein we describe the methods for generating hippocampal slice cultures from postnatal rodent brain, administering toxic stimuli to induce neuronal injury, and assaying and quantifying hippocampal neuronal death.
Protocol
1. Preparation
Before starting, assemble the required surgical instruments, dissection and culture media, and 7 day old rat or mouse pups. The protocol is the same for mouse and rat preparations.
(i) Materials and equipment
- Operating scissors (straight length 6″, cat# RS6818, Roboz, Gaithersburg, MD)
- Micro dissecting scissors, (length 4′”, cat# RS5910. Roboz, Gaithersburg, MD)
- Carbon Steel Dumont Tweezers (cat# RS-5045.Roboz, Gaithersburg, MD)
- Modified glass “transfer” pipettes (bottom of Pasteur pipette removed and edge smoothed)
- Aclar plastic film cut to 2″in x 2″ in squares (Honeywell, # 812071, Pottsville, PA)
- Cover sponges 4 in by 3 in ( Kendall , cat# 3157,Mansfield, MA)
- 30 mm Millicell membrane inserts (0.4 μm; Millipore, Bedford, MA)
- Mcllwain Tissue chopper with double-edged razor blade (McILwain tissue chopper, Vibratome Company, St. Louis, MO)
- Inverted microscope with CCD camera and image analysis software
(ii) Dissection Medium (DM; 1 liter at pH 7.3)
| Hank’s Balanced Salt | 1 vial ( 95.2 g/1L) ( Sigma : H2387 ) |
| NHCO3 ( 4.2 mM) | 350 mg |
| HEPES (10 mM) | 2.83 g |
| Glucose ( 33.3 mM) | 6.0 g |
| Penicillin/streptomycin (100x) | 10 mL |
| BSA (0.3%) | 300 mg |
| MgSO4-7H2O | 1.44 g |
(iii) Culture medium (400 mL)
| MEM (Invitrogen 11575-032) | 200 mL |
| ***Hank’s Balance Salt(Invitrogen 24020-117) | 100 mL |
| Horse serum (Invitrogen 26050-070) | 100 mL |
| HEPES buffer (1 M; Invitrogen 15630-080) | 5 mL |
| Penicillin/Streptomycin (100x) | 4 mL |
| ***Add 12.8 g glucose to 500 mL Hank’s Balance Salt before making culture medium | |
(iv) Animals
7 day old rat or mouse postnatal pups, generally one litter per experiment.
2. On the Day of Culture
- All dissection tools and Aclar film must be soaked in 70% ETOH for 30 minutes before beginning. The surface of the dissection hood, blade, and tissue chopper must be disinfected with ethanol.
- Prepare several (3-4) 6 well tissue culture plates; add 1 mL culture medium per well, and insert a Millipore permeable membrane in each well. Place 6-well plates in the 37° incubator until ready to transfer slices.
- Prepare several (4-6) 60 mm plates with DM and place on ice.
- Rapidly decapitate with scissors and dip in 70% ETOH, and quickly expose the brain with a saggital incision of the skull. Carefully remove the brain and place immediately in cold DM. Separate it into two hemispheres and place each hemisphere medial side down on a cover sponge for 2-3 seconds.
- Gently lift and place the hemisphere onto the Aclar film square and place on the tissue chopper; cut the brain coronally in 350 μm sections. When finished, gently take the Alcar square with the sectioned hemisphere and submerge in cold DM.
- Using a dissecting microscope, separate out the hippocampal sections. For rat, there should be 5 sections spanning rostral to caudal hippocampus, and for mouse there should be 3-4 sections.
- Take out the 6-well plates with membrane inserts from the 37° tissue culture incubator. Transfer hippocampal slices individually onto the permeable membranes using the glass transfer pipette. If too much DM is released onto the membrane, remove excess DM with a Pasteur pipette. Place five hippocampal slices on each membrane. Allow at least 3 wells per condition, or 15 slices.
- Incubate the culture plates at 37° in a 5% CO humidified incubator for 12-14 days before beginning the toxicity experiment. The culture medium can be changed twice a week.
3. Inducing and Quantifying Hippocampal Neuronal Injury in Slice Culture
- After 12-14 days in culture, add 5 μg/mL propidium iodide (PI) to the culture media. This step will allow visualization and quantification of basal cell death.
- The following day, quantify PI fluorescence in the CA1 subregion (and/or CA3 or dentate if desired) of each hippocampal slice and obtain mean the PI fluorescence measurements representing basal cell death (referred to as time=0 hours, or T0). For image acquisition and quantification, we use OpenLab software (Improvision, U.K.), but other image acquisition and analysis software can be used to quantify fluorescence intensity.
- Immediately after image acquisition of T0 PI fluorescence, induce neuronal injury by your method of choice (we have used 10 μM NMDA, oxygen-glucose deprivation, or LPS for one hour to induce neuronal toxicity 2,5). After the toxic stimulus, replace medium with fresh medium containing 5 μg/mL PI and maintain slices overnight.
- On day 3, quantify mean PI fluorescence of the experimental condition at 24h, or T24.
- After this image acquisition, immediately change medium and add 10 μM NMDA and 5 μg/mL PI and incubate slices overnight to achieve maximal neuronal death.
- On day 4, quantify mean PI fluorescence of maximal neuronal death, defined as Tmax. Calculate percent cell death as follows: (T24 – T0) / (Tmax-T0) .
4. Representative Results
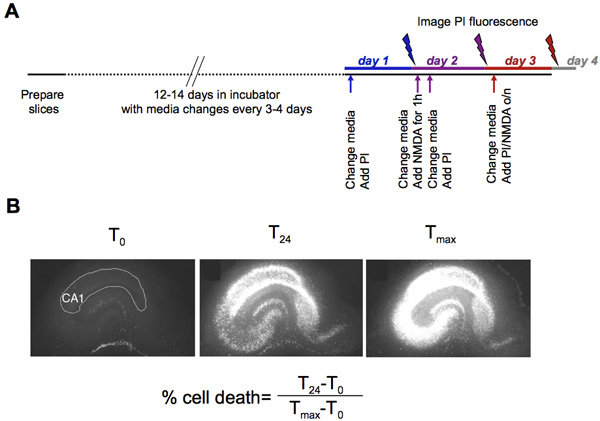
Figure 1. (A) Time line for generation of hippocampal and subsequent experimental injury and image acquisition. (B) Representative images of hippocampal slices basally (T0), 24 hours following a 1 hour exposure to 10 μM NMDA (T24), and after a further 24h or 10 μM NMDA to obtain maximal neuronal injury (Tmax).
Discussion
There are two important points to follow to ensure reproducible and consistent results when replicating experiments. First, it is important to let the hippocampal slices age for 12-14 days before experimentation. With slice isolation, there is a significant microglial response, and microglia remain activated for some time, with a return to a resting ramified state occurring by day 10 in vitro 3,6. Secondly, mean PI fluorescence intensity is proportional to the number of injured cells 4. Sequential fluorescence is measured at three time points: (i) at T0, before NMDA or OGD stimulation to measure basal levels of spontaneous neuronal death; (ii) at T24, or 24 hours after stimulation with NMDA or OGD or other stimulus, and (iii) at Tmax, following a final treatment of slices with a 24 hour overnight lethal treatment with 10 μM NMDA which produces the maximal amount of neuronal death. The percent cell death is then calculated using the formula (T24 – T0) / (Tmax-T0). It is critical to normalize each slice to itself using this method because the size of the hippocampus and the glutamate receptor expression and distribution change significantly as one goes from rostral to caudal brain, and a failure to normalize each slice to itself may result in artifactual data.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Funded by NINDS, American Heart Association, American Federation for Aging Research, March of Dimes
References
- Stoppin, L., Buchs, P. A., Muller, D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 37 (2), 173-173 (1991).
- McCullough, L., Wu, L., Haughey, N. Neuroprotective Function of the PGE2 EP2 Receptor in Cerebral Ischemia. J Neurosci. 24 (1), 257-257 (2004).
- Czapiga, M., Colton, C. A. Function of microglia in organotypic slice cultures. J Neurosci Res. 56, 644-644 (1999).
- Newell, D. W., Barth, A., Papermaster, V. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J Neurosci. 15, 7702-7702 (1995).
- Wu, L., Wang, Q., Liang, X. Divergent Effects of prostaglandin recetor signaling on neuronal survival. Neuroscience letter. 421, 253-253 (2007).
- Hailer, N. P., Jarhult, J. D., Nitsch, R., R, . Resting microglial cells in vitro: Analysis of morphology and adhesion molecule expression in organotypic hippocampal slice cultures. Glia. 18, 319-319 (1996).

