膜运输过程由单一的蛋白质解析一个高度并行的纳米孔芯片分析系统
Summary
The presented protocol describes the analysis of membrane protein mediated transport on the single transporter level using pore-spanning solvent-free lipid bilayers. This is achieved by the creation of bulk produced nanopore array chips, combined with highly parallel data acquisition and analysis, enabling the future establishment of membrane protein effector screenings.
Abstract
上的单个蛋白质水平的膜蛋白运输仍然逃避详细的分析,如果易位衬底是非电的。相当大的努力在这一领域已经取得,但技术实现自动化高通量运输分析与膜转运的分析所需的无溶剂型脂质双层技术组合是罕见的。然而,这类转运体是细胞动态平衡至关重要的,因此迫切需要在药物开发和方法,以获得新的见解的主要目标。
在这里介绍稿件描述了一种新的生物芯片的膜蛋白介导的运输过程中的单一转运分辨率分析建立和处理。生物芯片是由通过纳米孔是在它的设计高度并行,可在工业级和数量来制造封闭微腔。蛋白窝藏脂质体可直接被应用到在芯片表面上形成使用SSM的技术自组装孔跨越脂质双层(固体支持的脂质膜)。孔横跨膜的部件是独立的,为基片易位接口进入或离开腔室空间,它可以随后在实时多光谱的荧光读数。标准操作规程(SOP)建立允许简单的建立几乎每一个膜蛋白的芯片表面,可以进行功能上的重组蛋白窝藏脂质双层的。唯一的先决条件是对非电的输送基板建立了荧光读出系统。
高内涵筛选的应用是通过使用自动化倒置荧光显微镜并行记录多个芯片的accomplishable。可以使用免费的定制设计分析软件进行分析大型数据集。三色多光谱荧光读出还允许无偏数据判别成不同的事件类,消除假阳性结果。
芯片技术目前基于SiO 2的表面上,但使用涂覆金的芯片表面进一步官能也是可能的。
Introduction
膜蛋白质的分析已经成为过去20年中增加了对基础和制药研究兴趣。新型药物的开发依赖于识别和新的目标细致的刻画,目前正在限制因素之一。即所有的药物靶标-约60%是膜蛋白1的事实,使得技术的发展,以阐明其功能最重要的。
在过去,电的通道和转运的研究技术已经开发在众多2 – 4。在相反的非电的基材呈现出更为艰巨的任务。然而,它们特别感兴趣作为首要的药物靶标,因为它们控制穿过细胞膜和功能键受体溶质和营养物质的熔剂在信号级联5。
相当大的努力已投入T的发展echniques研究膜转运蛋白6,7的功能。 10,包括固体支持的脂质双层,拴系双层11,12 microblack脂膜13,14和原生泡囊阵列15,16仅举几–使用固体支持膜系统已在本领域8成为最有希望的工具。他们中的一些甚至可以作为商业设置17,18。一些例子已经发表结合研究单膜蛋白以高度平行的方式14,19,为筛选应用的先决条件的能力。然而,这些方法很少从基础研究到工业环境弥合。的困难往往在于该系统是自动化的能力,成本密集的生产和/或费力的制剂。一种方法Òvercoming上述所有障碍是最终目的。
这里介绍的技术的开发,研究膜通道和载体在体外对单个蛋白质水平20受控的环境– 22。 26或黑脂膜27 –净化膜蛋白的重建成的LUV是远远超过了GUVs 23类似的方法建立的。它们可以直接施加到芯片表面,其中双层形式经由自组装过程的发生。纳米多孔芯片( 图 1)的玻璃底设计允许空气显微镜,其允许系统的简单的自动化。在用电动载物台相结合的多个芯片可以在同一时间进行测定,用包含数千个密封腔的用于分析每个视野。
<p class="jove_content" fo:keep-together.within-page="“1”">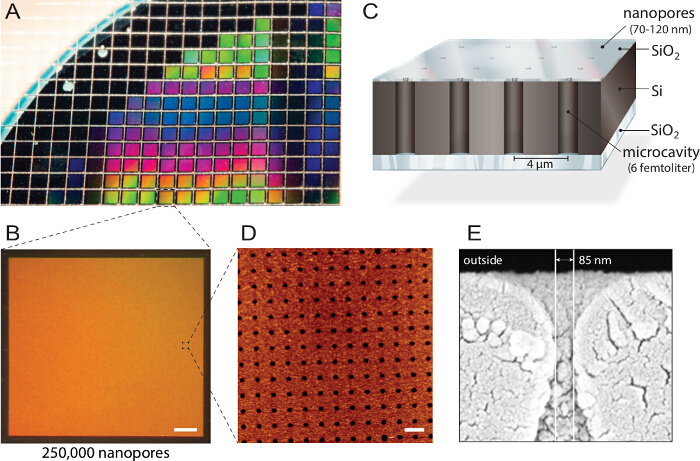
图 1。复纳米孔生物芯片 。A) 设计硅绝缘体(SOI)晶片由反应离子刻蚀结构。大约1150个人的芯片是由具有相同属性和质量。B)每个芯片包含250,000纳米孔径个人微腔每片晶圆制造。比例尺:200微米C)每个腔是通过多光谱荧光读出寻址。一个不透明的顶层块从缓冲储荧光信号,使得生物芯片倒置荧光显微镜。D)的原子力显微镜兼容(AFM)成像揭示均匀排列孔开口和3.6纳米的氧化硅层的表面粗糙度(N = 40)最优的囊泡融合。比例尺:5微米E)扫描电子麦克风roscopy(SEM)图像示出了通过纳米孔允许访问在硅芯片内的飞升腔的横截面。这个数字是从21重复使用许可。 请点击此处查看本图的放大版本。
使用免费软件,以保证最终用户不受限制的访问进行所有的数据分析。时间序列使用免费的图像处理软件和一个自定义生成曲线分析软件,使批量处理和曲线的多种荧光渠道和数千家大型数据集的简单分析相关性。
在这个协议中使用的模型蛋白是大电导(元富)通道蛋白从大肠杆菌产生的机械敏感性通道大肠杆菌 。它用作一个阀以释放在自然界渗透压休克,但在设计合理SYNT这样一种方式被修改hetic官能可共价地附连到信道收缩侧。通过信道被触发以打开共价结合活化剂(MTSET)的电荷斥力,产生的纳米阀。像离子,水,小分子蛋白,但也小的荧光小分子可以通过渠道渗透。这里,该蛋白质作为模型来演示系统来检测蛋白介导的转运的能力。
Protocol
Representative Results
Discussion
这里提出的技术允许膜蛋白转运的一个高度并行分析。重构膜蛋白系统可以直接应用到生物芯片,使得理论上每膜转运的适应或信道成为可能。运输分析仅由建立的荧光读出系统的任通过直接荧光变化(荧光团的易位或荧光标记的底物)或间接荧光变化(pH敏感染料,次级酶反应)的限制。后者,但是还没有建立。
上的单个蛋白质水平膜通道或转运的功能特性是该技术的主要…
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Barbara Windschiegl for her help in establishing SOPs; Dennis Remme for his work on the NanoCalcFX software and Alina Kollmannsperger, Markus Braner and Milan Gerovac for helpful suggestions on the manuscript. The German-Israeli Project Cooperation (DIP) provided by the DFG and the Federal Ministry of Education and Research to R.T., as well as the Federal Ministry of Economics and Technology (ZIM R&D Project) to R.T. and Nanospot GmbH supported this work.
Materials
| Reagent | |||
| [2-(Trimethylammonium)ethyl] methanethiosulfonate |
Toronto Research Chemicals Inc. | T792900 | MTSET; hydrolized by water. Keep as dry pouder aliquot at -80 °C. Use immediately (30 minutes) after solubilization in buffer. |
| 1 ml gas-tight syringe | Hamilton | #1001 | |
| 10 ml round flask | Schott Duran | ||
| 2.7 mm glas beads | Roth | N032.1 | |
| 2-Propanole | Roth | 9866.5 | |
| 30 cm Luer-Lock Extension Tube | Sarstedt | 744304 | |
| Acetone | Roth | 5025.5 | |
| Bio-Beads SM-2 Adsorbent | Bio-Rad | 152-3920 | need to be activated before first use |
| CaCl2 Dihydrat | Roth | HN04.3 | |
| Calcein | Sigma | C0875 | store dark at -20 °C |
| Chloroform reagent grade | VWR Chemicals | 22711324 | |
| DOPEATTO390 | ATTO-TEC | AD 390-165 | store dark at -20 °C |
| Ethanol absolute | Sigma-Aldrich | 32205 | |
| Injekt Single-use syringe | Braun | 460 60 51V | |
| Injekt-F single-use syringe | Braun | 91 66 017V | |
| Keck clips | Schott | KC29 | |
| L-α-Phosphatidylcholine, 20% (Soy) | Avanti Polar Lipids | 5416016 | store under inert gas at -20 °C |
| NaCl 99.5% p.a. | Roth | 3957.2 | |
| Nanopore E100 wafer/chips | Micromotive (Mainz/Germany) | available on request | |
| Nucleopore Track-Etch Membrane 0.4 µm | Whatman | 800282 | |
| Oregon Green Dextran 488 (70 kDa) | life Technologies | D-7173 | store dark at -20 °C |
| Oy647 | Luminartis (Münster/Germany) | OY-647-T-1mg | store dark at -20 °C |
| Rotilabo-syringe filtration, unsterile, pore-size 0.22 µm | Roth | P 818.1 | |
| Sephadex G-50 | Sigma-Aldrich | G5080 | column material for size exclusion chromatography |
| Silastic MDX4-4210 | Dow Corning | curing agent for chip fixation onto cover glass support | |
| sticky-Slide 8-well | ibidi | 80828 | multi-well chamber for the mounting onto glass slides (chip holder) |
| Three-way stopcock blue | Sarstedt | 744410001 | |
| Tris Pufferan 99.9% Ultra Quality | Roth | 5429.2 | |
| Triton-X 100 | Roth | 6683.1 | |
| Whatman 0.2 µm cellulose nitrate membrane filter | Roth | NH69.1 | |
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Büchi 461 water bath | Büchi | ||
| Büchi Rotavapor RE 111 | Büchi | ||
| Cary Eclipse Fluorescence Spectrophtometer | Varian | ||
| LiposoFast Mini Extruder | Avestin | ||
| Membrane pump | Vaccubrand | 15430 | |
| Nanosight Nanoparticle Tracking Microscope | Malvern / Nanosight | LM 14C | |
| NyONE microscope | Synentec | available on request | |
| Pump control | Vaccubrand | CVC 2II | |
| Sonicator bath Sonorex RK100H | Brandelin electronic | 31200001107477 | |
| Vaccum pump RC5 | Vaccubrand | 1805400204 | |
| Water bath W13 | Haake | 002-9910 | |
| Plasma Cleaner PDC-37G | Harrick Plasma | PDC-37G | |
| Name | Company | Catalog Number | Comments |
| Software | |||
| ImageJ | Open Source | http://imagej.nih.gov/ij/ | scientific image processing software |
| NanoCalcFX | Freeware | http://sourceforge.net/projects/nanocalc/ | data analysis/evaluation software for massive transport kinetic datasets |
| NTA 2.3 Analytical Software | Nanosight | data acquisition and analysis software for nanoparticle tracking microscope | |
| NTA 2.3 Temperature Comms | Nanosight | temperature controle software for nanoparticle tracking microscope |
References
- Yildirim, M. A., Goh, K. I., Cusick, M. E., Barabasi, A. L., Vidal, M. Drug-target network. Nat Biotechnol. 25, 1119 (2007).
- Hamill, O. P., Marty, A., Neher, E., Sakmann, B., Sigworth, F. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85-100 (1981).
- Osaki, T., Suzuki, H., Le Pioufle, B., Takeuchi, S. Multichannel simultaneous measurements of single-molecule translocation in α-hemolysin nanopore array. Anal. Chem. 81, 9866-9870 (2009).
- Carrillo, L., et al. High-resolution membrane capacitance measurements for studying endocytosis and exocytosis in yeast. Traffic. , (2015).
- Giacomini, K. M., et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 9, 215-236 (2010).
- Castell, O. K., Berridge, J., Wallace, M. I. Quantification of membrane protein inhibition by optical ion flux in a droplet interface bilayer array. Angew. Chem. Int. Ed. 51, 3134-3138 (2012).
- Zollmann, T., et al. Single liposome analysis of peptide translocation by the ABC transporter TAPL. Proc. Natl. Acad. Sci. U.S.A. 112, 2046-2051 (2015).
- Tamm, L. K., McConnell, H. M. Supported phospholipid bilayers. Biophys. J. 47, 105-113 (1985).
- Sackmann, E. Supported membranes: scientific and practical applications. Science. 271, 43-48 (1996).
- Castellana, E. T., Cremer, P. S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 61, 429-444 (2006).
- Wagner, M. L., Tamm, L. K. Tethered polymer-supported planar lipid bilayers for reconstitution of integral membrane proteins: silane-polyethyleneglycol-lipid as a cushion and covalent linker. Biophys. J. 79, 1400-1414 (2000).
- Naumann, C. A., et al. The polymer-supported phospholipid bilayer: tethering as a new approach to substrate-membrane stabilization. Biomacromolecules. 3, 27-35 (2002).
- Weiskopf, D., Schmitt, E. K., Klühr, M. H., Dertinger, S. K., Steinem, C. Micro-BLMs on highly ordered porous silicon substrates: Rupture process and lateral mobility. Langmuir. 23, 9134-9139 (2007).
- Watanabe, R., et al. Arrayed lipid bilayer chambers allow single-molecule analysis of membrane transporter activity. Nat. Commun. 5, (2014).
- Stamou, D., Duschl, C., Delamarche, E., Vogel, H. Self-Assembled Microarrays of Attoliter Molecular Vessels. Angew. Chem. Int. Ed. 115, 5738-5741 (2003).
- Lohr, C., et al. Single Liposomes Used to Study the Activity of Individual Transporters. Biophysical Journal. 106, 229a (2014).
- Dunlop, J., Bowlby, M., Peri, R., Vasilyev, D., Arias, R. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nat. Rev. Drug Discov. 7, 358-368 (2008).
- Milligan, C. J., et al. Robotic multiwell planar patch-clamp for native and primary mammalian cells. Nat. Protoc. 4, 244-255 (2009).
- Soga, N., Watanabe, R., Noji, H. Attolitre-sized lipid bilayer chamber array for rapid detection of single transporters. Sci. Rep. 5, (2015).
- Kleefen, A., et al. Multiplexed parallel single transport recordings on nanopore arrays. Nano Lett. 10, 5080-5087 (2010).
- Wei, R., Gatterdam, V., Wieneke, R., Tampé, R., Rant, U. Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat. Nanotechnol. 7, 257-263 (2012).
- Urban, M., et al. Highly parallel transport recordings on a membrane-on-nanopore chip at single molecule resolution. Nano Lett. 14, 1674-1680 (2014).
- Kusters, I., Van Oijen, A. M., Driessen, A. J. Membrane-on-a-Chip: Microstructured Silicon/Silicon-Dioxide Chips for High-Throughput Screening of Membrane Transport and Viral Membrane Fusion. ACS Nano. 8, 3380-3392 (2014).
- Hansen, J. S., Thompson, J. R., Hélix-Nielsen, C., Malmstadt, N. Lipid directed intrinsic membrane protein segregation. J. Am. Chem. Soc. 135, 17294-17297 (2013).
- Heinemann, F., Schwille, P. Preparation of Micrometer-Sized Free-Standing Membranes. ChemPhysChem. 12, 2568-2571 (2011).
- Lazzara, T. D., Carnarius, C., Kocun, M., Janshoff, A., Steinem, C. Separating attoliter-sized compartments using fluid pore-spanning lipid bilayers. ACS nano. 5, 6935-6944 (2011).
- Winterhalter, M. Black lipid membranes. Curr. Opin. Colloid Interface Sci. 5, 250-255 (2000).
- Koçer, A., Walko, M., Feringa, B. L. Synthesis and utilization of reversible and irreversible light-activated nanovalves derived from the channel protein MscL. Nat. Protoc. 2, 1426-1437 (2007).

