Sample Preparation Method of Scanning and Transmission Electron Microscope for the Appendages of Woodboring Beetle
Summary
In order to observe ultrastructure of insect sensilla, scanning and transmission electron microscopy (SEM and TEM, respectively) sample preparation protocol were presented in the study. Tween 20 was added into the fixative to avoid sample deformation in SEM. Fluorescence microscopy was helpful for improving slicing accuracy in TEM.
Abstract
This report described sample preparation methods that scanning and transmission electron microscope observations, demonstrated by preparing appendages of the woodboring beetle, Chlorophorus caragana Xie & Wang (2012), for both types of electron microscopy. The scanning electron microscopy (SEM) sample preparation protocol was based on sample chemical fixation, dehydration in a series of ethanol baths, drying, and sputter-coating. By adding Tween 20 (Polyoxyethylene sorbitan laurate) to the fixative and the wash solution, the insect body surface of woodboring beetle was washed more cleanly in SEM. This study's transmission electron microscopy (TEM) sample preparation involved a series of steps including fixation, ethanol dehydration, embedding in resin, positioning using fluorescence microscopy, sectioning, and staining. Fixative with Tween 20 enabled penetrate the insect body wall of woodboring beetle more easily than it would had been without Tween 20, and subsequently better fixed tissues and organs in the body, thus yielded clear transmission electron microscope observations of insect sensilla ultrastructures. The next step of this preparation was determining the positions of insect sensilla in the sample embedded in the resin block by using fluorescence microscopy to increase the precision of target sensilla positioning. This improved slicing accuracy.
Introduction
Scanning electron microscopy is an important tool in many morphology studies, that SEM shows surface structures1,2. Transmission electron microscopy's appeal is that it can be used to study a wide range of biological structures at the nanometer scale, from the architecture of cells and the ultrastructure of organelles, to the structure of macromolecular complexes and proteins. TEM shows inner structures3,4,5.
Coleoptera is the largest group of insects, including about 182 families and 350,000 species. Most of the coleopteran insects, in particular woodboring beetle, feed on plants, many of which are important pests of forests and fruit trees, causing devastating damage to trees6. At present, prevention and control population of pests based on chemical ecology theory have received increasing attention7. Efficient, low-toxic, pollution-free pheromone control methods have become an effective way8. Studying the sensilla morphology and ultrastructure of insects is an important part of insect chemical ecology research. The scanning and transmission electron microscopy (SEM and TEM, respectively) are used to great effect to study their morphology and internal anatomy. However, during preparation of insect samples for electron microscopy (EM), the objectivity and authenticity of the observation site may be affected9. In general, SEM sample preparation of insects requires cleaning, tissue fixation, dehydration, metathesis, drying, and sputter-coating10. Due to the complex environment in which woodboring beetle live, the body surface often has various pollutants and their appendages often have many fine long sensilla or bristles. In particular, some woodborers are not available from laboratory raising, which collected directly in the field, and then put into fixing fluid to ensure freshness and subsequently washed in the laboratory. If the sample is first fixed and then washed, obviously it is much more difficult to remove debris because glutaraldehyde strongly fixes it to the sample. Tween 20 is a surfactant11,12,13,14, which plays an important role in the washing process, including reducing the surface tension of water and improving the wettability of water on the surface of the laundry. In this study, Tween 20 was added to the fixing solution and PBS cleaning solution to reduce the surface tension of the liquid, and prevent the dirt from depositing on the body surface of the woodboring beetle, which made the body surface cleaner in SEM.
Using TEM, sensilla on different organs of insects can be sliced to reveal the clear structures inside them, thus providing a basis for analyzing sensilla functions. When the subject insect, such as woodboring beetle, is large, and its body wall has a substantial degree of sclerotization, so the fixative may not fully saturate organ tissues inside the insect body. Tween 20 can enhance the dispersion and suspension capacity of the dirt. In this study, Tween 20 was added to the fixative to enhance fixative fluid penetration into the insect body wall of woodboring beetle, avoiding deformation and collapse of the epidermi11,12,13. In addition, using general slicing technology, it is difficult to accurately locate different types of sensilla, in particular for some small sensilla15. Based on traditional TEM sample preparation, this study combined fluorescence microscopy and SEM to determine the position of insect sensilla in the embedded block, thus improving slicing accuracy.
Protocol
CAUTION: Consult the material safety data sheets of reagents before using them. Several of the chemicals used during sample preparation are toxic, mutagenic, carcinogenic, and/or reprotoxic. Use personal protective equipment (gloves, lab coat, full-length pants, and closed-toe shoes) and work under a fume hood while handling the sample.
1. SEM Sample Preparation and Imaging
- Sample Fixation and Cleaning
- Working in an area where C. caragana occur, attract adults into field traps baited with plant attractants, such as isophorone16. Preserve clean bodies of adult C. caragana in 0.1 mol L-1 phosphate-buffered saline (PBS, pH 7.2), 2.5% (wt/vol) glutaraldehyde (Anhydrous EM Grad), and 0.06% (vol/vol) Tween 20. Fix the sample at 4 °C over the weekend.
- Remove the bodies from the preservation liquid and rinse in phosphate buffer. Using an stereomicroscope, remove the appendages and clean them ultrasonically (40 kHz) in a 0.1 mol L-1 phosphate-buffered saline (pH 7.2) with 0.06% (vol/vol) Tween 20 (PBST). After cleaning for 100 s, transfer the sample to the microscope to check if it was clean. Under normal circumstances, clean for 400s to ensure that the sample was clean enough to observe and not damaged.
- Sample Dehydration, Mounting and Drying
- Dehydrate the samples by using 20 min successive treatments in 50%, 60%, 70%, 80%, 85%, 90%, 95%, 100%, and 100% (all vol/vol) ethanol. Under a stereomicroscope, use carbon double-sided adhesive tape to separately fix 3 observation surfaces (dorsal ventral and lateral) onto stubs. Note that all viewing surfaces must be kept clean and free of contamination. Place the sample stage in a petri dish containing a silica gel desiccant for 48 h.
- Sputter-coat and Sample Insertion
- Using Hitachi Koki (E-1010) ion sputtering instrument, rotate MAIN VALVE to OPEN position, remove the sample chamber cover, and put the sample into chamber. Put the POWER switch on, and READY light was on. Set Sputtering time as 45 seconds, and coating thickness as 70.875 Å. Once mechanical pump vacuum dial index dropped below 7, press DISCHARGE and start spraying platinum. At the end of experiment, turn off the power supply and take the sample out of chamber. Spray film thickness: d = KIVt ("d" is the thickness of the film in the unit of " Å "; "K" is a constant, depending on the sputtered metal and gas. For example, K of air is 0.07; "I" is the unit mA of plasma flow; "V" is the voltage applied in the unit of "KV". "t" is time in seconds.
- Insert the stub containing the sample onto the stage of SEM. Make sure the sample stage with the sample stub had enough height to allow a good image. Open the SEM software and select a desired operating voltage, beginning at 20 kV.
2. TEM Sample Preparation and Imaging
- Obtain and fix the sample as in steps 1.1.1 and 1.1.2.
- Cleaning, Secondary Fixation and Dehydration
- Remove adult C. caragana from the preservation liquid. Using a stereomicroscope, remove the appendages, wash the samples in PBST for 3 h, and then post-fix them in 1% (wt/vol) osmium tetroxide in PBS for 1h at 25 °C. Dehydrate the samples by using 20 min successive treatments in 50%, 60%, 70%, 80%, 85%, 90%, 95%, 100%, and 100% (all vol/vol) ethanol at room temperature.
- Resin Embedding and Polymerization
- Embed the samples in resin in a flat embedding moulds. The sample was at the bottom of the plate and was placed as close as possible to the edge of the recessed groove. Place the label in the blank then incubate the plate containing the sample at 60 °C for 72 h. Remove the capsule from the incubator and verify that the resin had polymerized.
- Sample Sectioning and Staining
- Once ensure the sample had been solidified, place each resin block under a fluorescence microscope and photograph them under blue light. Move the microscope's fluorescent light source so it irradiated the sample from above. Enable the sensilla in the resin block to be clearly observed. Photographed and measure distances to target the sensilla (Figure 1).
- Refer to SEM image of the palps (Figure 2A), and cut roughly the resin block with a razor blade to close the target receptor (Figure 2B).
- Next, using blue-light fluorescence microscopy, photograph the roughly cut resin block, adjusting the light source from above so that the sensilla was observed clearly. Green light excited by the blue light created a favorable observation. When imaging, the objective micrometer (DIV 0.01mm) was added to the fluorescence microscope stage, and then the distance of the target was measured by ImageJ software (U.S. National Institute of Health) (Figure 2C). The image ruler was made by Adobe Photoshop CS5 (Adobe Systems, Inc., San Jose, CA, USA). Then, for ultramicrotome slicing, set the cutting distance, using 50-60 nm slice thicknesses, until the target position was reached. Use fluorescence microscopy to pinpoint the target receptor.
- Mount the sections on Formvar-coated, 100-mesh copper grids, double-stained with uranyl acetate and lead citrate.
- Firstly, add 3.75 g uranyl acetate to 50 mL of 50 % methanol. Stain grids with a filtered (0.45 µm) syringe of a saturated solution of uranyl acetate at room temperature for 10 min. Cover sections during staining to block light induced precipitates. Rinse 2x in 50 % methanol; 2x filtered degassed water.
- Secondly, add 0.02 g lead citrate to 10 mL of degassed distilled water in centrifuge tube. Add 0.1 mL of 10 N sodium hydroxide, seal and shake to dissolve. Stain grids with a solution of lead citrate for 8 min. Centrifuge before use. Staining must be done in a carbon dioxide free environment to prevent the formation of lead carbonate precipitates. Place drops of stain on squares of plastic petri dishes. Rinse in degassed filtered water and dry17. Observe them via TEM operating at 80 kV.
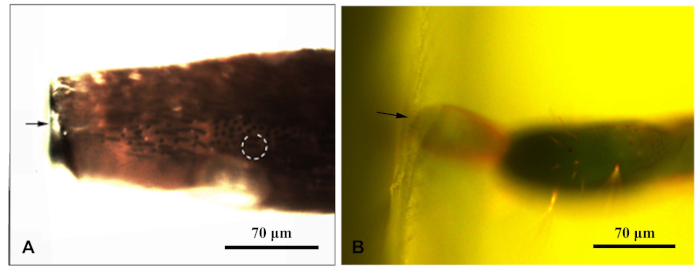
Figure 1: A fluorescent microscope photographed a resin block enclosing the appendage of the Chlorophorus caragana. (A) Antenna resin block; (B) Resin block at the end of the ovipositor. The arrow indicated the edge of the resin block; dotted circle indicates the target sensilla. Please click here to view a larger version of this figure.
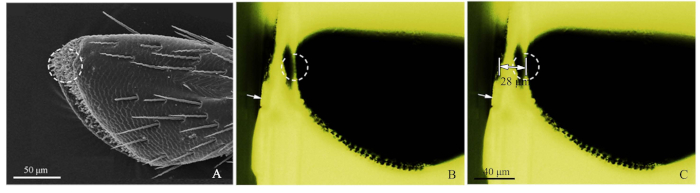
Figure 2: Procedures of the precise sensilla location method. (A) The 4th sub-segment of a maxillary palp of Chlorophorus caragana, the dotted circle showed the sensilla targeted by SEM. (B) The 4th sub-segment of a maxillary palp of C. caragana viewed by fluorescence microscopy. White arrow showed the roughly cut edge of the resin block and the dotted circle showed the accurate location. (C) The marked distance from the edge of the resin block to the maxillary palp target location (28 µm in this sample). Please click here to view a larger version of this figure.
Representative Results
Using cleaning and fixative solution with Tween 20, cleaner SEM image was observed than that without Tween 20 (Figure 3). Tween 20 fixing solution penetrated the glutaraldehyde fixing solution into the tissue. Microtubule structure was clearly seen. TEM image of the internal structure of the sample was blurred without Tween 20 (Figure 4).
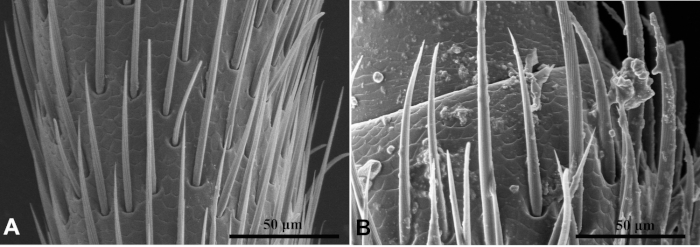
Figure 3: The sensilla locating on antenna of Chlorophorus caragana under SEM. Comparison of SEM image with Tween 20 (A) and without Tween 20 (B), which showed that picture A is cleaner than picture B in general. Please click here to view a larger version of this figure.
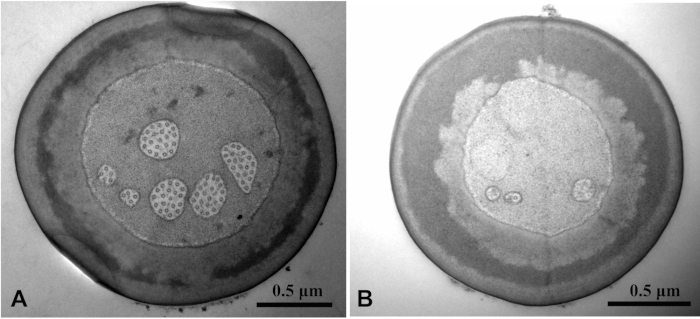
Figure 4: Chlorophorus caragana viewed by transmission electron microscopy of sensilla twig basiconica on the labial palps. Comparison of TEM image with Tween 20 (A) and without Tween 20 (B). Microtubule structure of picture A is clear, while that of picture B is blurred. Please click here to view a larger version of this figure.
We used SEM to study the types and ultrastructures of sensilla on the palps of C. caragana, finding 4 types of sensilla including 10 sub-types: 1 Böhm's bristles (BB.), 3 sensilla chaetica (Ch.1-Ch.3), 1 digitiform sensilla (Dig.), and 5 sensilla twig basiconica (S.tb.1-S.tb.5) (Table 1). Sensilla identification and ultrastructure was based on their morphology and size18,19,20,21,22,23. Our sample preparation methods rendered clear images of the surfaces and internal ultrastructures of insect sensilla.
| Number | Type | Length (μm)a | Diameter at base (μm)a | Wall | Tip | Socket | Cuticular pores | Distribution |
| 1 | BB. | 5.18 ± 1.25 | 1.70 ± 0.47 | Smooth | Sharp | Wide | No | maxillary palp, labial palp |
| 2 | Ch.1 | 38.59 ± 8.20 | 3.15 ± 0.84 | Grooved | Sharp | Wide | No | maxillary palp, labial palp |
| 3 | Ch.2 | 81.54 ± 18.07 | 3.75 ± 0.88 | Grooved | Sharp | Wide | No | maxillary palp, labial palp |
| 4 | Ch.3 | 282.06 ± 22.60 | 6.10 ± 0.70 | Grooved | Sharp | Wide | No | labial palp |
| 5 | Dig. | 24.77 ± 2.98 | 1.24 ± 0.32 | Smooth | Blunt | Wide | No | maxillary palp |
| 6 | S.tb.1 | 6.51 ± 1.01 | 2.31 ± 0.25 | Grooved | With protrusions | Raised and tight | Tip pore | maxillary palp, labial palp |
| 7 | S.tb.2 | 5.91 ± 0.90 | 2.24 ± 0.30 | Smooth | Blunt | Raised and tight | Tip pore | maxillary palp, labial palp |
| 8 | S.tb.3 | 6.84 ± 0.98 | 1.96 ± 0.35 | Smooth | With protrusions | Raised and tight | Tip pore | maxillary palp, labial palp |
| 9 | S.tb.4 | 2.21 ± 0.59 | 2.86 ± 0.46 | Grooved | With protrusions | Raised | Tip pore | maxillary palp, labial palp |
| 10 | S.tb.5 | 1.16 ± 0.29 | 1.05 ± 0.19 | Smooth | Blunt | Raised and wide | Tip pore | maxillary palp, labial palp |
Table 1: Types of sensilla on the palps of C. caragana.
To examine the ultrastructure inside the sensilla on C. caragana palps, we used TEM. One example of these studies was continuous cross-sectional views of the peg of the S.tb.1 on maxillary palps. The views showed that the dendritic sheath surrounded the outer dendritic segments and extended until the tip pore (Figure 5A-D). Seven unbranched outer dendritic segments existed inside the inner receptor lymph cavity, which was surrounded by an outer cavity (Figure 5D). The tubular body was separated by a dendritic sheath from other outer dendritic segments at each sensillar socket base (Figure 5E). In the ciliary region, we noted 8 dendrites of different diameters, indicating the presence of 8 bipolar neurons. Finally, the ciliary segment contained 9 peripheral microtubule doublets (Figure 5F).
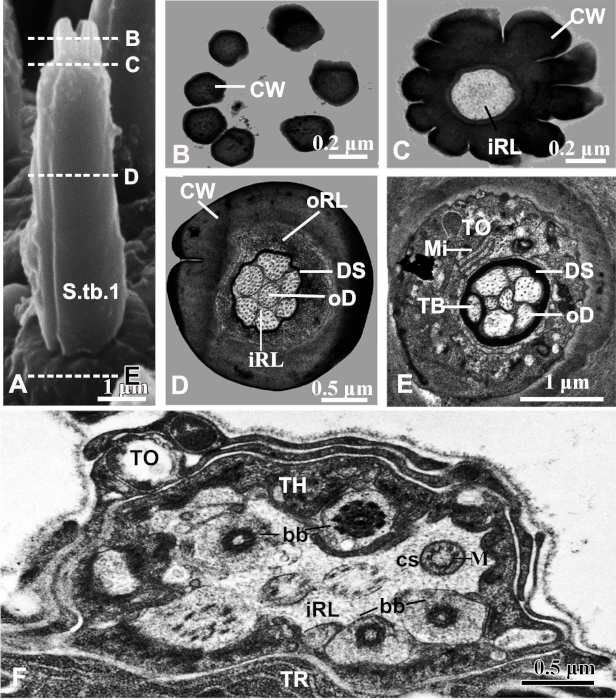
Figure 5: TEM views of sensilla type 1 twig basiconica (S.tb.1) on a Chlorophorus caragana maxillary palp30. (A) S.tb.1 with dotted lines marking regions close to the cross-sections taken for Figs. B-E. (B) Cross-section of finger-shaped protrusions showing scattered cuticula. (C) Cross-section of the basal region of finger-shaped protrusions showing inner receptor lymph cavities without outer dendritic segments. (D) Cross-section of the middle region of the peg showing the dendritic sheath dividing the sensillum-lymph cavity into both inner and outer cavities with 7 outer dendritic segments in the inner cavity. (E) Basal region of the peg showing the tubular body surrounded by a dendritic sheath and separated from outer dendritic segments. A tormogen cell forms the outside of the dendritic sheath. (F) Cross-section of the ciliary region showing 8 dendrites of different diameters. Abbreviations: bb, basal body; cs, ciliary segment; CW, cuticular wall; DS, dendritic sheath; iRL, inner receptor lymph cavity; M, microtubule; Mi, microvilli; oD, outer dendritic segment; oRL, outer receptor lymph cavity; S.tb.1, type 1 sensilla twig basiconica; TB, tubular body; TH, thecogen cell; TO, tormogen cell; TR, trichogen cell. Please click here to view a larger version of this figure.
Discussion
In this article, we presented a sample preparation scheme for scanning and transmission electron microscopy for woodboring beetle. Using insect appendage as a representative study subject, we demonstrated several improvements over traditional sample preparation methods.
The liquid oil detached from the solid surface is emulsified into small droplets, which can be well dispersed and suspended in the washing medium to reduce redepositing on the surface of the object. Washing performance of surfactant includes all the basic characteristics such as wettability, permeability, emulsifying property, dispersibility, solubilization11,12,13,14. Effects of different detergents on the electron microscopic samples preparation of Golden Nematode showed that Tween 20 had the best cleaning effect, followed by sodium bicarbonate, and distilled water24. In this study, we found that Tween 20 can be used to reduce the surface tension of the liquid, and prevent the dirt from depositing on the body surface of the insect, in particular for woodboring beetle collected directly in the field. Insect body surface was washed more cleanly in SEM. Fixative with Tween 20 penetrated the insect body wall more easily, and subsequently better fixed tissues and organs in the body in TEM. The advantage of surfactant in electron microscopy sample preparation has been extensively studied24,25,26,27,28,29,30,31.
Also, we adopted a modified air-drying method for SEM sample preparation, in which the dehydrated sample was placed in a petri dish containing a silica gel desiccant that gradually evaporates the dehydrating agent. The biggest advantage of this method is that it is simple, easily maintained, and it keeps the microenvironment air dry and no special equipment required. The natural drying method is a simple, practical and effective method for seed, nut and long-term preservation of insect specimens. Although the sample volume shrinks during the natural drying process, the basic morphology of the sample is retained32. In general, Coleoptera insects have relatively low water content, and their surface is surrounded by hard chitin walls. Air-dry is able to meet requirements. However, this drying method is not suitable for the drying of tissues with a large water content, such as louse, mites, and larvae, because the surface tension will deform the sample during the drying process.
In order to observe and calculate the type and number of sensilla distributed across the surface of the appendage, dorsal, ventral and lateral of the appendage must be considered. Some sensilla were few, small, and sometimes covered, scan and observe carefully from all angles to find those sensilla fully protruding the epidermis or arising from the depression. Since many sensilla were relatively long and hairlike, the tip effect can be significant. So, the electron microscope acceleration voltage must not be too high, 5-20kV was best and we used 20kV.
In the TEM sample embedding, the sample had better close to the edge of the flat embedding moulds groovein order to save time when roughing the resin block. The traditional TEM method forcontinuously cutting the resin is extensive, and it is usually blindly cut using an optical microscope17,33. To improve on this, we first explored an insect sensilla localization technique in resin-embedded blocks by using fluorescence microscopy to view and measure the target distance to the cut. Compared with the traditional TEM trimming method, this technology can save sample preparation time and more accurately locate the target sensor. In the absence of measurement software, a scaled ruler can be placed in the field of view to roughly measure the target distance. The combination of an ultramicrotome with a fluorescence microscope provides clear observations of the cutting process, yielding accurate cuts of target sensilla and other appropriate subjects.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We appreciate the generous assistance of the Beijing Vocational College of Agriculture, the Institute for the Application of Atomic Energy (Chinese Academy of Agricultural Science), the Bioresearch Center of Beijing Forestry University and Professor Shan-gan Zhang of the Institute of Zoology, Chinese Academy of Sciences. This research was supported by National Key R&D Program of China (2017YFD0600103), the National Natural Science Foundation of China (Grant No. 31570643, 81774015), Forest Scientific Research in the Public Welfare of China (201504304), Inner Mongolia Agricultural University High-level Talent Research Startup Plan (203206038), and Inner Mongolia Autonomous Region Higher Education Research Project (NJZZ18047), Inner Mongolia Autonomous Region Linxue "Double First-class" Construction Project (170001).
Materials
| Anatomical lens | Chongqing Auto Optical limited liability company |
1425277 | |
| Carbon adhesive tape | SPI Supplies, Division of Structure Probes, Inc. | 7311 | |
| Carbon tetrachloride | Sigma | 56-23-5 | |
| Copper grids | GilderGrids | G300 | |
| Disodium hydrogen phosphate | Sinopharm group chemical reagent co., LTD | 10039-32-4 | |
| Ethanol | J.T. Baker | 64-17-5 | |
| Flat embedding molds | Hyde Venture (Beijing) Biotechnology Co., Ltd. | 70900 | |
| Fluorescence microscope | LEICA | DM2500 | |
| Glutaraldehyde | Sigma-Aldrich | 111-30-8 | Anhydrous EM Grade |
| Isophorone | Sigma | 78-59-1 | |
| Lead citrate | Sigma | 512-26-5 | |
| Methanol | Sigma | 67-56-1 | |
| Monobasic sodium phosphate | Its group chemical reagent co., LTD | 7558-80-7 | |
| Objective micrometer | Olympus | 0-001-034 | |
| Osmium tetroxide | Sigma | 541-09-3 | |
| Petri dish | Aldrich | 1998 | |
| Razor blade | Gillette | ||
| Resin | Spurr | ERL4221 | |
| Scalpel | Lianhui | GB/T19001-2008 | |
| SEM | Hitachi | S-3400 | |
| Silica gel desiccant | Suzhou Longhui Desiccant Co., Ltd. | 112926-00-8 | |
| Small brush | Martol | G1220 | |
| Sodium hydroxide | Sigma | 1310-73-2 | |
| Sputter ion instrument | Hitachi Koki Co. Ltd., Tokyo, Japan | E-1010 | |
| Stereo microscope | Leica | EZ4 HD | |
| TEM | Hitachi | H-7500 | |
| Tween 20 | Tianjin Damao Chemical Reagent | 9005-64-5 | |
| Ultramicrotome | Leica | UC6 | |
| Ultrasonic cleaner | GT Sonic | GT-X1 | |
| Uranyl acetate | Sigma | 6159-44-0 |
References
- Song, Y. Q., Dong, J. F., Sun, H. Z. Scanning Electron Microscope Technology of Insect Material. Hubei Agricultural Sciences. 52, 1064-1065 (2013).
- Liu, C. The development of the scanning electron microscopy (sem) and its application in polymer materials research. Journal of the Graduates Sun Yat-Sen University (Natural Sciences Medicine). 34, 7-12 (2008).
- Gan, L., Jensen, G. J. Electron tomography of cells. Quarterly Reviews of Biophysics. 45, 27-56 (2011).
- Lucic, V., Rigort, A., Baumeister, W. Cryo-electron tomography: the challenge of doing structural biology in situ. The Journal of Cell Biology. 202, 407-419 (2013).
- Trepout, S., Bastin, P., Marco, S. Preparation and Observation of Thick Biological Samples by Scanning Transmission Electron Tomography. Journal of Visualized Experiments. (121), e55215 (2017).
- Zhang, X. J., Sun, W., Zhang, J., Zuo, T. T., Wang, Z. Q., Zhao, H. W. Research progress of coleopteran insect species antennal sensilla. Journal of Anhui Agricultural Sciences. 41, 2932-2935 (2013).
- Aldrich, J. R., Bartelt, R. J., Dickens, J. C., Knight, A. L., Light, D. M., Tumlinson, J. H. Insect chemical ecology research in the United States Department of Agriculture-Agricultural Research Service. Pest Management Science. 59, 777-787 (2003).
- Thomas, C. B., Marlin, E. R. Pheromone mating disruption: Novel, non-toxic control of the European corn borer. Leopold Center. 8, 57-60 (1999).
- Chen, X. F., Hu, M. Y. Studies on the specimen preparation techniques of scanning electron microscope of Ficus simplicissima Lour. Journal of Zhongkai Agrotechnical College. 14, 68-70 (2001).
- Zhou, W., Apkarian, R., Wang, Z. L., Joy, D. Fundamentals of Scanning Electron Microscopy (SEM). Scanning Microscopy for Nanotechnology. , 1-40 (2006).
- Kothekar, S. C., Ware, A. M., Waghmare, J. T., Momin, S. A. Comparative Analysis of the Properties of Tween-20, Tween-60, Tween-80, Arlacel-60, and Arlacel-80. Journal of Dispersion Science and Technology. 28, 477-484 (2007).
- Chai, J. L., Liu, N., Bai, T. T., Zhang, H. M., Liu, N. N., Wang, D. D. Compositions and Physicochemical Properties of Tween Type Surfactants-Based Microemulsions. Journal of Dispersion Science and Technology. 35, 441-447 (2014).
- Zhang, L. D., Zhao, L., Han, F., Xu, B. C. Performance and applications of surfactants (XV) Detergency of surfactants and its applications. China Surfactant Detergent and Cosmetics. 45, 132-137 (2015).
- Waghmare, P. R., Das, S., Mitra, S. K. Under-water superoleophobic glass: unexplored role of the surfactant-rich solvent. Scientific Reports. 3, 1-25 (2013).
- Zhang, Y. R., Ren, L. L., Luo, Y. Q. Microtomy of insect sensilla embedded in resin blocks for transmission electronic microscopy. Chinese Journal of Applied Entomology. 50, 1479-1483 (2013).
- Zong, S. X., Liu, X. H., Cao, C. J., Luo, Y. Q., Ren, L. L., Zhang, H. Development of semiochemical attractants for monitoring and controlling Chlorophorus caragana. Zeitschrift für Naturforschung. 68, 243-252 (2013).
- Sumner, M. J. Epoxy resins for light and transmission electron microscopy. Plant Microtechniques and Protocols. , 83-101 (2015).
- Schneider, D. Insect antennae. Annual Review of Entomology. 9, 103-122 (1964).
- Zacharuk, R. Antennae and sensilla. Comprehensive Insect Physiology, Biochemistry and Pharmacology. 6, 1-69 (1985).
- Zacharuk, R., Albert, P., Bellamy, F. Ultrastructure and function of digitiform sensilla on the labial palp of a larval elaterid (Coleoptera). Canadian Journal of Zoology. 55, 569-578 (1977).
- Shanbhag, S., Müller, B., Steinbrecht, R. Atlas of olfactory organs of Drosophila melanogaster: 1, Types, external organization, innervation and distribution of olfactory sensilla. International Journal of Insect Morphology and Embryology. 28, 377-397 (1999).
- Tarumingkeng, R. C., Coppel, H. C., Matsumura, F. Morphology and ultrastructure of the antennal chemoreceptors and mechanoreceptors of worker Coptotermes formosanus Shiraki. Cell Tissue Res. 173, 173-178 (1976).
- Zacharuk, R. Y. Ultrastructure and function of insect chemosensilla. Annual Review of Entomology. 25, 27-47 (1980).
- Li, Y. Z., Zhong, G. Q. Screening of detergents and floating carriers for treating potato golden nematode cysts to improve the original appearance of electron microscopy. Plant quarantine. 8, 72-75 (1994).
- Marzio, L. D., Marianecci, C., Petrone, M., Rinaldi, F., Carafa, M. Novel pH-sensitive non-ionic surfactant vesicles: comparison between tween 21 and tween 20. Colloids and Surfaces B: Biointerfaces. 82, 18-24 (2011).
- Ren, L. L., Wu, Y., Shi, J., Zhang, L., Luo, Y. Q. Antenna morphology and sensilla ultrastructure of Tetrigus lewisi Candèze (Coleoptera: Elateridae). Micron. 60, 29-38 (2014).
- Ren, L., Shi, J., Zhang, Y., Luo, Y. Antennal morphology and sensillar ultrastructure of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). Micron. 43, 921-928 (2012).
- Teng, X. H., Liu, X. L., Xie, G. Y., Tang, Q. B., Li, W. Z., Zhao, X. C. Morphology and distribution of ovipositor sensilla of female Helicoverpa armigera (Lepidoptera: Noctuidae). The 11th Henan Plant Protection Society, the 10th Henan Insect Society, and the 5th Member Congress and Academic Symposium of Henan Plant Pathology Society. , 138-142 (2017).
- Yang, R., Zhang, L. N., Fan, J. W., Wang, J. L., Fang, K. F., Yu, T. Q., Wang, S. H., Du, Y. L. Insect specimens for scanning electron microscopy. Journal of Beijing University of Agriculture. 29, 33-36 (2014).
- Zhang, Y. R., Ren, L. L., Zhang, L., Wang, R., Yu, Y., Lu, P. F., Luo, Y. Q. Ultrastructure and distribution of sensilla on the maxillary and labial palps of Chlorophorus caragana (Coleoptera: Cerambycidae). Journal of Morphology. 279, 574-588 (2018).
- Harrison, J. D. G. Cleaning and preparing adult beetles (Coleoptera) for light and scanning electron microscopy. African Entomology. 20, 395-401 (2012).
- Xiao, Y., Liu, W., Wang, Y., Zuo, Y. X., Hu, R., Li, T. T., Cui, Z. B. Drying methods of biological sample preparation for scanning electron microscope. Research and Exploration Laboratory. 32, 46-53 (2013).
- Graef, M. D. . Introduction to Conventional Transmission Electron Microscopy. , 1 (2003).

