Isolation of Neural Stem/Progenitor Cells from the Periventricular Region of the Adult Rat and Human Spinal Cord
Summary
The adult mammalian spinal cord contains neural stem/progenitor cells (NSPCs) that can be isolated and expanded in culture. This protocol describes the harvesting, isolation, culture, and passaging of NSPCs generated from the periventricular region of the adult spinal cord from the rat and from human organ transplant donors.
Abstract
Adult rat and human spinal cord neural stem/progenitor cells (NSPCs) cultured in growth factor-enriched medium allows for the proliferation of multipotent, self-renewing, and expandable neural stem cells. In serum conditions, these multipotent NSPCs will differentiate, generating neurons, astrocytes, and oligodendrocytes. The harvested tissue is enzymatically dissociated in a papain-EDTA solution and then mechanically dissociated and separated through a discontinuous density gradient to yield a single cell suspension which is plated in neurobasal medium supplemented with epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and heparin. Adult rat spinal cord NSPCs are cultured as free-floating neurospheres and adult human spinal cord NSPCs are grown as adherent cultures. Under these conditions, adult spinal cord NSPCs proliferate, express markers of precursor cells, and can be continuously expanded upon passage. These cells can be studied in vitro in response to various stimuli, and exogenous factors may be used to promote lineage restriction to examine neural stem cell differentiation. Multipotent NSPCs or their progeny can also be transplanted into various animal models to assess regenerative repair.
Introduction
NSPCs are multipotent cells committed to the neural lineage that can self renew and readily expand in vitro. We refer to these cells as a mixed population of neural stem/progenitor cells since they display properties of self-renewing multipotent stem cells and more restricted progenitors. NSPCs are found in both the fetal and adult brain and spinal cord1,2. In the adult, NSPCs are normally quiescent and reside within specific niches including the subventricular zone lining the lateral ventricles2-4, and the periventricular region surrounding the central canal of the spinal cord5,6.
Typically, NSPCs are cultured as free-floating neurospheres or as adherent monolayers in serum-free medium supplemented with EGF and bFGF, mitogens which select for the stem/progenitor cell populations. The neurosphere assay originally developed by Reynolds and Weiss2, is most commonly used to culture and expand neural stem cells. NSPCs show multipotency when they are plated in growth factor-free medium containing serum, differentiating into neurons, astrocytes, and oligodendrocytes. Multipotent, self-renewing NSPCs can be isolated and cultured from the adult rodent spinal cord when the cultured tissue includes regions of the central canal6,7. A potential advantage in using NSPCs generated from the adult spinal cord as opposed to generating cells from other regions is that these tissue-specific cells most closely resemble cells in the spinal cord that are lost or damaged following injury or disease.
Previous work showed that neurospheres derived from the adult human spinal cord could not be propagated long-term or passaged to generate sufficient numbers of cells8,9. However, with modifications in culturing conditions, we reported the expansion and transplantation of adult human spinal cord-derived NSPCs, demonstrating that self-renewing and multipotent NSPCs can be isolated from the adult human spinal cord of organ transplant donors10. Primarily, the removal of most of the white matter during dissection and culturing these cells on an adherent substrate in growth factor-enriched medium selected for proliferating adult human spinal cord NSPCs. In this protocol we describe the harvesting of the spinal cord from adult rat and from human organ transplant donors, dissection of the periventricular tissue, and the isolation, culture and expansion of NSPCs.
Protocol
All animal procedures are approved by the Animal Care Committee of the University Health Network, Toronto ON Canada, in accordance with the policies established in the Guide to the Care and Use of Experimental Animals prepared by the Canadian Council on Animal Care. For the harvesting of human spinal cord tissue, approval was obtained from the University Health Network Research Ethics Board and from the Trillium-Gift of Life Foundation which oversees organ donation in Ontario, Canada.
1. Preparation of Dissection Buffers and Culture Media
- For the isolation of rat spinal cord, prepare 100 ml dissection buffer (1x PBS + 0.6% glucose + 2% penicillin-streptomycin) and refrigerate. For human spinal cord prepare 100 ml dissection buffer (1x HBSS + 0.6% glucose + 2% penicillin-streptomycin) and refrigerate.
- Prepare 100 ml serum-free medium (SFM) and warm at 37 °C. To prepare SFM, add 2 mM L-glutamine, 100 µg/ml penicillin-streptomycin, 2% B27, and 10% hormone mix to Neurobasal-A medium. To prepare hormone mix, make a 1 : 1 DMEM/F12 medium containing 0.6% glucose, 3 mM NaHCO3, 5 mM HEPES, 25 µg/ml insulin, 100 µg/ml apo-transferrin, 10 µM putrescine, 30 nM selenium, and 20 nM progesterone.
- Prepare the EFH growth factor-enriched plating media (to SFM add 20 ng/ml EGF, 20 ng/ml bFGF, and 2 µg/ml heparin) and warm at 37 °C. Ensure that human EFH contains human recombinant growth factors (see Table of Materials).
2. Harvesting and Dissection of Adult Rat Periventricular Spinal Cord Tissue
- Sterilize dissecting instruments in ethanol and rinse in sterile saline, or autoclave instruments in advance. Give rat (6 – 8 weeks old) a lethal injection of sodium pentobarbital (1 cc at 65 mg/ml) or overdose of anesthetic (i.e., 4% isofluorane) according to one’s institution approved animal protocol. Douse the back of the rat with 70% ethanol and remove the skin on the dorsal surface with large dissection scissors.
- Holding the scissors perpendicular to the dorsal surface, transversely cut the vertebral column above the hindlimbs. Use smaller scissors to longitudinally cut the dorsal muscle overlying the vertebral column in a rostral direction to expose the spinous processes.
- Starting at the exposed caudal end, insert rongeurs or a small blunt bone-cutting instrument extradurally into the lateral aspect of the spinal canal in the space between the spinal cord and the vertebral column. Make small cuts into the lamina (bony arches left and right of the spinous processes on each side of the vertebrae) and carefully peel away the lamina to expose the spinal cord (Figure 1A-D). Ensure the angle of the blades is shallow and parallel to the cord so the underlying spinal cord is not damaged.
- Continue laminectomy in the rostral direction to expose the thoracic and cervical spinal cord.
- Gently excise the spinal cord from the vertebral column with blunt tissue forceps and use microscissors to carefully sever the roots to release the cord. Place the excised spinal cord in a Petri dish containing 4 °C sterile rat dissection buffer (described above in 1.1). Rinse the tissue in freshly prepared 4 °C dissecting buffer and using scissors cut the spinal cord transversely into 1 cm segments (Figure 1E).
- For each segment of tissue, use one hand to hold the tissue with fine forceps. With the other hand, use microscissors to carefully remove the overlying meninges, white matter, and most of the grey matter with the aid of a dissecting scope. Alternatively, use fine forceps (Dumont #4) to peel away most of the grey and white matter, leaving only the periventricular region of the spinal cord which includes the ependyma and a small amount of surrounding grey matter (Figure 1F-H).
- Pool the dissected periventricular tissue into a 10 cm sterile Petri dish containing cold rat dissection buffer.
3. Harvesting and Dissection of Adult Human Periventricular Spinal Cord Tissue
Note: Human spinal cord tissue is harvested from adult organ transplant donors (donors ranged in age from 2 to 60 years of age) after the other organs have been removed for organ transplantation. The Trillium-Gift of Life Program obtains consent for removal of tissue for research purposes from the patient’s family and notifies our harvesting team in a timely fashion such that we have been able to harvest the cords within 2 hrs of aortic cross-clamping. Male and female adult donors with negative serology and no infections are accepted.
- Harvest tissue in a sterile fashion in the operating room through an anterior approach using the same anterior exposure already dissected for organ removal by the organ transplant harvesting team.
- Expose the anterior spinal column through retraction with large rib spreaders and large paddle retractors of the remaining tissues and organs including the large blood vessels.
- Use a sternal saw mounted with a long blade to cut through the intervertebral discs at the rostral and caudal ends of the desired length of the vertebral column to be resected. Angle the saw about 45° medially to cut a triangular trough from the lateral part of the vertebral bodies stopping just short of the spinal canal.
- Remove en bloc the medial aspects of the vertebral bodies of the desired segments with the aid of curved osteotomes and mallet taking care to avoid cutting through the dura. Then gently lift the dura covered spinal cord with toothed forceps and sharply incise the rostral and caudal ends of the cord. Use long scissors and forceps to bilaterally transect the individual roots.
- Place the excised segment of the spinal cord in 4 °C sterile buffer (1x HBSS + 0.6% glucose + 2% penicillin-streptomycin) in a large test tube for transportation to the tissue culture room.
- Note: Generally, a 3 – 6 cm segment of spinal cord from the upper thoracic and/or mid-to-low thoracic level is removed in the operating room under sterile conditions as soon as possible after cessation of circulation and placed in cold sterile buffer. The time elapsed between cessation of circulation and removal of the spinal cord varies with the time required to harvest the targeted organs for donation and has ranged from 45 min to 2 hrs. If the heart and lungs have also been removed, the dissection can be taken far rostrally to harvest a portion of the lower cervical cord as well.
- Dissect the human spinal cord tissue as soon as possible after harvesting, generally within 3 hrs. Remove the dura and other meninges with forceps. Rinse the spinal cord tissue in freshly made 4 °C dissection buffer and cut transversely into 1 cm segments.
- With the aid of a dissecting scope, excise the overlying meninges, white matter, and most of the grey matter using tissue forceps, fine forceps and microscissors for each tissue segment. Pool the dissected periventricular tissue, which includes the ependyma and a small amount of surrounding grey matter, into a 10 cm sterile Petri dish containing cold human dissection buffer.
4. Isolation and Culturing of Adult Rat and Human Spinal Cord NSPCs
- Perform the following steps aseptically in a laminar flow hood.
- Mince the dissected rat or human periventricular tissue into 1 mm3 pieces with microscissors.
- Enzymatically dissociate the minced tissue with proteolytic enzymes using the papain dissociation kit as indicated in the table of materials/reagents. Note: Reagent components include 4 vials; Vial 1: Earle's Balanced Salt Solution (EBSS), Vial 2: Papain containing L-cysteine and EDTA, Vial 3: Deoxyribonuclease I (DNase), Vial 4: Ovomucoid protease inhibitor with bovine serum albumin (BSA).
Note: Note: Papain is a sulfhydryl protease that has wide specificity for protein substrates. The papain herein is derived from the latex from the Carica papaya plant and degrades most protein substrates more extensively than pancreatic proteases. During the dissociation process there is some cell damage causing DNA to be released into the dissociation medium which will increase viscosity and make pipetting difficult. Thus, DNase is included in the cell isolation procedure to digest the DNA without damaging the intact cells. Ovomucoids are glycoprotein protease inhibitors used to inhibit the papain activity after the dissociation step.
- At first use, reconstitute the albumin ovomucoid inhibitor mixture (vial 4) with 32 ml of EBSS (vial 1), and then store at 4 °C and use for subsequent isolations. Note: This yields a solution at an effective concentration of 10 mg of ovomucoid inhibitor and 10 mg of albumin per ml.
- Add 5 ml of EBSS (vial 1) to a papain vial (vial 2), yielding a solution at 20 units of papain/ml in 1 mM L-cysteine with 0.5 mM EDTA. Check that the papain solution appears clear when completely dissolved, otherwise place the vial in a 37 °C water bath for ten minutes until the papain is completely dissolved to ensure full activity of the enzyme.
- Add 500 µl of EBSS (vial 1) to a DNase vial (vial 3) and mix gently as DNase is sensitive to shear denaturation. Add 250 µl of the DNase solution to the vial containing the papain, resulting in a final concentration of approximately 20 units/ml papain and 0.005% DNase. Save the balance of the DNase vial to use later.
- Place the minced rat or human tissue in the papain solution as prepared in step 4.4 above. For the human tissue isolation, split the minced tissue equally between two papain vials (approximately 0.2 g/vial).
- Incubate at 37 °C with constant agitation on a rocker platform to dissociate the tissue in the activated papain solution. Incubate rat tissue for 45 min to 1 hr, and human tissue for 1 to 2 hrs depending on the amount of minced tissue, about 0.2 – 0.4 g respectively.
- Triturate the mixture with a 10 ml pipette to dissociate any remaining tissue pieces to yield a cloudy cell suspension. Transfer the cell suspension (do not include any pieces of undissociated tissue) into a sterile 15 ml conical tube and centrifuge at 300 x g for 5 min at RT.
- Resuspend the pelleted cells in medium containing ovomucoid, a papain inhibitor.
- Prepare the ovomucoid solution by mixing 2.7 ml EBSS (vial 1) with 300 µl of the reconstituted albumin-ovomucoid inhibitor solution (vial 4) in a 15 ml conical tube. Add 150 µl of DNase solution (vial 3) saved at step 4.4 above.
- Discard the supernatant from the pelleted cells and immediately resuspend the cell pellet in the diluted DNase albumin-inhibitor mixture.
- Separate intact cells from cell membranes by centrifugation through a single step discontinuous density gradient.
- Prepare the discontinuous density gradient by adding 5 ml of albumin-inhibitor solution (vial 4) to a 15 ml tube, and using a 5 ml pipette, gently and slowly layer the cell suspension (prepared as described above in step 4.8) on top of the albumin-inhibitor solution.
- Centrifuge at 70 x g for 6 min at RT.
- Note: The interface between the two layers of the gradient should be clearly visible, although minimal mixing at this boundary does not affect the result. Membrane fragments remain at the interface and dissociated cells pellet at the bottom of the tube.
- For rat cells, discard the supernatant and resuspend the cell pellet in 1 ml of rat EFH medium (pre-warmed at 37 °C). Count live cell density with a haemocytometer and plate the cells into a T25 culture flask at a density of 10 cells/µl in EFH. The typical yield of cells from rat tissue is about 2 x 106 cells with about 80% viability.If clonal cultures are desired, seed cells at a density of less than 10 cells/µl. Incubate the flasks at 37 °C in 5% CO2 and allow the cultures to grow undisturbed for 1 week to avoid aggregation of spheres.
- For human cells, discard the supernatant and resuspend the cell pellet in 10 ml of human EFH medium (pre-warmed at 37 °C). Filter the cell suspension through a 40 µm nylon cell strainer followed with 30 ml of EFH to remove myelin and cell membrane fragments. Centrifuge at 300 x g for 5 minutes and resuspend the cell pellet in 1 ml of EFH medium.
- Count live cell density with a haemocytometer and plate the human cells into 6-well culture plates at a density of 105 cells/well in a total volume of 5 ml EFH/well. The typical yield of cells from human tissue is about 1 x 106 cells with about 70% viability. Pre-coat wells with an adherent substrate such as Matrigel (see Note below). Dilute at a ratio of 40 µl Matrigel : 1 ml SFM.
- Note: Alternatively, other adherent substrates such as poly-D-lysine/laminin, fibronectin, or collagen may be used.
- Incubate the plates at 37 °C in 5% CO2 and allow the cultures to grow undisturbed for 1 week.
5. Passaging of Adult rat and human spinal cord NSPCs
- Rat NSPCs will form small neurospheres approximately 70 µm in diameter within 1 week of initial seeding; passage rat NSPCs weekly by collecting the cell suspension, centrifuging at 300 x g for 5 min, and mechanically triturating the cell pellet to dissociate the neurospheres.
- Seed the dissociated cells into T25 flasks containing fresh rat EFH medium. Alternatively, use 50% conditioned rat medium for passaging. The typical yield of rat NSPCs at passaging is about 5 x 106 cells which increases exponentially with passage number.
- For the human cultures, one week after the initial plating, replace half of the culture medium with fresh EFH twice weekly to allow the cells to adhere to the substrate. Generally, 1 – 2 weeks later once the cells have attached to the substrate, replace the entire volume of culture medium twice a week with fresh EFH.
- Subculture cells before reaching confluence between 4 – 8 weeks.
- Subculture cells by detaching cells from the substrate enzymatically (see Table of Materials) with 2 ml of enzyme per well incubated for approximately 10 min at 37 °C. Collect cell suspension, centrifuge at 300 x g for 5 min, and gently resuspend the cell pellet in 1 ml of freshly made EFH medium.
- Count live cells with a haemocytometer and plate the cells into 6-well culture plates (pre-coated as above in step 4.12) at a density of 105 cells/well in a total volume of 5 ml EFH/well. The typical yield of human NSPCs at passaging is about 2 x 106 cells and generally this will double with passage. Generally, maintain cultures with 50% conditioned EFH medium 2 – 3 times per week between passaging.
Representative Results
Adult rat spinal cord cells grown in suspension culture in EFH medium will form small neurospheres (colonies of undifferentiated cells) within 1 week of initial plating. In primary cultures, most of the cells plated will die and growth factor-responsive stem cells will proliferate and are selected for in the EFH medium. By passage 3, there will be numerous free-floating neurospheres about 100 µm in diameter (Figure 2A). Neurospheres are round and phase-bright, and under high magnification, cilia-like microspikes are seen protruding from cells on the outside of spheres which are characteristic of neurospheres unlike clumps of cell debris (Figure 2B). The typical yield of cells from rat tissue is about 2 x 106 cells with about 80% viability. The neurospheres should not be seeded at too high a density to avoid aggregation of cell clusters. Also, large neurospheres become difficult to dissociate and cells will become necrotic in the center of the sphere. This will also occur if cultures are left too long before passaging. In EFH culture, adult rat spinal cord NSPCs proliferate (Figure 2C) and express nestin (Figure 2D) a marker for precursor cells, and low levels of mature neural markers (data not shown).
Adult human spinal cord NSPC cultures do not grow as rapidly as rat NSPC cultures. If human spinal cord cells are initially cultured in suspension, they will form aggregates and irregular clusters of small numbers of cells combined with debris (Figure 3A). Subsequent passaging of these cultures does not promote enrichment of the NSPC population. However, when the human cells are plated in EFH on an adherent monolayer, the growth factor-responsive NSPCs adhere to the substrate (Figure 3B) and subsequent media replacement removes myelin and cell debris (Figure 3C). The typical yield of cells from human tissue is about 1 x 106 cells with about 70% viability. When the human NSPC cultures become well established as an adherent monolayer, the NSPCs can then also be plated in suspension cultures to form free-floating neurospheres. In EFH culture, adult human spinal cord NSPCs proliferate (Figure 3D) and express nestin (Figure 3E) and Sox2 (Figure 3F), a transcription factor shown to be expressed by neural stem cells, and very low levels of mature neural markers (data not shown).
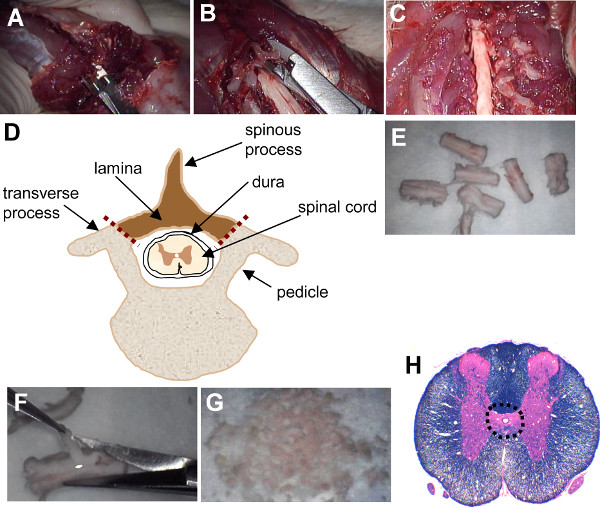
Figure 1. Laminectomy of rat spinal cord and dissection of periventricular region. (A) At the exposed caudal end of the spinal cord, rongeurs are inserted extradurally into the lateral aspect of the spinal canal. (B) Small cuts are made into the lamina on each side of the vertebrae and the lamina is carefully peeled away to expose the spinal cord. (C) The exposed cervical spinal cord after laminectomy. (D) Schematic of thoracic vertebral cross-section showing spinal cord and location of cuts made for laminectomy (depicted with red dotted lines). The laminae and spinous process (shaded region) are removed. (E) The spinal cord is cut transversely into 1 cm segments. (F) The overlying meninges, white matter, and most of the grey matter is carefully removed using microscissors. (G) Minced periventricular tissue. (H) Transverse section of rat spinal cord stained with luxol fast blue and hemotoxylin and eosin with dotted outline showing dissected periventricular region. Please click here to view a larger version of this figure.
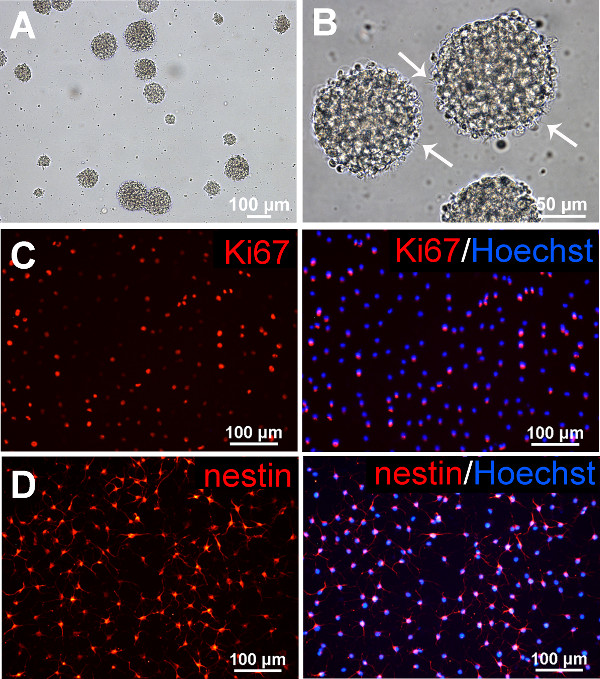
Figure 2. Adult rat spinal cord NSPCs. (A) By passage 3, numerous neurospheres are seen when rat spinal cord NSPCs are grown in free-floating suspension culture. (B) Neurospheres are phase-bright and microspikes protruding from the neurospheres (arrows) are apparent at high magnification. (C) Dissociated neurospheres (passage 3, day 4) are plated on Matrigel-coated wells in EFH medium, fixed, and immunostained and counterstained with Hoechst to visualize nuclei (blue). In EFH conditions, adult rat spinal cord NSPCs proliferate (as shown with Ki67 immunostaining), and (D) primarily express nestin. Please click here to view a larger version of this figure.
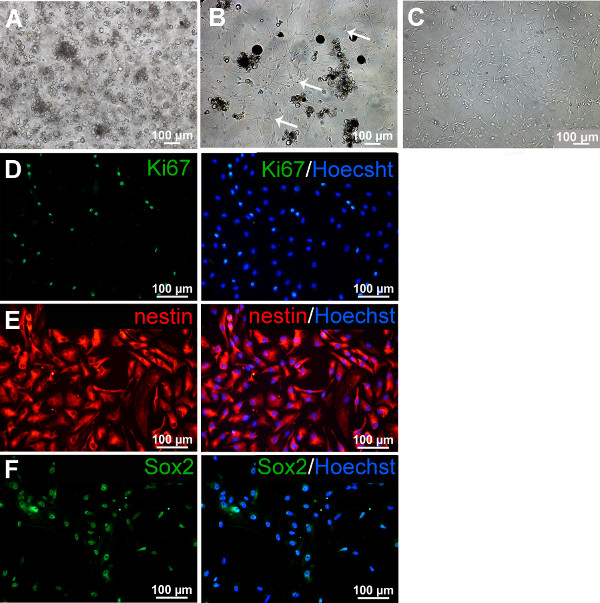
Figure 3. Adult human spinal cord NSPCs. (A) When initially plated in culture flasks, adult human spinal cord NSPCs do not grow well. Primary free-floating cultures in EFH medium (13 days after plating) show aggregates of cells and debris. (B) In contrast, in primary adherent cultures in EFH, NSPCs have attached to the Matrigel substrate (arrows), shown at 17 days after plating, and (C) at 41 days in vitro. (D) In EFH medium, adult human spinal cord NSPCs proliferate, as shown with Ki67 immunostaining (26 days in vitro shown), and primarily express (E) nestin (26 days in vitro shown) and (F) Sox2 (66 days in vitro shown). Nuclei are counterstained with Hoechst (blue). Please click here to view a larger version of this figure.
Discussion
During the dissection of the rat spinal cord tissue care should be taken not to damage the spinal cord while performing the laminectomy. It is easier to isolate the periventricular tissue when the spinal cord segments are intact. The tissue segments should be fully immersed in dissection buffer and the overlying meninges and white matter can be cut away as longitudinal strips with microscissors. Alternatively, fine tissue forceps can be used to peel the white matter away.
For the isolation procedure for NSPCs, we used a papain-EDTA dissociation method combined with mechanical trituration to dissociate the rat and human spinal cord tissue. Compared to other proteases, papain is less damaging but effective, as previously shown with dissociation of postnatal rat cortical neurons11. We found that dissociation with only mechanical trituration, often used with fetal tissue, is not as effective with tissue from the adult. After the enzymatic dissociation, mechanical trituration is important to break up the remaining tissue fragments. Trituration with repetitive cell dispersion with a pipette should be performed so there are no remaining intact tissue fragments resulting in a cloudy cell suspension. However, trituration should not be performed too strongly to avoid bubbling of the cell suspension and resultant cell death.
For passaging, rat neurospheres are dissociated into a single cell suspension via mechanical trituration, as was originally described2. However, neurospheres can also be dissociated using enzymatic methods such as trypsin-EDTA which will not damage cell surface receptors or interfere with sphere formation as long as enzymatic exposure is brief12.
There are a number of advantages in using the neurosphere assay to culture NSPCs. It is relatively simple, reproducible, and allows for the rapid expansion of neural stem cells2,12. However, neurospheres are heterogenous comprised primarily of progenitor cells that are more restricted and only a small number of stem cells. Several factors such as cell density and passaging technique may affect the phenotype of the cultures, and neurospheres may also form from progenitor cells13. Neurospheres are highly motile and may fuse even under conditions of low cell density14. Thus, the number of neurospheres in culture does not represent the number of stem cells. To discriminate neural stem cells from progenitor cells, one should use the neural-colony forming cell assay15,16.
Adult rat spinal cord NSPCs grow well as neurospheres in suspension culture in EFH supplemented medium and are easily expandable. In contrast, we have found that adult human spinal cord NSPCs grow better as an adherent monolayer10. Human brain-derived neural stem cells can be sustained long-term in monolayer culture in a chemically defined medium in the presence of mitogens which promote their proliferative capacity17,18. As described in this protocol, NSPCs can be isolated from the adult human spinal cord from organ transplant donors and passaged and expanded as an adherent monolayer in the presence of EGF, bFGF, and heparin10. It is easier to isolate NSPCs from younger than older donors as the cells expand faster, although NSPCs could still be isolated from donors up to our maximum age of 60 years10. We have examined a variety of adherent substrates such as Matrigel, fibronectin, collagen type I, and poly-D-lysine/laminin, and found these to be effective for adherence of adult human spinal cord NSPCs10. Initially both neurosphere and adherent cultures contain cellular aggregates, debris, and dead cells. However this debris is progressively removed with subculturing and the growth factor-enriched neurobasal medium selects for the proliferating NSPCs. Also debris and myelin contamination can be reduced with careful dissection and removal of white matter from the spinal cord tissue segments. Filtering the resultant cell suspension through a strainer prior to plating also removes myelin.
We have cultured adult human spinal cord NSPCs as adherent monolayers for at least 9 months involving approximately 10 passages, although in older cultures there is increased risk for karyotypic abnormalities. We performed karyotype analysis on human NSPCs cultured for 4 months and found a normal diploid karyotype with no chromosomal abnormalities (data not shown). We also found that cell proliferation and capacity to differentiate into oligodendrocytes decreased with increased time in culture. For example, the relative percentage of O4 positive cells decreased from 1.3% at 1 month in culture to 0.01% at 4 months in culture10. Both rat and human NSPCs are amenable to cryopreservation using standard freezing methods and show no significant differences in phenotypic profile.
The isolation and expansion of multipotent NSPCs in culture allows for several in vitro applications including the examination of various factors on adult neural stem cell differentiation. Increased numbers of NSPCs can be generated in vitro and transplanted into animal models such as spinal cord injury, stroke, and neurodegenerative disease to examine the reparative response of these neural stem cells in vivo.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge support from Spinal Cord Injury Ontario, the Ontario-China Research and Innovation Fund, the Toronto General and Western Hospital Foundation, and Physicians’ Services Incorporated Foundation. The authors thank Dr. Tasneem Zahir for expert advice in human spinal cord NSPC culture, and Drs. Cindi Morshead and Iris Kulbatski for their expert advice in rat spinal cord NSPC isolation.
Materials
| 1X PBS | Life Technologies | #10010023 | Dissection buffer |
| 1X HBSS | Life Technologies | #14175095 | Dissection buffer |
| D-glucose | Sigma | # G-6152 | Prepare 30% glucose stock solution for dissection buffer and hormone mix |
| Penicillin-Streptomycin | Life Technologies | #15140-148 | Dissection buffer and culture medium |
| Neurobasal-A | Life Technologies | #10888-022 | Culture medium |
| L-glutamine, 200mM | Life Technologies | #25030-081 | Culture medium |
| B27 | Life Technologies | #12587010 | Culture medium |
| DMEM | Life Technologies | #11885084 | Hormone mix |
| F12 | Life Technologies | #21700-075 | Hormone mix |
| NaHCO3 | Sigma | # S-5761 | Prepare 7.5% NaHCO3 stock solution for hormone mix |
| HEPES | Sigma | #H9136 | Prepare 1M HEPES stock solution for hormone mix |
| Insulin | Sigma | #I-5500 | Hormone mix |
| Apo-transferrin | Sigma | #T-2252 | Hormone mix |
| Putrescine | Sigma | # P7505 | Hormone mix |
| Selenium | Sigma | #S-9133 | Hormone mix |
| Progesterone | Sigma | #P-6149 | Hormone mix |
| EGF, mouse | Sigma | #E4127 | Prepare 100 μg/ml stocks in B27 and aliquot; EFH medium |
| EGF, human recombinant | Sigma | #E9644 | Prepare 100 μg/ml stocks in B27 and aliquot; EFH medium |
| bFGF, human recombinant | Sigma | #F0291 | Prepare 100 μg/ml stocks in B27 and aliquot; EFH medium |
| Heparin, 10000U | Sigma | #H3149 | Prepare 27.3 mg/ml stocks in hormone mix and aliquot; EFH medium |
| Papain dissociation kit | Worthington Biochemicals | #LK003150 | Contains EBSS, papain, DNase, ovomucoid protease inhibitor with BSA |
| Sodium Pentobarbital | Bimeda – MTC Animal Health Inc | DIN 00141704 | |
| Tissue Forceps: Addisons | Fine Science Tools | #11006-12 | Serrated standard tip; micro-tip also available |
| Fine Forceps: Dumont #4 | Fine Science Tools | #11241-30 | |
| Microscissors | Fine Science Tools | #15024-10 | Round-handled Vannas |
| Rongeurs | Bausch & Lomb | N1430 | |
| 10mm Petri dishes | Nunc | 1501 | |
| T25 Culture flasks | Nunc | 156367 | |
| 40 μm nylon cell strainer | VWR | CA21008-949 | |
| 6 well plates | Nunc | CA73520-906 | |
| Matrigel, growth factor reduced (100X) | Corning | 354230 | Thaw according to directions and freeze aliquots; use diluted at a ratio of 40 μl Matrigel: 1 ml SFM |
Referências
- Gage, F. H. Mammalian neural stem cells. Science. 287, 1433-1438 (2000).
- Reynolds, B. A., Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 255, 1707-1710 (1992).
- Gritti, A., et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J Neurosci. 16, 1091-1100 (1996).
- Morshead, C. M., et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 13, 1071-1082 (1994).
- Weiss, S., et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 16, 7599-7609 (1996).
- Martens, D. J., Seaberg, R. M., van der Kooy, D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 16, 1045-1057 (2002).
- Kulbatski, I., et al. Oligodendrocytes and radial glia derived from adult rat spinal cord progenitors: morphological and immunocytochemical characterization. J Histochem Cytochem. 55, 209-222 (2007).
- Akesson, E., et al. Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiol Behav. 92, 60-66 (2007).
- Dromard, C., et al. Adult human spinal cord harbors neural precursor cells that generate neurons and glial cells in vitro. J Neurosci Res. 86, 1916-1926 (2008).
- Mothe, A. J., Zahir, T., Santaguida, C., Cook, D., Tator, C. H. Neural stem/progenitor cells from the adult human spinal cord are multipotent and self-renewing and differentiate after transplantation. PLoS One. 6, e27079 (2011).
- Huettner, J. E., Baughman, R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 6, 3044-3060 (1986).
- Azari, H., Rahman, M., Sharififar, S., Reynolds, B. A. Isolation and expansion of the adult mouse neural stem cells using the neurosphere assay. J Vis Exp. , (2010).
- Reynolds, B. A., Rietze, R. L. Neural stem cells and neurospheres–re-evaluating the relationship. Nat Methods. 2, 333-336 (2005).
- Singec, I., et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 3, 801-806 (2006).
- Louis, S. A., Azari, H., Sharififar, S., Vedam-Mai, V., Reynolds, B. A. Neural-colony forming cell assay: an assay to discriminate bona fide neural stem cells from neural progenitor cells. J Vis Exp. , (2011).
- Louis, S. A., et al. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 26, 988-996 (2008).
- Conti, L., et al. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 3, e10 (2005).
- Pollard, S. M., Conti, L., Sun, Y., Goffredo, D., Smith, A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 16, i112-i120 (2006).

