Genotyping of Staphylococcus aureus by Ribosomal Spacer PCR (RS-PCR)
Summary
Here, ribosomal spacer PCR (RS-PCR) is used together with a miniaturized electrophoresis system as a fast and high resolution method for genotyping S. aureus at moderate costs allowing a high throughput.
Abstract
The ribosomal spacer PCR (RS-PCR) is a highly resolving and robust genotyping method for S. aureus that allows a high throughput at moderate costs and is, therefore, suitable to be used for routine purposes. For best resolution, data evaluation and data management, a miniaturized electrophoresis system is required. Together with such an electrophoresis system and the in-house developed software (freely available here) assignment of the pattern of bands to a genotype is standardized and straight forward. DNA extraction is simple (boiling prep), setting-up of the reactions is easy and they can be run on any standard PCR machine. PCR cycling is common except prolonged ramping and elongation times. Compared to spa typing and Multi Locus Sequence Typing (MLST), RS-PCR does not require DNA sequencing what simplifies the analysis considerably and allows a high throughput. Furthermore, the resolution for bovine strains of S. aureus is at least as good as spa typing and better than MLST or pulsed-field gel electrophoresis (PFGE). The RS-PCR data base includes presently a total of 141 genotypes and variants. The method is highly associated with the virulence gene pattern, contagiosity and pathogenicity of S. aureus strains involved in bovine mastitis. S. aureus genotype B (GTB) is contagious and causes herds problems causing large costs in the Switzerland and other European countries. All the other genotypes observed in Switzerland infect individual cows and quarters. Genotyping by RS-PCR allows the reliable prediction of the epidemiological and the pathogenic potential of S. aureus involved in bovine intramammary infection (IMI), two key factors for clinical veterinary medicine. Because of these beneficial properties together with moderate costs and a high sample throughput the goal of this publication is to give a detailed, step-by-step protocol for easily establishing and running RS-PCR for genotyping S. aureus in other laboratories.
Introduction
Staphylococcus (S. aureus) is known as the most important pathogen worldwide responsible for intramammary infection (IMI) in cattle 1. The study by Fournier et al. 2 demonstrated in Swiss cows and the studies by Cosandey et al. 3 and Boss et al. 4 in cows of 12 European countries that S. aureus isolated from bovine IMI is a genetically heterogeneous group. By PCR amplification of the 16S-23S rRNA intergenic spacer region (RS-PCR), a total of 17 genotypes were initially detected in 101 epidemiologically independent isolates 2. Genotype B and genotype C (GTC) were most common (80%), whereas the other 15 genotypes (GTs) occurred rarely, each making 1 to 4% of all the isolates. At the European level 3, GTB, GTC, GTF, GTI, and GTR were the most important genotypes comprising 76% of all analyzed strains (n = 456). GTB was situated in central Europe whereas the other genotypes were widely disseminated. The remaining 24% of the strains included 41 genotypes, many of them, however, were observed only once.
The studies by Fournier et al. 2, Cosandey et al. 3, and Graber et al. 5 further demonstrated that the genotypes were highly associated with their virulence gene pattern, as well as at the Swiss as at the European level. The studies further revealed massive differences in IMI prevalence among S. aureus GTB, GTC and the other genotypes 2,5: considering GTB, up to 87% of cows in a herd were infected by this genotype. In the case of GTC and of the other genotypes, however, IMI was only found in 1 to 2 cows of a herd.
IMI caused by S. aureus, normally results in inflammation of the corresponding mammary glands and an increase of inflammatory cells in milk. As a consequence, the somatic cell counts in milk (SCC), the key indicator of milk quality in most countries, is increased leading to a decreased milk price or even delivery stop.
Besides the RS-PCR of Fournier et al. 2, other typing methods have been described for subtyping bovine strains of S. aureus 8-11. Two common ones include spa typing 12 and Multi Locus Sequence Typing (MLST) 13. The former one is based on DNA sequencing the variable spacer region of the staphylococcal spa gene coding for protein A, whereas the latter one requires the sequencing of 7 housekeeping genes. This means that one 12 and seven PCRs 13, respectively, need to be performed, followed by amplicon purification, sequencing reaction and analysis using specific equipment. Compared to RS-PCR, these methods require a considerable additional effort in the laboratory resulting in a low sample throughput and high costs. The throughput is particularly low for PFGE. Considering the resolution of these methods for bovine strains of S. aureus, RS-PCR is at least as good as spa typing 4 and better than MLST 4 or PFGE 15.
All these methods including binary typing 13 demonstrated their effectiveness in obtaining further insight into the pathogenesis of bovine IMI caused by S. aureus, but because of the mentioned restrictions they are less appropriate for large clinical investigations. In addition, genotyping by RS-PCR as described by Fournier et al. 2 allows the reliable prediction of the epidemiological and the pathogenic potential of S. aureus involved in bovine IMI, two key values in clinical veterinary medicine.
Protocol
1. Staphylococcus aureus Isolates
- Spread 10 µl of S. aureus stock culture or one colony grown on a selective medium on Columbia agar plates containing 5% sheep blood (BA).
- Incubate aerobically at 37 °C for 18 hr to 24 hr.
2. DNA Extraction
- Prepare TEL buffer containing 10 mM Tris-HCl and 10 mM Na2EDTA, pH 8.5. Prepare aliquots and autoclave at 121 °C for 15 min.
- Collect 1 colony from BA using a sterile plastic loop or a sterile wooden tooth pick and resuspend in 100 µl TEL. Use 1.5 ml tubes with screw caps.
- Incubate at 95 °C for 10 min. Afterwards, place the samples immediately on wet ice.
- Dilute the samples 1:100 in H2O suitable for PCR (= template DNA for PCR). The non-diluted and diluted samples may be kept at -20 °C for 1 month.
3. RS-PCR
- For each sample pipette into a PCR tube 1.5 µl H2O, 12.5 µl Taq Master Mix, 2 µl G1 primer 10 µM, 2 µl L1 primer 10 µM, and 7 µl of template DNA that corresponds to about 30 ng of pure DNA 2.
- Run the following cycling program in a standard PCR cycler: 15 min 95 °C, followed by 27 cycles comprising 94 °C for 1 min, followed by a 2 min ramp and annealing at 55 °C for 7 min. After a further 2 min ramp, extend at 72 °C for 2 min. Terminate PCR by a final extension at 72 °C for 10 min followed by cooling down to 4 °C.
NOTE: The PCR products can be stored at -20 °C for 5 days. - For each run of RS-PCR include a positive control, i.e., a sample with a known genotype (normally GTB), and a negative control (H2O).
4. Electrophoresis
- Use a commercial miniaturized electrophoresis kit for DNA (MED kit) together with a bioanalyzer connected to a PC and the installed software. Study the corresponding manuals of the manufacturer carefully.
- Set up the chip priming station and adjust the syringe clip according to the manual of the manufacturer.
- Prepare the gel-dye mix according to the manual of the manufacturer.
- Loading the gel-dye mix (according to the manual of the manufacturer).
- Equilibrate the gel-dye mix to RT for 30 min before use.
- Put new DNA chip on the chip priming station. Pipette 9 µl of gel-dye in the well-marked G.
- Make sure that the plunger is positioned at 1 ml and then close the chip priming station. Press plunger until it is held by the syringe clip. Wait exactly for 30 sec then release the syringe clip.
- Wait for 5 sec. Slowly pull back plunger to 1 ml position.
- Open chip priming station and pipette 9 µl of gel-dye in both wells marked 'G'.
- Loading the Marker
- Pipette 5 µl of marker in all 12 sample wells and in the ladder well. Do not leave any of these 13 wells empty.
- Loading the Ladder and the Samples.
- Pipette 1 µl of DNA ladder to the well-marked with the ladder sign.
- Pipette 1 µl of sample (used wells) or 1 µl of deionized water (unused wells).
- Put the chip horizontally in the adapter and vortex for 1 min at 2,400 rpm.
- Run the chip in the bioanalyzer within 5 min.
- Running the Analysis
- Start the bioanalyzer and the connected computer. Open the software.
- Open the lid of the bioanalyzer and place the chip into the chip holder (the chip fits only one way). Close the lid carefully.
- In the Instrument context of the software select "Electrophoresis" > "dsDNA" > "DNA 7500". Accept the current File Prefix of the software or modify it.
- Click the start button present in the software. Enter the sample names in the software. When the chip run is finished (after approximately 30 min) the End of Run message appears in the bioanalyzer software.
- Remove the chip and clean the electrodes of the bioanalyzer according to the instructions of the manufacturer.
5. Inferring the Genotype from the Electrophoresis Profile
- Load the Mahal software (software is freely available from the authors).
- Import reference data into the Mahal software: "File" > "Import Reference Data"
- Open the electrophoresis run in the Data context of the bioanalyzer software. Select an electrophoresis profile of one sample.
- Check for the relevant peaks.
- Use the Mahal software to check for the relevant peaks: "Tools" > "Mean Fluorescence". Insert into the "Fluorescence" box the fluorescence values indicated as fluorescence units (FU) as they can be read out from the bioanalyzer software. Insert the 4 (3) peaks with highest fluorescence in ascending order and separated by comma. Example: 126,130,132,137.
NOTE: If not all of these peaks FU are indicated by the bioanalyzer software, they need to be estimated by reading them out from the plot.
- Use the Mahal software to check for the relevant peaks: "Tools" > "Mean Fluorescence". Insert into the "Fluorescence" box the fluorescence values indicated as fluorescence units (FU) as they can be read out from the bioanalyzer software. Insert the 4 (3) peaks with highest fluorescence in ascending order and separated by comma. Example: 126,130,132,137.
- Press the "Calculate" button. A peak is relevant if the observed fluorescence exceeds the value given in the box "Minimal peak height".
- Infer the genotype.
- Insert into the Mahal textbox "Input Peaks (bp)" the sizes in bp of all relevant peaks in ascending order and separated by comma. Example: 440,462,519,579,637.
NOTE: If not all of these peak sizes are indicated by the bioanalyzer software, they need to be estimated by reading them out from the plot.
- Insert into the Mahal textbox "Input Peaks (bp)" the sizes in bp of all relevant peaks in ascending order and separated by comma. Example: 440,462,519,579,637.
- Press the "Calculate" button.
- In the list box, look for the minimal squared Mahalanobis distance "d2m". If the corresponding P value right to it is ≥0.05 then the probability is ≥5% that the null hypothesis H0 is rejected (H0: the observed and the reference profile are identical) meaning that the observed profile is accepted to be identical to the reference profile of the indicated genotype.
- If d2m is minimal but the field of the corresponding P value is empty, reject H0 with a probability <5%.
NOTE: This means that the profiles are unequal although d2m is minimal. Such an outcome may result from non-average electrophoresis conditions.- To correct for these deviations, insert into the text box "Observed Peak (bp)" the value 395 and press the "Calculate" button. Check again for minimal d2m and a corresponding P value ≥0.05. If not successful, try the values 405, 415, and 425.
NOTE: Sometimes smaller steps are even indicated. If still not successful, the observed profile is not present in the Reference Data, it may be a new genotype or variant.
- To correct for these deviations, insert into the text box "Observed Peak (bp)" the value 395 and press the "Calculate" button. Check again for minimal d2m and a corresponding P value ≥0.05. If not successful, try the values 405, 415, and 425.
- Various Profiles
- Repeat steps 5.6 to 5.9 for each electrophoresis profile separately.
Representative Results
Definition of a genotype and its variants
An electrophoretic profile differing in more than one band from all identified genotypes is considered as a new genotype. New genotypes are named and extended according to Fournier et al. 5 leading to the genotypes GTA to GTZ, followed by the genotypes GTAA to GTAZ, and GTBA to GTBZ, and GTCA to GTCC at present. Variation in just one band is regarded as a genotypic variant. It is indicated with roman numerals superscripted after the name of the genotype (e.g., GTRI, GTRII). In total, there are presently 141 genotypes and variants in the reference data base.
Under the conditions outlined above, RS-PCR by Fournier et al. 5 is highly reproducible to genotype strains of S. aureus. Definitive identification of a known genotype or variant is always possible if technical problems can be excluded. A typical outcome is presented in Figure 1 demonstrating the electrophoresis of 12 RS-PCR products and presented in the pseudo gel mode by the bioanalyzer software. Each lane is characterized by a pattern of two or more PCR bands and a marker band in front and a marker band at the end. Double clicking into a lane opens the corresponding, actually measured electrophoretic profile in a separate window. The bioanalyzer software allows, among other possibilities, the observed peaks being read as size as fluorescent units FU (Figure 2) or in bp (Figure 3) whereby one peak corresponds exactly to one band in the pseudo-gel mode. Peak reading in FU is required for evaluating the relevant peaks of a profile in the Mahal software (Figure 4), reading in bp for inferring the genotype. Irrelevant peaks are defined in the Mahal software as small peaks whose observed fluorescence FU is below 40% of the geometric mean of the 4 (3) peaks with highest fluorescence. They are normally observed when there is an excess of DNA in RS-PCR (>30 ng pure DNA/assay) 2.
As an example, the genotype of sample 211 present in Figure 1 is inferred. Visual analysis of the electrophoretic profile in Figure 2 (bioanalyzer software) and analysis with the Mahal software using the tool to test for irrelevant peaks revealed 6 relevant peaks with peak sizes of 395 bp, 432 bp, 453 bp, 505 bp, 567 bp, and 622 bp (Figure 3). These values are filled in and separated by comma in the textbox "Input Peaks (bp)" of the Mahal software (Figure 4). To correct for run-specific deviations, the value 415 was inserted into the text box "Observed Peak (bp)" (Figure 4). After having pressed the "Calculate" button, a list is presented ordered by genotype together with the squared Mahalanobis distance d2m between the queried and indicated genotypic profile. Scroll the list looking for minimal d2m. In the present case, minimal d2m is equal to 4.499 (Figure 4) with a value of P ≥0.05, meaning that the queried profile does not significantly differ from the one of GTB.
Variants of genotypes are indicated as _I to _XV in the Mahal software. In the case of genotype B, there are the three variants GBTI, GBTII, and GBTIII indicated as B_I, B_II, and B_III in the software (Figure 4).
A chip of the MED kit allows analyzing products of 12 RS-PCR at a time. If less products are available, the remaining wells need to be loaded with deionized water. Results may only be interpreted if the controls included in RS-PCR are appropriate.
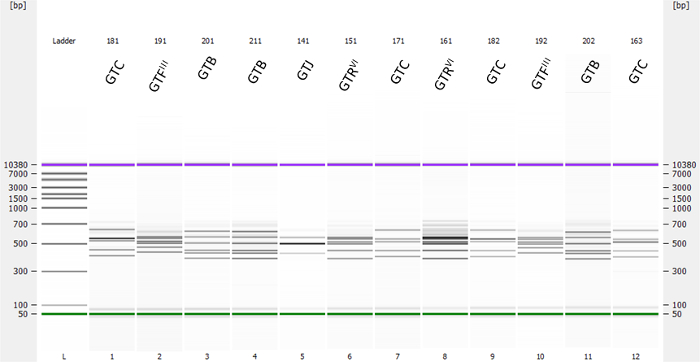
Figure 1. Pseudo-gel representing 12 RS-PCR products and their inferred genotypes. Twelve different strains of S. aureus were analyzed by RS-PCR using the MED kit together with a bioanalyzer and the included software. The lane on the left side shows the marker or "ladder" for size calibration of the system in bp. On the top of the graphics, the sample names and the corresponding genotypes are indicted that were obtained by the Mahal software. Please click here to view a larger version of this figure.
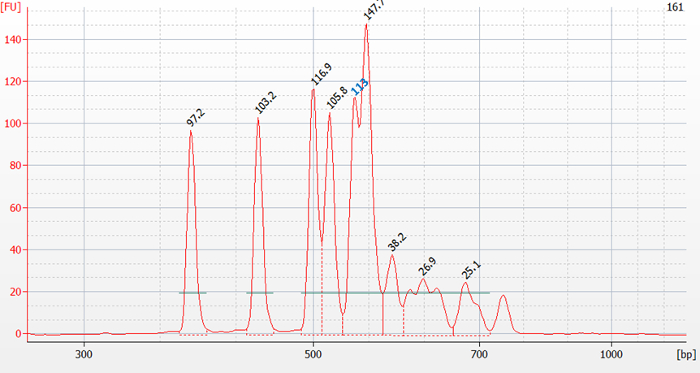
Figure 2. Electrophoretic profile of sample 161 present in Figure 1. On top of the peaks the measured fluorescence is indicated in FU. Visually distinguishable peaks that were not detected by the bioanalyzer software need to be estimated by reading them out from the plot as performed for the peak with an estimated fluorescence value of 113 FU (in blue). Please click here to view a larger version of this figure.
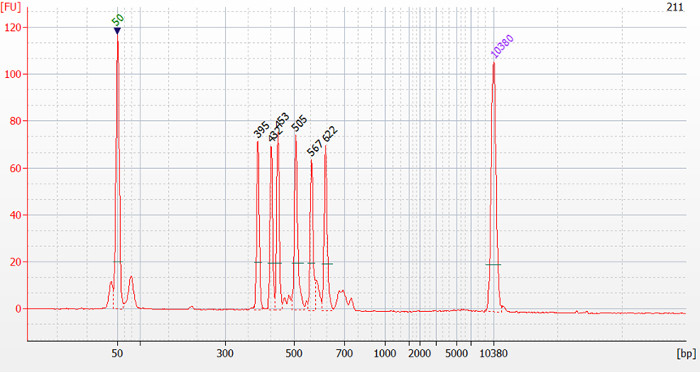
Figure 3. Electrophoretic profile of sample 211 present in Figure 1. On top of the peaks their size is indicated in bp. Please click here to view a larger version of this figure.
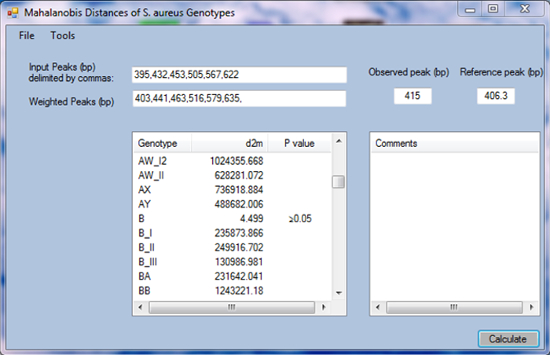
Figure 4. Screenshot of the Mahal software. In the text box "Input Peaks (bp)" the peak sizes of sample 211 from Figure 3 are filled in. Please click here to view a larger version of this figure.
Discussion
The most critical step of the whole procedure is when there is an excess of DNA in RS-PCR (>30 ng pure DNA/assay) 2. In general, resolution of a profile is optimal if all of its peaks start and end at the baseline. If two peaks are too close together, resolution is incomplete. Under these conditions, the bioanalyzer software may lead to inappropriate peak identification so that manual identification is required.
All the visibly separated and relevant peaks expressed as bp or FU are included for further analyses. Too much template DNA for RS-PCR results in broad peaks and therefore to peak overlapping and impaired resolution of the peaks. In addition, migration is slower leading to peak sizes that are falsely increased so that the genotype can no longer be inferred. Finally, the number of irrelevant peaks is bigger. For profiles with more than 3 peaks, the peak with maximum fluorescence should normally not exceed 150 FU.
Genotyping by RS-PCR as described is limited by the fact that investment in machines is necessary and the free Mahal software is required. In fact, a standard PCR machine and the bioanalyzer are needed. The former is rather cheap nowadays whereas the latter is more expensive. The resolution of agarose-based electrophoresis is not sufficient even if high resolution agarose is used. Under these conditions it is just possible to distinguish between GTB, GTC, and all the other genotypes. The costs for electrophoresis using the MED kit or agarose electrophoresis, however, are similar. The MED kit together with the bioanalyzer further outperforms standard agarose electrophoresis in a way that the latter method is handy, straight forward, and obtained data is present in electronic form that simplifies storage and further analysis considerably. Principally all commercially available bioanalyzers are suitable for this type of analysis provided that the electrophoretic resolution is adequate and the obtained size of the bands is accurate and unbiased.
The resolution of RS-PCR for bovine strains of S. aureus is high. It is as good as spa typing, and better than MLST and PFGE 4,14. In addition, compared to all the other typing methods commonly used for subtyping S. aureus (spa typing, MLST, PFGE), its sample throughput is high: DNA extraction is easy and fast, 96 samples can be processed in one RCR run (normally O/N) and electrophoresis and genotype inferring is straight forward. The complete analysis of 96 samples requires one night for PCR and one day for electrophoresis and evaluation. Other advantages of RS-PCR by Fournier et al. 2 are the low costs, particularly when compared to MLST that requires seven genes to be sequenced in both directions 15. Furthermore, RS-PCR is the only method predicting contagiosity and pathogenicity of strains of S. aureus involved in bovine mastitis 2,5, key predictors in clinical veterinary medicine. Finally, RS-PCR alone is sufficient to investigate the epidemiology of bovine S. aureus 3,4,5,14. Interpretation of the data is straight forward even in an international context as only a few major genotypes are observed 2,3. For epidemiological comparisons between bovine and human strains, RS-PCR and spa typing together are preferable as the latter method is widely used for subtyping human strains so that a lot of data is available. MLST, however, is suitable for assessing the clonality of the strains 15 and for phylogenetic analyses 4.
For troubleshooting regarding the PCR cycler, MED kit, and the bioanalyzer, the corresponding manuals are to be consulted. In the case of the RS-PCR method, troubleshooting is massively simplified if appropriate positive and negative PCR controls are included in each run. If the positive control is negative, the PCR mix was not adequately composed and/or the control DNA was degraded. If the negative control (H2O) generated one or more bands, the PCR mix was contaminated with some DNA. In both cases, the profiles must not be interpreted and RS-PCR has to be repeated. If interpretation is impossible because of an electrophoretic problem, electrophoresis needs to be repeated using a new chip. Electrophoretic problems are normally characterized by inadequate migration and alignment of one or both marker bands resulting from inappropriate loading and manipulating of the chip. If the obtained electrophoresis profile cannot be interpreted by the Mahal software, the profile file generated by the bioanalyzer software is sent to the author for further evaluation. Normally, too much template DNA was used for RS-PCR or the profile represents a new genotype or genotypic variant.
Declarações
The authors have nothing to disclose.
Acknowledgements
The author thanks R. Boss, A. Cosandey, C. Fournier, I. Ivanovic, and J. Naskova for their excellent work.
Materials
| Tris(hydroxymethyl)-aminomethan | Merck | 1.08382.0500 | Mw =121.14g/mol |
| Titriplex III | Merck | 1.08418.0250 | Mw = 372.24g/mol; Substance is equivalent to Na2EDTA·2H2O |
| Hydrochloric acid 5mol/l | Merck | 1.09911.0001 | |
| Columbia agar+5% sheep blood | bioMérieux | 43049 | |
| Agilent DNA7500 Kit | Agilent Technologies | 5067-1504 | |
| Agilent 2100 Bioanalyzer | Agilent Technologies | G2940CA | |
| Agilent 2100 expert sofware | Agilent Technologies | ||
| HotStarTaq master mix | Qiagen | 203445 | |
| Primer L1 | Mycrosinth | 5'CAA GGC ATC CAC CGT3' | |
| Primer G1 | Mycrosinth | 5'GAA GTC GTA ACA AGG3' |
Referências
- Sears, P. M., McCarthy, K. K. Management and treatment of staphylococcal mastitis. Vet.Clin.North Am.Food Anim.Pract. 19 (1), 171-185 (2003).
- Fournier, C., et al. Bovine Staphylococcus aureus: association of virulence genes, genotypes and clinical outcome. Res.Vet.Sci. 85 (3), 439-448 (2008).
- Cosandey, A., et al. Staphylococcus aureus genotype B and other genotypes isolated from cow milk in European countries. J.Dairy Sci. 99 (1), 529-540 (2016).
- Boss, R., et al. Bovine Staphylococcus aureus: Subtyping, evolution, and zoonotic transfer. J.Dairy Sci. 99 (1), 515-528 (2016).
- Graber, H. U., et al. Mastitis-related subtypes of bovine Staphylococcus aureus are characterized by different clinical properties. J.Dairy Sci. 92 (4), 1442-1451 (2009).
- Heiniger, D., et al. Kosten-Nutzen-Analyse einer Intervention zur Verbesserung der Eutergesundheit in Schweizer Milchviehbetrieben. Schweiz.Arch.Tierheilkd. 156 (10), 473-481 (2014).
- Hamann, J. Definition of the physiological cell count threshold based on changes in milk composition. IDF Mastitis Newsletter. 25, 9-12 (2003).
- Hwang, S. Y., Park, Y. K., Koo, H. C., Park, Y. H. spa typing and enterotoxin gene profile of Staphylococcus aureus isolated from bovine raw milk in Korea. J.Vet.Sci. 11 (2), 125-131 (2010).
- Sakwinska, O., et al. Link between genotype and antimicrobial resistance in bovine mastitis-related Staphylococcus aureus strains, determined by comparing Swiss and French isolates from the Rhone Valley. Appl.Environ.Microbiol. 77 (10), 3428-3432 (2011).
- Rabello, R. F., et al. Multilocus sequence typing of Staphylococcus aureus isolates recovered from cows with mastitis in Brazilian dairy herds. J.Med.Microbiol. 56, 1505-1511 (2007).
- Tavakol, M., et al. Bovine-associated MRSA ST398 in the Netherlands. Acta Vet.Scand. 54, 28 (2012).
- Harmsen, D., et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J.Clin.Microbiol. 41 (12), 5442-5448 (2003).
- Zadoks, R., et al. Application of pulsed-field gel electrophoresis and binary typing as tools in veterinary clinical microbiology and molecular epidemiologic analysis of bovine and human Staphylococcus aureus isolates. J.Clin.Microbiol. 38 (5), 1931-1939 (2000).
- Cremonesi, P., et al. Genomic characteristics of Staphylococcus aureus strains associated with high within-herd prevalence of intramammary infections in dairy cows. J.Dairy Sci. 98 (10), 6828-6838 (2015).
- Enright, M. C., Day, N. P., Davies, C. E., Peacock, S. J., Spratt, B. G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J.Clin.Microbiol. 38 (3), 1008-1015 (2000).

