Organ Ischemia-Reperfusion Injury by Simulating Hemodynamic Changes in Rat Liver Transplant Model
Summary
This paper provides a detailed description of how to build an animal model of the anhepatic phase (liver ischemia) in rats to facilitate basic research into ischemia-reperfusion injury after liver transplantation.
Abstract
Orthotopic liver transplantation (OLT) in rats is a tried and proven animal model used for preoperative, intraoperative, and postoperative studies, including ischemia-reperfusion injury (IRI) of extrahepatic organs. This model requires numerous experiments and devices. The duration of anhepatic phase is closely related to the time to develop IRI after transplantation. In this experiment, we used hemodynamic changes to induce extrahepatic organ damage in rats and determined the maximum tolerance time. The time until the most severe organ injury varied for different organs. This method can easily be replicated and can also be used to study IRI of the extrahepatic organs after liver transplantation.
Introduction
Ischemia-reperfusion injury (IRI) is a common complication after liver transplantation. Hepatic IRI is a pathological process involving ischemia-mediated cell damage and abnormal deterioration of liver reperfusion. Hepatic IRI and the local innate immune response can be divided into hot and cold IRI, according to differences in the clinical environment1. Hot IRI is induced by stem cell injury, usually as a result of liver transplantation, shock, and trauma2. Cold IRI is a complication of liver transplantation caused by endothelial cells and peripheral circulation3. Clinical reports have shown that hepatic IRI is associated with 10% of early organ failures and may increase the incidence of acute and chronic rejection4,5. In addition, hepatic IRI may also induce multiple organ dysfunction syndromes or systemic inflammatory response syndrome, with high mortality6. Patients with extrahepatic organ involvement tend to stay longer in the hospital, spend more money, and have a worse prognosis7. The development of complications is closely related to the length of the anhepatic phase of liver transplantation8.
Orthotopic liver transplantation (OLT) in rats was first reported by the American professor Lee in 1973. The experimental operation simulated the steps of clinical liver transplantation and the anastomosis of blood vessels and the common bile duct (CBD) using the suture method. The procedure is difficult and time-consuming with a low rate of success9. In 1979, Kamada et al. made a significant improvement to OLT in rats by creatively using the 'two-cuff method' for anastomosis of the portal vein to control the anhepatic phase within 26 minutes10. In the same year, Zimmermann proposed the 'single biliary stent method.' On the basis of Lee's work, Zimmermann used polyethylene tubes to directly anastomose the CBD of the donor and recipient, simplified the reconstruction of CBD, and preserved the function of the sphincter, and this method became the standard for biliary reconstruction of OLT models11. In 1980, Miyata et al. proposed the 'three-cuff method' where the portal vein (PV), suprahepatic vena cava (SVC), and intrahepatic vena cava (IVC) were anastomosed by the cuff method. However, there is a risk of distortion of the cannula with this method, which can lead to the obstruction of inferior vena cava reflux12. In 1983, the 'two-cuff method' was proposed using the cuff method for anastomosis of the PV and IVC, but adopting the suture method for the SVC13. This method was adopted by scholars globally to establish OLT models. Since then, the cuff anastomosis steps have been improved to shorten the anhepatic phase and improve the survival rate of the rats14. Similarly, improved methods are used in clinical practice to shorten the anhepatic phase15. However, basic research into IRI after liver transplantation has shown that the survival rate is inversely related to the degree of injury to extrahepatic organs. Therefore, further research is required, and a simple and reproducible animal model is needed to simulate IRI after liver transplantation.
Based on the definition of the anhepatic phase, we simulated the hemodynamic changes in liver transplantation resulting in IRI of extrahepatic organs in rats. Herein, we provide a detailed description of how to build an animal model of the anhepatic phase (liver ischemia) in rats to facilitate basic research into IRI after liver transplantation.
Protocol
The Animal Ethics Committee approved the experiment of Guangxi Medical University (No20190920). All animals were supplied by the Animal Experiment Center of Guangxi Medical University. We used SPF male Sprague Dawley rats (200-250 g, 10-12 weeks), kept under the room temperature of 25 ± 2°C and humidity of 50 ± 10%. Feeding was stopped 24 hours before operation; however, water was provided.
NOTE: One operator can perform all operations without a microsurgery basis or surgical microscope.
1. Operation
- After weighing, anesthetize the rats with isoflurane (5%) using an animal anesthesia machine.
- After 1-2 minutes, gently clamp the toes of the rat with tweezers. If the rat does not respond after pinching, it has entered a state of anesthesia. Use vet ointment on the eyes to prevent dryness. Use animal heating lamps to keep the rats' body temperature at 37-38 °C.
- Following abdominal disinfection (povidone iodine solution), fix the rat on the animal dissection table. Make a median incision of 3 cm below the xiphoid process using forceps and scissors.
- Open the abdominal cavity, expose the liver using a retractor, and mobilize the hepatogastric ligament. Use cotton swabs to flip the middle lobe of the liver gently and turn it upward to expose the porta hepatis. Identify the CBD, PV, and HA.
- Push the small intestine toward the left lower abdominal cavity using cotton swabs, cover it with wet gauze, and move the intrahepatic vena cava to the right renal vein.
- Isolate the portal vein, hepatic artery, and the inferior vena cava above the right renal vein with an intraocular lens and forceps marked with 3-0 silk thread, each with a slip knot.
- Cut open the left and right lower extremity skin and expose the femoral vein using ophthalmic forceps. Slowly inject low molecular weight heparin 625 IU/kg through the femoral vein to heparinize the whole body.
- Ligate the portal vein, hepatic artery, and inferior vena cava above the right renal vein with No. 3-0 sutures, lasting 45 minutes (Figure 1). Replace the small intestine in the abdominal cavity and cover it with gauze. Reduce inhalation anesthesia during these periods.
- After 45 minutes, release the portal vein, hepatic artery, and the inferior vena cava above the right renal vein.
- Suture the muscle and skin, layer by layer, and terminate the inhalational anesthesia. Provide postoperative analgesia using subcutaneous morphine of 5 mg/kg every 4 hours.
- Observe the rat until it is awake and feed under a temperature of 25 ± 2 °C and humidity of 50 ± 10%. Animal heating lamps are necessary.
Representative Results
Rats' tolerance to liver ischemia
In this animal model, the sites at which blood vessels were ligated during operating are shown in Figure 1. The rats were randomly divided into 5 groups for ischemia for 15 minutes (I15 group), 30 minutes (I30 group), 45 minutes (I45 group), 60 minutes (I60), and sham group, with 10 rats in each group. The survival rate of each group was observed 14 days after the operation. All rats survived in the I15 group, I30 group, and sham group. Eight survived for 14 days in the I45 group, and only 2 survived in the I60 group. These results suggest that the rats could tolerate the anhepatic phase for a maximum of 45 minutes (Table 1).
Effects of vascular ligation on circulation in rats
During the experiment, Biosystems recorded the heart rate and blood pressure (right internal carotid artery intubation) before and after the anhepatic phase. We found that the heart rate and mean arterial pressure (MAP) of rats changed dramatically after vascular ligation (Figure 2).
Effects on extrahepatic organs
Hepatic ischemia congestion and edema were found in the intestines, gastric varices, and splenomegaly after ligation. Eighty rats were randomly divided into 8 groups for ischemia for 45 minutes (T0), reperfusion for 6 hours (T6), 12 hours (T12), 24 hours (T24), 48 hours (T48), 72 hours (T72), 7 days (D7), and 14 days (D14). After the rats were sacrificed, tissue from the kidney, pancreas, small intestine, heart, and lung were taken and stained with hematoxylin-eosin (HE). The whole staining process consists of five steps: dewaxing, staining, dehydration, transparency, and sealing. Except for the heart, pathological scores were assigned as previously described16,17,18,19.
The time until maximum injury to the extrahepatic organs varied; it was 6-24 hours after the operation for the pancreas and 24-48 hours for the lungs. The intestinal tract and kidney were most severely injured after 45 minutes of ischemia. There was no obvious abnormality of the intestinal mucosa 24 hours after the operation, and the kidneys recovered after 48 hours. After reperfusion, local myocardial cell necrosis, cell fragmentation and dissolution, inflammatory cell infiltration, and local vasodilation and congestion were found in the heart by 24-48 hours after the operation (Figure 3).
Lungs
Neutrophil infiltration was found in the lung tissue after ischemia. With the increase of reperfusion time(T0,T6), mucus of the bronchial lumen could also be seen in the lung tissue. Inflammatory cell infiltration occurred in the alveolar wall, which became severely thickened. Alveolar collapse and disappearance of the alveolar cavity could also be found in some tissue. There was no significant alveolar edema or capillary congestion in the alveolar walls. They were most severely injured 24-48 hours after the operation, with some rats showing dyspnea and other manifestations 7 days post-operation. HE staining results suggested lymphadenitis in the airway, mild inflammatory cell infiltration in the alveolar wall, and local hemorrhage (Figure 3A, Figure 4).
Kidneys
A small amount of eosinophilic substance was found in renal tubules after ischemia at phase T0, but no inflammatory cell infiltration and other abnormalities were seen. However, swollen renal tubular epithelial cells, porous or vacuolated cytoplasm, necrotic cells in few lumens, karyopyknosis, fragmentation, brush border loss, and tube-shaped acidic group in many lumens were seen 6-48 hours after the operation. In addition, a small number of renal tubular epithelial cells were seen with granular degeneration, and porous and lightly stained cytoplasm seen 48 hours after the operation. Significant interstitial telangiectasia, yet no severe inflammatory cell infiltration, was found (Figure 3B, Figure 5).
Small intestine
The small intestine was most severely injured after ischemia (T0). There was severe inflammatory cell infiltration, mucosa epithelium shedding, and telangiectasia. With the increase of reperfusion time, the injury healed quickly. The mucosal epithelium was restored completely 24 hours after the operation, and only mild inflammatory cell infiltration was seen (Figure 3C, Figure 6).
Pancreas
Severe inflammatory cells infiltrated around the pancreatic tissue at phase T0. However, the pancreatic lesions were not uniform. Six out of 10 had pancreatic necrosis and inflammatory infiltration 24 hours after surgery, and the other 4 had no apparent abnormalities. Twenty-four hours after the operation, in addition to the infiltration of inflammatory cells, there was edema, widening of interlobular space, hemorrhage, necrosis of a small number of acinar cells, unclear demarcation of the cells, nuclear fragmentation and dissolution, and mild inflammatory cell infiltration in the visual field. Then, the inflammation disappeared slowly (Figure 3D, Figure 7 ).
Heart
By phase T0, cardiac myocytes were arranged regularly with clear demarcation, normal cell morphology, local interstitial congestion, and mild brown-yellow pigment deposition. Moreover, inflammatory cell infiltration was observed in myocardial interstitial and perivascular regions. With the increase in reperfusion time, local myocardial cell necrosis, cell fragmentation and dissolution, inflammatory cell infiltration, local vasodilation, and congestion were found in tissues 24-48 hours after the operation. Ventricular dilatation, porous structure, increased myocardial interstitium, and mild inflammatory cell infiltration were seen in some specimens. After 48 hours, local cardiomyocytes disappeared and were replaced by a small amount of fibrous connective tissue with mild inflammatory cell infiltration. By then, no other obvious abnormalities were seen (Figure 8).
Effects of hemodynamic changes on liver, kidney, pancreas, and heart serological indexes
Serum was collected, and the levels of alanine aminotransferase(ALT),aspartate aminotransferase(AST), creatinine, and amylase were detected by an automatic biochemical analyzer. All indicators peaked at 24-48 hours, unlike the pathological changes. Although these levels were normal 48 hours after the operation, pathological damage continued (Figure 9).
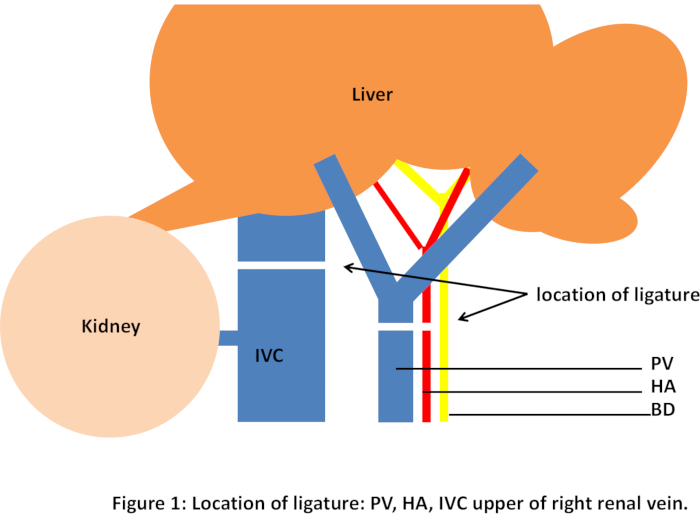
Figure 1: Location of ligature: PV, HA, IVC upper of right renal vein. Please click here to view a larger version of this figure.
| Group | n | survival at 24 h, n (%) | survival at 7 d, n (%) | survival at 14 d, n (%) |
| sham | 10 | 10 | — | — |
| I 15 min | 10 | 10/10 | 10 (100) | 10 (100) |
| I 30 min | 10 | 10/10 | 10 (100) | 10 (100) |
| I 45 min | 10 | 8/10 | 8/10 (80) | 8/10 (80) |
| I 60 min | 10 | 2/10 | 2/10 (20) | 2/10 (20) |
Table 1: Rats' tolerance to liver ischemia
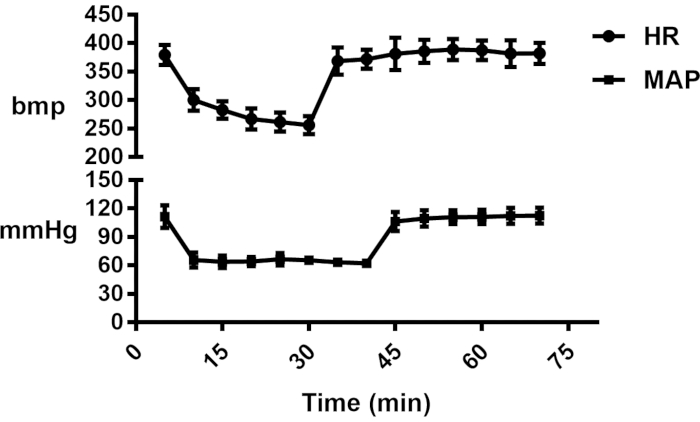
Figure 2: Hemodynamic changes in I45 min group. Please click here to view a larger version of this figure.
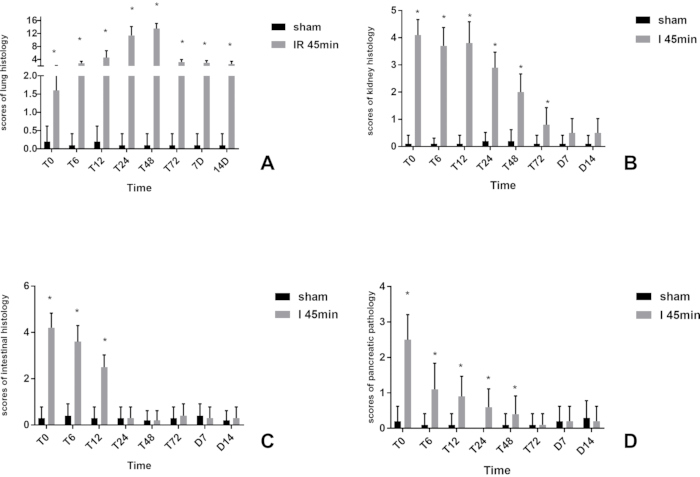
Figure 3: Scores of organ histology. (A) lung; (B) kidney; (C) intestine; (D) pancreas; *Statistically significant compared to the sham group (P < 0.05). Please click here to view a larger version of this figure.
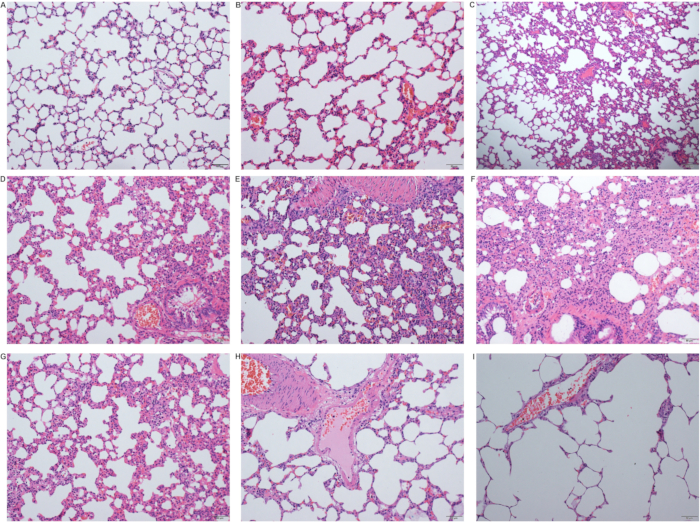
Figure 4: Pathological changes in the lungs after the operation. (A) Sham group; (B) Ischemia group (T0 group); (C) Reperfusion 6 hours (T6) group; (D) Reperfusion 12 hours (T12) group; (E) Reperfusion 12 hours (T12) group; (F) Reperfusion 24 hours (T24) group; (G) Reperfusion 48 hours (T48) group; (H) Reperfusion 7 days (D7) group; (I) Reperfusion 14 days (D14) group (scale 50 µm). Please click here to view a larger version of this figure.
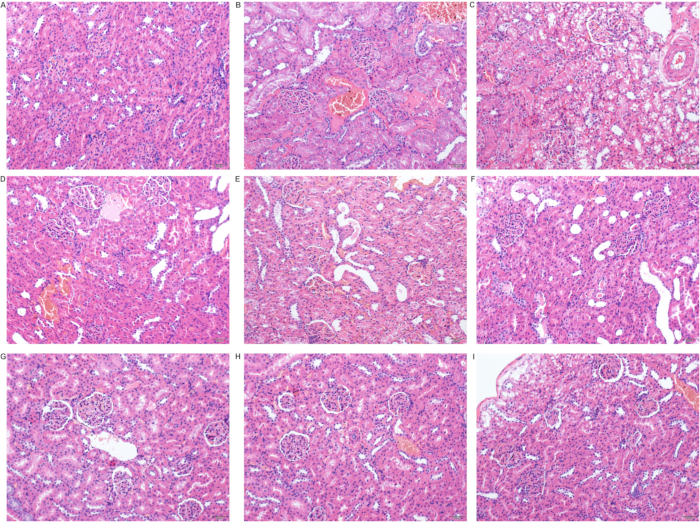
Figure 5: Pathological changes in the kidneys after the operation. (A) Sham group; (B) Ischemia group (T0 group); (C) Reperfusion 6 hours (T6) group; (D) Reperfusion 12 hours (T12) group; (E) Reperfusion 12 hours (T12) group; (F) Reperfusion 24 hours (T24) group; (G) Reperfusion 48 hours (T48) group; (H) Reperfusion 7 days (D7) group; (I) Reperfusion 14 days (D14) group (scale 50 µm). Please click here to view a larger version of this figure.
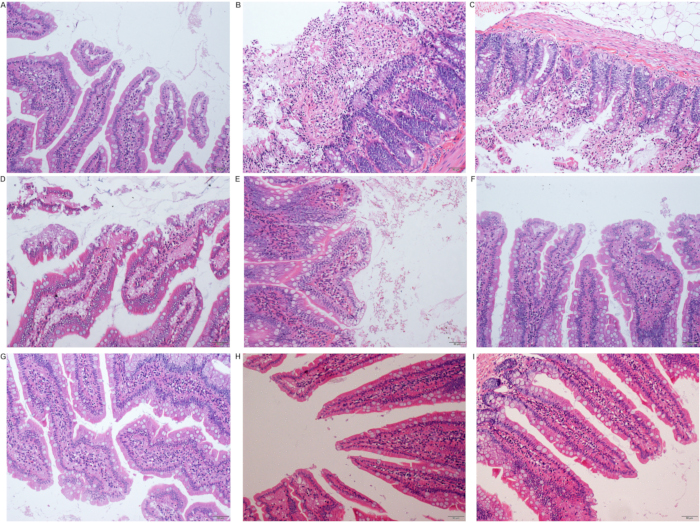
Figure 6: Pathological changes in the small intestine after the operation. (A) Sham group; (B) Ischemia group (T0 group); (C) Reperfusion 6 hours (T6) group; (D) Reperfusion 12 hours (T12) group; (E) Reperfusion 12 hours (T12) group; (F) Reperfusion 24 hours (T24) group; (G) Reperfusion 48 hours (T48) group; (H) Reperfusion 7 days (D7) group; (I) Reperfusion 14 days (D14) group (scale 50 µm). Please click here to view a larger version of this figure.
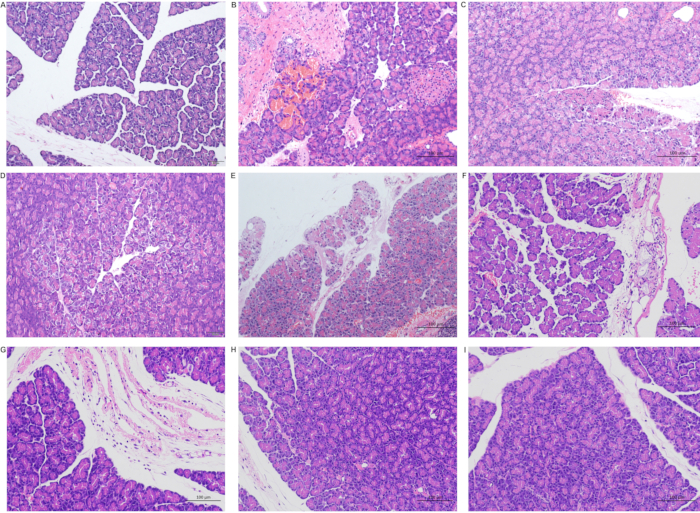
Figure 7: Pathological changes in the pancreas after the operation. (A) Sham group; (B) Ischemia group (T0 group); (C) Reperfusion 6 hours (T6) group; (D) Reperfusion 12 hours (T12) group; (E) Reperfusion 12 hours (T12) group; (F) Reperfusion 24 hours (T24) group; (G) Reperfusion 48 hours (T48) group; (H) Reperfusion 7 days (D7) group; (I) Reperfusion 14 days (D14) group (A and D scale 50 µm; BCEFGHI scale 50 µm). Please click here to view a larger version of this figure.
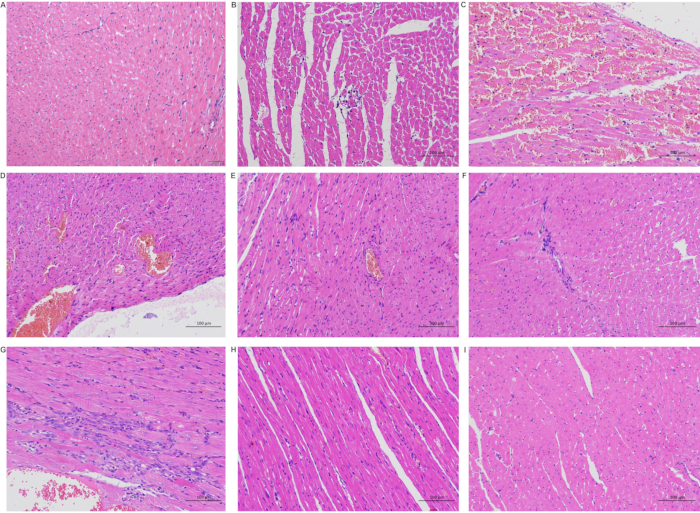
Figure 8: Pathological changes in the heart after the operation. (A) Sham group; (B) Ischemia group (T0 group); (C) Reperfusion 6 hours (T6) group; (D) Reperfusion 12 hours (T12) group; (E) Reperfusion 12 hours (T12) group; (F) Reperfusion 24 hours (T24) group; (G) Reperfusion 48 hours (T48) group; (H) Reperfusion 7 days (D7) group; (I) Reperfusion 14 days (D14) group (A scale 50 µm; BCDEFGHI scale 100 µm). Please click here to view a larger version of this figure.
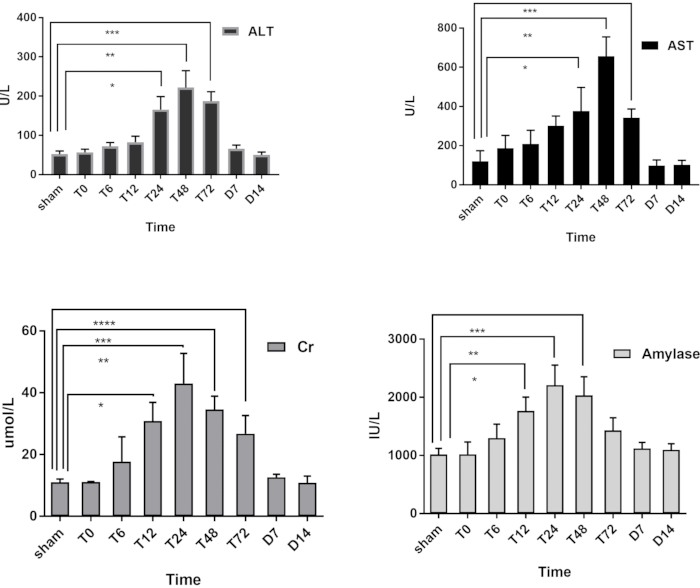
Figure 9: Changes of ALT, AST, creatinine (Cr) and amylase in each group; *Statistically significant compared to the sham group (P<0.05). Please click here to view a larger version of this figure.
Discussion
OLT in rats is an ideal model for studying organ preservation in liver transplantation, IRI, transplant rejection, immune tolerance, transplantation pathology and pharmacology, homotransplantation, and xenotransplantation. At present, it is widely used in the experimental research of liver transplantation.
During pilot studies we first administered pentobarbital sodium intraperitoneal anesthesia and found that this led to high postoperative mortality and short tolerance to hemodynamic changes. Thus, we used inhalation anesthesia in subsequent trials for rapid onset of action and rapid exclusion of in vitro characteristics. The switch to inhalation anesthesia significantly improved the tolerance time and the postoperative survival of rats. Investigators should pay attention to the breathing and heart rates of the rat to prevent overdosing the anesthetic. Biosystems can be used to monitor the heart rate and blood pressure. We also observed the impact of surgical suture thickness on the ligation of blood vessels. Although lines smaller than 3-0 could perfectly ligate the blood vessels, they were difficult to loosen and may lead to the rupture of blood vessels. On the contrary, lines larger than 3-0 may result in incomplete vascular occlusion, which prevents hemodynamic changes. These material problems will be improved upon in future experiments. There are some limitations to our protocol. Heat lamps are not recommended for temperature maintenance due to their potential for overheating; alternate heating suggestions, such as recirculating water blankets, are recommended for the animal's benefit.
There are many reasons for distal organ injury after OLT. First, injury can be caused by cold preservation of the donor liver in vitro20. Second, IRI can occur and cause tissue damage when blood supply returns to tissue (reperfusion) after a long period of ischemia. Ischemia is the leading cause of injury, and reperfusion is the process where the injury occurs. After simultaneously blocking the IVC and PV during the anhepatic phase, a large amount of blood stasis occurred in the lower limbs and internal organs. The effective circulating volume (ECV) decreased sharply, and the MAP decreased. However, due to vagus nerve stimulation, there was no compensatory increase in heart rate in rats. In this experiment, we found that rats underwent significant hemodynamic changes within 5 minutes of ligation and vessel release, which met the definition of the ischemia-reperfusion syndrome.
Ischemia occurred in some tissues outside the liver. After the anhepatic phase, ECV increased. MAP returned to normal after the IVC and PV were unblocked, with injury occurring outside the liver after reperfusion. Furthermore, IRI of the donor's liver produced inflammatory mediators (TNF-α, interleukin-1, interleukin-6, interleukin-8) that attacked the distal organs21. In this experiment, the hemodynamics during the anhepatic phase were simulated, which caused the passive congestion of IVC and kidney, damage to the gastrointestinal barrier, bacterial translocation, ischemia of the organs (e.g., lung, heart, pancreas, kidney, etc.) where the SVC is located, and IRI to the extrahepatic organs.
The pathological findings showed that the peak of ischemic injury and the recovery time were different in each organ. Although cold storage and damage caused by immune factors could not be simulated in this study, anhepatic IRI can be replicated and compared with other animal models to research extrahepatic organ injury. Our model and OLT model can be compared and observed to provide a basis for the research on extrahepatic organ injury. Furthermore, our model is similar to some clinical liver operations, such as that for Hilar cholangiocarcinoma. Hilar cholangiocarcinoma is a malignant tumor that frequently invades the PV or the IVC and often requires PV clamping during surgery22. Hepatic portal reconstruction was performed; when the tumor invades the IVC, intraoperative clamping of the IVC is also required, and the resulting hemodynamic changes are consistent with our model.
To summarize, our model in rats is easy-to-use and straightforward, without microsurgery, and provides the basis for fundamental research into IRI of extrahepatic organs after hepatic ischemia.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge the useful suggestions given by Dr. Wen-tao Li and Dr. Ji-hua Wu of the Second Affiliated Hospital of Guangxi Medical University. The authors would like to thank our team-mates for useful comments and discussions. The authors would also like to thank the anonymous reviewers and editors of JoVE for their comments. Special thanks should go to Dr Yuan's parents for their continuous support and encouragement. The work was supported by Ningbo Natural Science Foundation (2014A610248).
Materials
| 4% paraformaldehyde solution | Shanghai Macklin Biochemical Co.,Ltd | P804536 | |
| air drying oven | Shanghai Binglin Electronic Technology Co., Ltd. | BPG | |
| Alanine aminotransferase (ALT)Kit | Elabscience Biotechnology Co.,Ltd | E-BC-K235-S | |
| ammonia | Sinopharm Chemical Reagents Co. Ltd | 10002118 | |
| amylase Kit | Elabscience Biotechnology Co.,Ltd | E-BC-K005-M | |
| anhydrous ethanol | Sinopharm Chemical Reagents Co. Ltd | 100092183 | |
| Animal anesthesia machine | Shenzhen Ruiwode Life Technology Co. Ltd | R640 | |
| aspartate aminotransferase (AST)kit | Rayto Life and Analytical Sciences Co., Ltd. | S03040 | |
| automatic biochemical analyzer. | SIEMENS AG FWB:SIE, NYSE:SI Co., Ltd. | 2400 | |
| Biosystems (when nessary) | Chengdu Taimeng Electronics Co., Ltd. | BL-420F | |
| Centrifuge | Baiyang Medical Instrument Co., Ltd. | BY-600A | |
| cover glass | Jiangsu Shitai Experimental Equipment Co. Ltd | 10212432C | |
| creatinine Kit | Rayto Life and Analytical Sciences Co., Ltd. | S03076 | |
| dewatering machine | Hungary 3DHISTECH Co.,Ltd | Donatello Series 2 | |
| embedding machine | Hubei Xiaogan Kuohai Medical Technology Co., Ltd. | KH-BL1 | |
| frozen machine | Wuhan Junjie Electronics Co., Ltd | JB-L5 | |
| hematoxylin-eosin dye solution | Wuhan Saiwell Biotechnology Co., Ltd | G1005 | |
| high-efficiency paraffin wax | Shanghai huayong paraffin wax co., Ltd | Q/YSQN40-91 | |
| hydrochloric acid | Sinopharm Chemical Reagents Co. Ltd | 10011018 | |
| intraocular lens (IOL)forceps | Guangzhou Guangmei Medical Equipment Co., Ltd. | JTZRN | |
| Isoflurane | Shenzhen Ruiwode Life Technology Co. Ltd | — | |
| micro Scissors(when nessary) | Shanghai Surgical Instrument Factory | WA1010 | |
| needle holders | Shanghai Surgical Instrument Factory | J32010 | |
| neutral gum | Shanghai Huashen Healing Equipment Co.,Ltd. | — | |
| normal optical microscope | Nikon Instrument Shanghai Co., Ltd | Nikon Eclipse CI | |
| ophthalmic forceps | Shanghai Surgical Instrument Factory | J3CO30 | straight |
| ophthalmic forceps | Shanghai Surgical Instrument Factory | JD1060 | bending |
| ophthalmic Scissors | Shanghai Surgical Instrument Factory | J1E0 | |
| pathological slicer | Shanghai Leica Instrument Co., Ltd | RM2016 | |
| pipettes | Dragon Laboratory Instruments Co., Ltd. | 7010101008 | |
| retractors | Beijing Jinuotai Technology Development Co.,Ltd. | JNT-KXQ | |
| scanner | Hungary 3DHISTECH Co.,Ltd | Pannoramic 250 | |
| slide | Wuhan Saiwell Biotechnology Co., Ltd | G6004 | |
| xylene | Sinopharm Chemical Reagents Co. Ltd | 1330-20-7 |
Referências
- Dar, W. A., Sullivan, E., Byon, J. S., Eltzschig, H., Ju, C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver International. 39 (5), 788-801 (2019).
- Qiao, P. F., Yao, L., Zhang, X. C., Li, G. D., Wu, D. Q. Heat shock pretreatment improves stem cell repair following ischemia-reperfusion injury via autophagy. World Journal of Gastroenterology. 21 (45), 12822-12834 (2015).
- Liu, Y., et al. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury. Journal of Hepatology. 71 (4), 719-730 (2019).
- Hirao, H., Dery, K. J., Kageyama, S., Nakamura, K., Kupiec-Weglinski, J. W. Heme Oxygenase-1 in liver transplant ischemia-reperfusion injury: From bench-to-bedside. Free Radical Biology and Medicine. 157, 75-82 (2020).
- Motiño, O., et al. Protective role of hepatocyte cyclooxygenase-2 expression against liver ischemia-reperfusion injury in mice. Hepatology. 70 (2), 650-665 (2019).
- Guo, W. A. The search for a magic bullet to fight multiple organ failure secondary to ischemia/reperfusion injury and abdominal compartment syndrome. Journal of Surgical Research. 184 (2), 792-793 (2013).
- Elham, M., Mahmoudi, M., Nassiri-Toosi, M., Baghfalaki, T., Zeraati, H. Post liver transplantation survival and related prognostic factors among adult recipients in tehran liver transplant center; 2002-2019. Archives of Iranian Medicine. 1 (23), 326-334 (2020).
- Kim, E. H., Ko, J. S., Gwak, M. S., Lee, S. K., Kim, G. S. Incidence and clinical significance of hyperfibrinolysis during living donor liver transplantation. Blood Coagulation and Fibrinolysis. 29 (3), 322-326 (2018).
- Czigány, Z. Improving research practice in rat orthotopic and partial orthotopic liver transplantation: a review, recommendation, and publication guide. European Surgical Research. 55 (1-2), 119-138 (2015).
- Kamada, N., Calne, R. Y. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 28 (1), 47-50 (1979).
- Zimmermann, F. A., et al. Techniques for orthotopic liver transplantation in the rat and some studies of the immunologic responses to fully allogeneic liver grafts. Transplantation Proceedings. 11 (1), 571-577 (1979).
- Miyata, M., Fischer, J. H., Fuhs, M., Isselhard, W., Kasai, Y. A simple method for orthotopic liver transplantation in the rat. Cuff technique for three vascular anastomoses. Transplantation. 30 (5), 335-338 (1980).
- Kamada, N. A., Calne, R. Y. Surgical experience with five hundred thirty liver transplants in the rat. Surgery. 93 (1), 64-69 (1983).
- Yang, L. F., et al. A rat model of orthotopic liver transplantation using a novel magnetic anastomosis technique for suprahepatic vena cava reconstruction. Journal of Visualized Experiments. 19 (133), e56933 (2018).
- Liu, L. X., He, C., Huang, T., Gu, J. Development of a new technique for reconstruction of hepatic artery during liver transplantation in sprague-dawley rat. PLoS One. 10 (12), 0145662 (2015).
- Paller, M. S., Hoidal, J. R., Ferris, T. F. Oxygen free radicals in ischemic acute renal failure In the rat. Journal of Clinical Investigation. 74 (4), 1156-1164 (1984).
- Schmidt, J., Lewandrowsi, K., Warshaw, A. L., Compton, C. C., Rattner, D. W. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat. International Journal of Pancreatology. 12 (1), 41-51 (1992).
- Chui, C. J., McArdle, A. H., Brown, R., Scott, H. J., Gurd, F. N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Archives of Surgery. 101 (4), 478-483 (1970).
- Kozian, A., et al. One-lung ventilation induces hyperfusion and alveolar damage in the ventilated lung:an experimental study. British Journal of Anaesthesia. 100 (4), 549-559 (2008).
- Shimada, S., et al. Heavy water (D2O) containing preservation solution reduces hepatic cold preservation and reperfusion injury in an isolated perfused rat liver (IPRL) model. Journal of Clinical Medicine. 8 (11), 1818 (2019).
- Nakamura, K. Sirtuin 1 attenuates inflammation and hepatocellular damage in liver transplant ischemia/reperfusion: from mouse to human. Liver Transplantation. 23 (10), 1282-1293 (2017).
- Blaire, A., et al. Surgical Considerations of Hilar Cholangiocarcinoma. Surgical Oncology Clinics of North America. 28 (4), 601-617 (2019).

