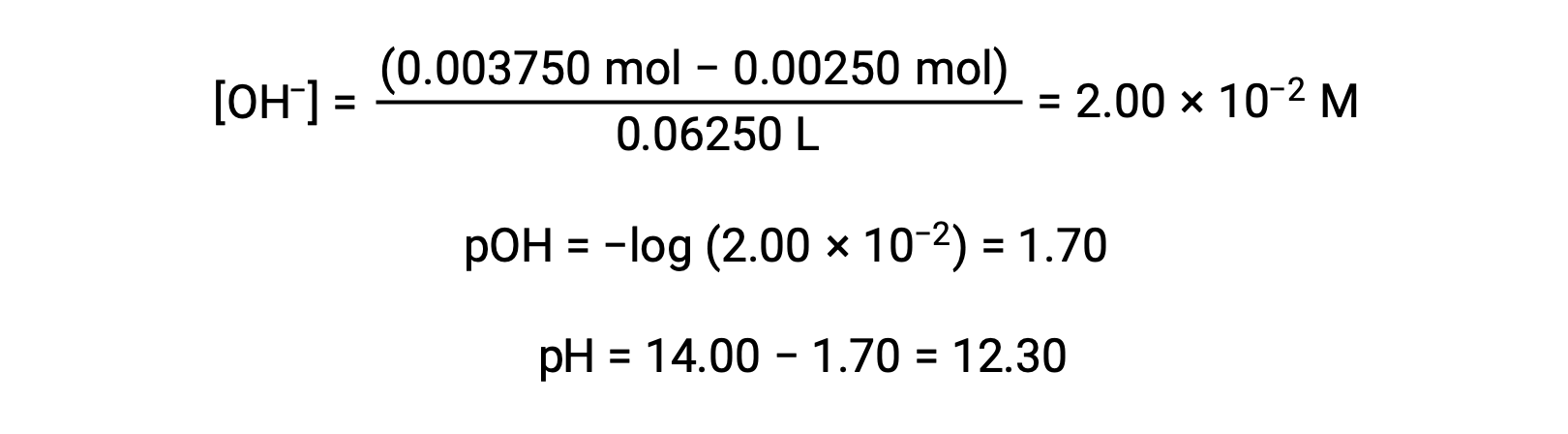
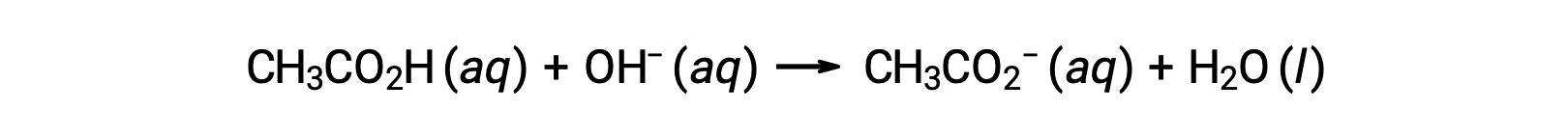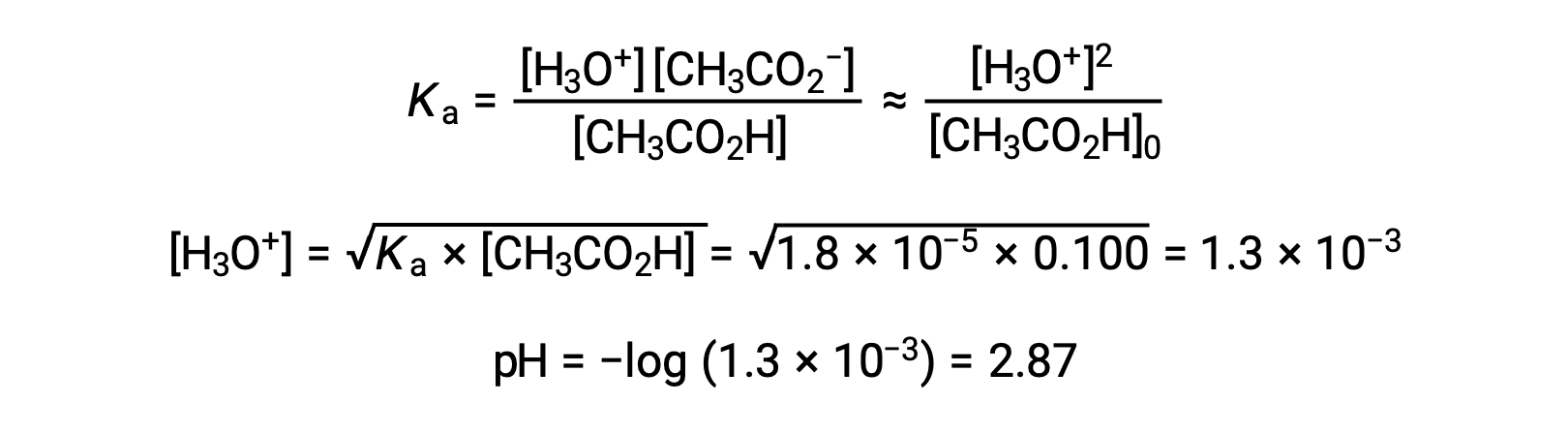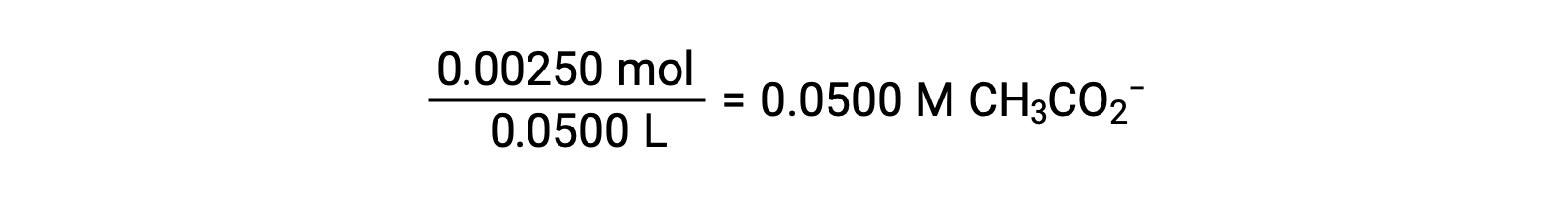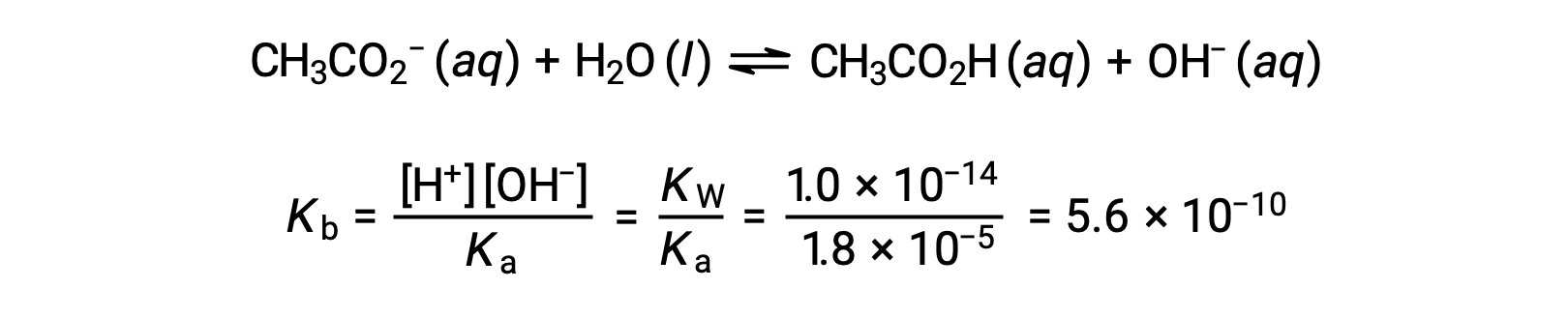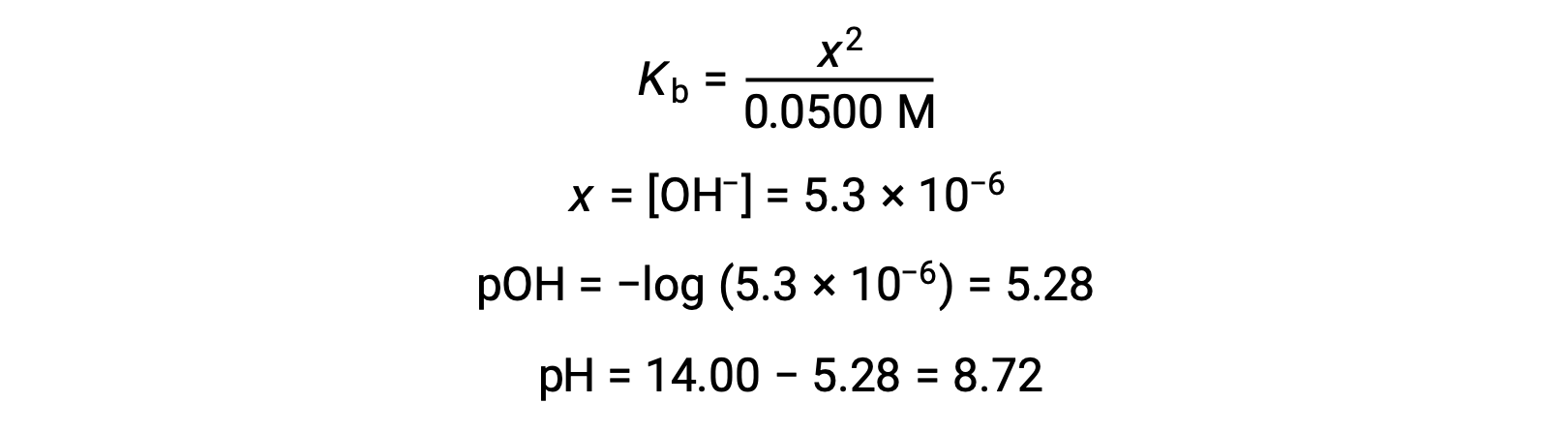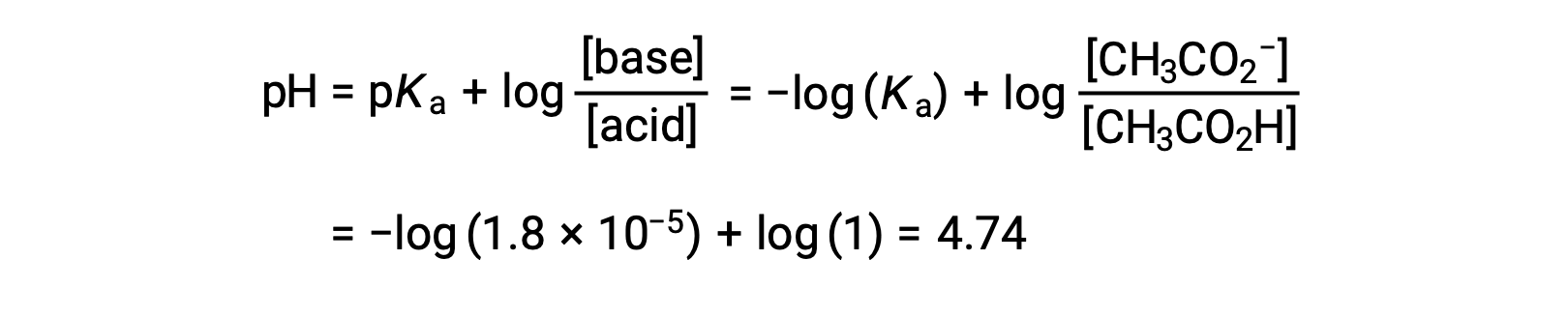The pH at different stages of a weak acid or base titration is calculated using different methods during various points of the titration. If a weak acid or base is the main determinant of the pH, the Ka or Kb and an ICE table, or the Henderson-Hasselbalch equation are used. If strong acid or base is present after the neutralization reaction, the concentration of the remaining hydronium or hydroxide ions is used to calculate the pH. The initial pH of 50 mL of a 0.10 M acetic acid solution is 2.87 and is calculated using the Ka and an ICE table, as acetic acid is the main contributor. If this solution containing 0.0050 moles of acetate is titrated with 0.10 M sodium hydroxide, the hydroxide ions react with acetic acid to produce acetate, resulting in a buffer. Therefore, when 10 mL of the sodium hydroxide containing 0.0010 moles of hydroxide ions is added, 0.0010 moles of acetate are formed, and 0.0040 moles of acetic acid remain. The pH of the buffer can be calculated by substituting these values into the Henderson-Hasselbalch equation and equals 4.14. When 25 mL of the sodium hydroxide is added, half of the initial moles of acetic acid are converted into acetate. At this point, the pH equals the pKa, as the amount of acetic acid and acetate ion are equal. The further addition of sodium hydroxide up to 50 mL converts all of the acetic acid molecules into acetate, and the equivalence point is reached. As acetate ions are basic, the equivalence point lies in the basic region. The concentration of the acetate ion is calculated by dividing the number of moles by the total volume of the solution. The pH is determined using the Kb for acetate ions and an ICE table, as the acetate ion is the main contributor to the pH at the equivalence point. The Kb for acetate is calculated using the formula, Kw = Ka × Kb, and equals 5.6 × 10−10. Substituting equilibrium concentrations in the expression for Kb gives the hydroxide concentration, 5.3 × 10−6 M. The pOH and pH of the solution are 5.28 and 8.72, respectively. Further addition of sodium hydroxide in the solution results in a mixture of acetate ions and sodium hydroxide. However, the final concentration of sodium hydroxide determines the pH of the solution, as it is a stronger base than acetate. Therefore, if 70 mL of sodium hydroxide is added into the solution, the final concentration of the hydroxide ions can be calculated by subtracting the total moles of acetic acid, 0.0050 moles, from the total moles of hydroxide ions added, 0.0070 moles, and dividing it by the total volume of the solution, 120 mL or 0.12 L. As the hydroxide ion concentration is 0.017 M, the pOH and pH of the solution are calculated to be 1.78 and 12.22, respectively.