A Method for High Fidelity Optogenetic Control of Individual Pyramidal Neurons In vivo
Summary
The recent development of neuroscience tools that combine genetics and optics, termed “optogenetics”, enables control over neural circuit activity with an unprecedented level of spatial and temporal resolution. Here we provide a protocol for integrating in vivo recording with optogenetic manipulation of genetically-defined subsets of prefrontal cortical and subicular pyramidal neurons.
Abstract
Optogenetic methods have emerged as a powerful tool for elucidating neural circuit activity underlying a diverse set of behaviors across a broad range of species. Optogenetic tools of microbial origin consist of light-sensitive membrane proteins that are able to activate (e.g., channelrhodopsin-2, ChR2) or silence (e.g., halorhodopsin, NpHR) neural activity ingenetically-defined cell types over behaviorally-relevant timescales. We first demonstrate a simple approach for adeno-associated virus-mediated delivery of ChR2 and NpHR transgenes to the dorsal subiculum and prelimbic region of the prefrontal cortex in rat. Because ChR2 and NpHR are genetically targetable, we describe the use of this technology to control the electrical activity of specific populations of neurons (i.e., pyramidal neurons) embedded in heterogeneous tissue with high temporal precision. We describe herein the hardware, custom software user interface, and procedures that allow for simultaneous light delivery and electrical recording from transduced pyramidal neurons in an anesthetized in vivo preparation. These light-responsive tools provide the opportunity for identifying the causal contributions of different cell types to information processing and behavior.
Introduction
The ability to activate or silence a specific cell type within a neural circuit in a temporally precise fashion is critical for understanding how neural circuits process different types of information underlying emotion and cognition. Experimental control over intact neural activity has employed loss-/gain-of-function tools (e.g., electrical stimulation, pharmacological modulation, lesion) that do not provide the required selectively for controlling specific populations of neurons, either on a temporal or spatial scale. Directly addressing these technological challenges, the development and application of genetically-encodable light-sensitive tools has enabled neuroscientists to control the electrical activity of select cell types during well-defined behavioral events. Many of these light-sensitive proteins are of microbial origin, with the light-gated cation channel, channelrhodopsin-2 (ChR2) 1, and the light-driven chloride pump, halorhodopsin (NpHR) 2,3, in widespread use.
A major advantage of optogenetics is the ability to genetically-target specific cell populations in heterogeneous brain regions and the technique has been successfully applied to a number of model organisms (invertebrates to nonhuman primates) 4-6. Many optogenetic transgenic animals have been generated and are commercially available 7,8, however establishing transgenic lines can be labor intensive and cost prohibitive. Here we describe a protocol for virally-mediated delivery of ChR2 and NpHR genes under the CaMKIIα promoter in rat prelimbic cortex and dorsal subiculum using a recombinant adeno-associated virus (AAV) vector. Within the forebrain, CaMKIIα expression is exclusive to glutamatergic pyramidal neurons 9. AAV is commonly used for basic research due to its relative ease of production and lack of pathogenicity, as well as the strong and persistent transgene expression that has been achieved with these vectors 10. Additionally we outline the steps and hardware for simultaneous light delivery and recording in anesthetized head-fixed rats.
Protocol
1. Virus Storage and Preparation
- The use of replication-incompetent AAV vectors for delivering opsin genes to rodent brain is approved for Biosafety Level 1 (BSL-1) and requires proper protection and handling procedures as outlined in the U.S. Government publication, Biosafety in Microbiology and Biomedical Laboratories, available at the Centers for Disease Control’s website at (http://www.cdc.gov/biosafety/publications/bmbl5/index.htm).
- In order to avoid repeated freeze-thaw cycles, pipette virus from manufacturer’s vial into smaller working aliquots in a Class II Biosafety Cabinet and store in a -80 °C freezer.
- Prior to stereotactic injection, allow an aliquot to thaw on ice and then briefly spin down with a bench top centrifuge.
- Slowly backfill a Hamilton glass syringe and needle with a small amount of silicone or mineral oil. Make sure there are no visible air bubbles in the syringe barrel. Insert the syringe into a microinjector pump and attach the pump directly to the vertical stereotaxic arm (i.e. stereotaxic manipulator).
- Lower the stereotaxic arm until the tip of the needle touches the bottom of the virus aliquot tube. Use the controller for the microinjector pump to withdraw the desired volume of virus (1.5 μl for a 1 μl injection).
- All material and surfaces that come into contact with virus should be decontaminated with a disinfectant such as chlorine bleach (10%).
2. Surgery and Virus Injection
- Anesthetize animal with a ketamine (rat; 125 mg/kg, i.p.) – xylazine (rat; 10 mg/kg, i.p.) cocktail. To gauge effectiveness of anesthesia, check for a reflex response to a toe pinch and give supplemental doses of ketamine if needed. Using standard aseptic surgical methods, place animal into a stereotaxic apparatus (David Kopf Instruments).
- Make a midline incision through the skin on top of the animal skull with small surgical scissors or scalpel. Gently separate connective tissue and clean the top of the skull with a small bone scraper.
- Verify that the head of the animal is level such that the z coordinates for bregma and lambda are equal. Determine the stereotaxic coordinates for the target brain area from a brain atlas such as The Rat Brain in Stereotaxic Coordinates (George Paxinos and Charles Watson, Academic Press, 2007) or The Mouse Brain in Stereotaxic Coordinates (Keith B.J. Franklin and George Paxinos, Academic Press, 2007). Mark the intended site of injection with a surgical pen.
- Carefully thin the skull over the target area using a hand-held drill and stop when the drill bit reaches the bottom of the skull. Using a pair of extra fine forceps, remove the thinned bone in order to expose the dura.
- Position the syringe needle over the target area and lower the needle until it touches the dura and use this point to calculate the z coordinate. Very slowly lower the injection needle into the brain until the proper z position is reached. In this protocol, the prelimbic region of the medial prefrontal cortex (2.7 mm anterior to bregma, ± 0.5 mm lateral to the midline, and -2.2 mm from dura) or the dorsal subiculum (-6.0 mm anterior to bregma, ± 3.0 mm lateral to the midline, and -2.0 mm from dura) is targeted in an adult Sprague Dawley male rat (approx. 275 g).
- Inject virus at a rate of 0.1 μl/min. After the injection is completed wait 10 min before withdrawing the needle to avoid backflow of the virus to the brain surface.
- Use a sewing suture with attached needle to seal the skin and apply antibiotics to the wound.
- The time course of ChR2 and NpHR functional expression from AAV vectors will vary depending on the experimental design and viral titer. For this experiment, in vivo recordings were performed 18-21 days post injection.
3. Light Delivery and In vivo Recording of ChR2 or NpHR-expressing Neurons
- Attach a tungsten electrode (~1-1.5 MΩ, MicroProbes) to a glass capillary tube (Fisher Scientific) using super glue.
- Use a fiber stripping tool (Thorlabs) to expose the bare end of a multimode optical fiber (200 μm diameter core, Thorlabs) and clean fiber tip with ethanol. Very lightly score fiber tip with a wedge shaped diamond knife (Thorlabs) and carefully remove the excess fiber (e.g. with fine forceps). The fiber should cleave easily at the score.
- Connect the FC end of the fiber to the PC connector on the output port of a 200 mW single diode laser (www.Lumiphy.com) with the desired wavelength. Make sure that appropriate protective eyewear is worn at all times when working with lasers.
- Measure the laser intensity at the tip of the optic fiber using an optical power meter (www.Lumiphy.com ). For in vivo recordings, light intensity as little as 20-100 mW/mm2 can reliably evoke ChR2- or NpHR-mediated activation or silencing, respectively, of neuronal activity.
- Insert the optical fiber into the capillary tube. Position the fiber tip approximately 500 μm above the tip of tungsten electrode and tie a thin suture thread twice at a few points near the tip so that the electrode and the fiber are straight. Make sure that the distance between the tip of electrode and the nearest knot is long enough for insertion to the target region.
- Prepare the animal as in steps 2.1 and 2.2 above. Use the initial craniotomy as a guide to make a new clean small window in the skull for positioning the optrode (www.Lumiphy.com ). Make a small incision on the dura using a fine needle.
- Carefully lower the optrode through the skull window and into the brain to the transduced region using a micromanipulator (Narishige). Then advance the optrode slowly in 10-50 μm steps until the emergence of a light-responsive cell.
- A custom software user interface (NeuroLux Pro www.Lumiphy.com) written in LabVIEW (National Instruments) is used to control the single diode laser and to visualize and record ongoing neural activity. Recorded signals are captured with an ExAmp-20K (Kation Scientific) amplifier band-pass filtered (0.3-8 kHz), and a National Instruments analog to digital board (NI USB-6009). The NeuroLux Pro user interface allows the choice of analog inputs (choose two channels for the neuronal and TTL signal) and digital output. The user may define the sampling rate (20 kHz), the baseline period (pre-light), and post-light period. The user may define the TTL laser stimulation pulse width, frequency and stimulation period (if frequency is 20 Hz and stimulation period is 500 msec, 10 laser pulses with defined pulse width are delivered). The user also can choose continuous light delivery (e.g., Figure 1). For repeated recordings, the user also may define the number of pulse trains and the interval between trains. Data can be acquired with or without recording to disk.
- Following recording, animals are anesthetized with the ketamine/xylazine cocktail and transcardially perfused with physiological saline and paraformaldehyde (4%) in order to verify optrode placement and opsin expression.
Representative Results
Figure 1 depicts a screenshot of the customized software (NeuroLux Pro) developed in LabVIEW environment (National Instruments) used for simultaneous recording of neuronal activity and controlling light pulse parameters (i.e. pulse frequency, pulse width, stimulation period). The software program sends a command signal to a digital acquisition device that generates TTL pulses for control over the single diode laser. Additionally the software program displays and stores the recorded neuronal activity and delivered TTL signals. These signals are digitized through the acquisition device at a defined sample rate (e.g., 20 kHz). Recorded signals are saved as a two-dimensional double-precision array in a binary file.
Figure 2 shows the custom optrode consisting of a tungsten electrode attached to an optical fiber. An optical fiber is fixed to the electrode using thin suture thread at three points. If needed, super glue can be used to adhere them. However, avoid permanently fixing the optical fiber to the electrode because although the electrode may be reused several times, light transmission through the fiber will degrade with repeated use and should be cleaved and cleaned or replaced with every insertion into the brain.
Left panels of Figure 3 show fluorescent images of enhanced yellow fluorescent protein, indicating actual ChR2 and NpHR expression. These photographs show representative NpHR expression in rat dorsal subiculum (Figure 3A) and ChR2 expression in prelimbic cortex (Figure 3B). Viral expression was restricted mostly to the dorsal subiculum and prelimbic cortex (e.g. some fluorescence was observed in dentate gyrus (Figure 3A, left)). The tracks of the electrode (thin track, arrow head) and optical fiber (thick track, arrow) were also observed.
Left panel of Figure 4 shows that ChR2 induced temporally precise spiking in rat prelimbic cortex. Figure 4A is an example trace of ChR2-triggered action potentials in response to 20 Hz delivery of 10 msec blue light pulses to rat prelimbic cortex. Raster plot (Figure 4B) shows ChR2-induced activation in six representative neurons. All recorded neurons showed light-evoked spiking with perfect fidelity. In contrast to ChR2, NpHR rapidly and reversibly silenced spontaneous activity in vivo in rat prelimbic cortex (right panel of Figure 4). Figure 4C is an example trace showing that continuous 532 nm illumination (10 s) of the prelimbic cortex expressing NpHR under the CaMKllα promoter eliminates spontaneous single-unit activity in vivo. Note that the complete silencing of prelimbic pyramidal cell activity is time-locked to the 10 sec continuous light delivery. Lower raster plot (Figure 4D) shows NpHR-induced silencing in six representative neurons. Both in vivo recordings are typical of those obtained from adult Sprague-Dawley rats in which AAV-mediated delivery of microbial opsin genes occurred 18-21 days prior.
Figure 5 shows the result of repeated ChR2 stimulation of a rat prelimbic pyramidal neuron. Blue light pulse (10 msec) was delivered at 20 Hz for 5 sec (100 pulses total). Voltage traces of the light-evoked spiking were repeatedly acquired every 2 min during a 2 hr recording session (61 repetitions total). Even when using this repetitive stimulation protocol, ChR2 induced stable and robust spiking in response to the light delivery.
Figures 6 and 7 show the results of NpHR-induced photoinhibition and ChR2-driven photoactivation of rat subicular neuron activity. As is the case in prelimbic cortex, NpHR and ChR2 were able to mediate the light-induced time-locked inhibition and activation of the opsin-transduced subicular neurons with high reproducibility.
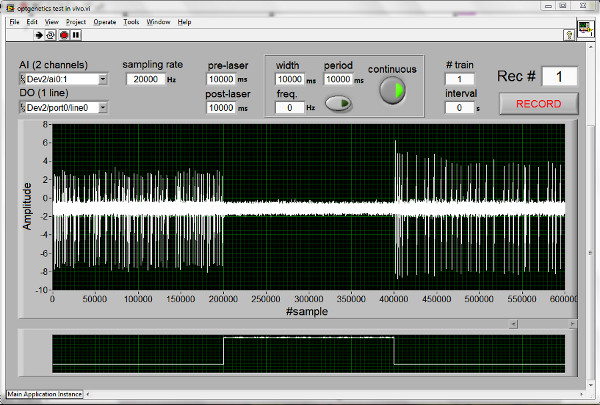
Figure 1. Screenshot of the NeuroLux Pro software interface for simultaneous light delivery and electrophysiological recording. Pictured trace shows NpHR induced silencing of spontaneous activity of rat prelimbic pyramidal cell in response to 10 sec of continuous 532 nm light delivery. Click here to view larger figure.
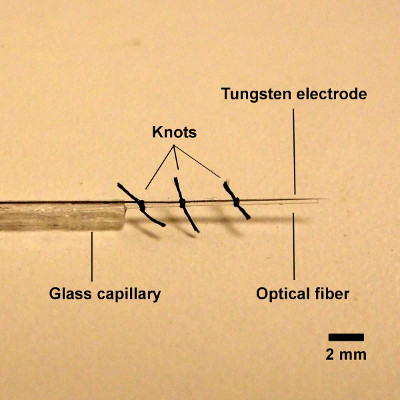
Figure 2. High power image of custom optrode. An optical fiber is inserted into glass capillary tube attached to a tungsten electrode and fixed to the electrode using suture thread. The center-to-center distance between the electrode tip and the fiber tip is approximately 500 μm.
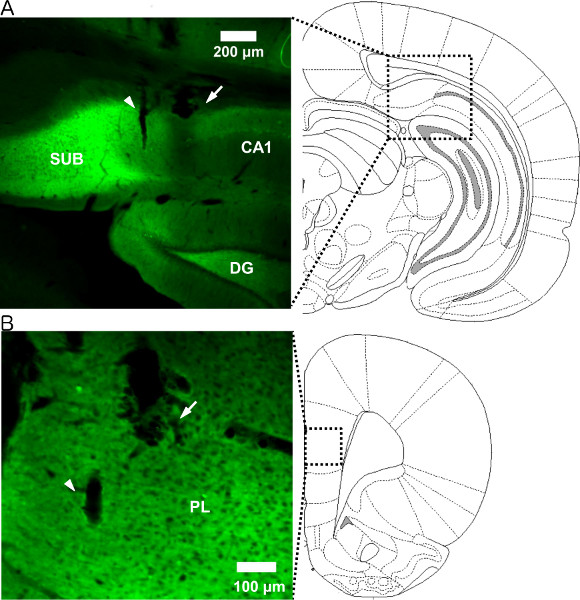
Figure 3. Viral expression and optrode placement. (Left) Photographs show representative NpHR expression in dorsal subiculum (A) and ChR2 expression in prelimbic cortex (B). (Right) Schematic that represents the location where each photograph was taken. The arrowhead and arrow in each photograph indicate the location of the tungsten electrode and optical fiber, respectively. SUB: subiculum, DG: dentate gyrus, PL: prelimbic cortex. Click here to view larger figure.
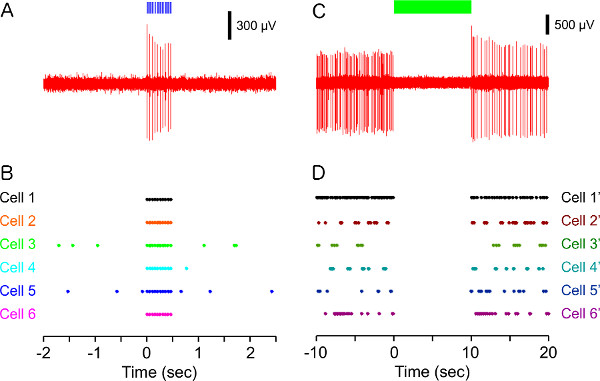
Figure 4. In vivo electrophysiological recordings from ChR2 or NpHR-transduced rat prelimbic cortex. (A) Example trace of ChR2-triggered action potentials in response to 20 Hz delivery of blue (473 nm) light pulses (10 msec, blue bar). (B) Raster plot showing ChR2-induced spiking in six representative neurons. Each unit activity is plotted as a dot. (C) Example trace of NpHR-induced suppression of spontaneous activity during continuous green (532 nm) light illumination (10 sec, green bar). (D) Raster plot showing NpHR-induced silencing in six representative neurons. Each unit activity is plotted as a dot. Click here to view larger figure.
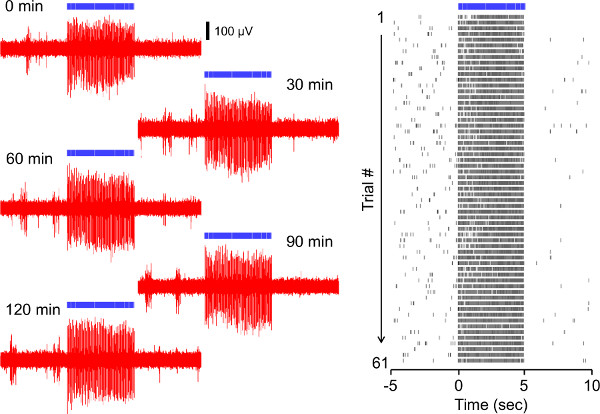
Figure 5. Repetitive 20 Hz ChR2-driven spiking of a rat prelimbic pyramidal neuron in vivo. (A) Voltage traces of the light-evoked spiking acquired at the time points 0, 30, 60, 90, 120 min after the beginning of the recordings. (B) Raster plot showing all 61 repetitions (2 min inter-trial interval, 120 min total) of the light-induced activation. Each unit activity is plotted as a dot. Click here to view larger figure.
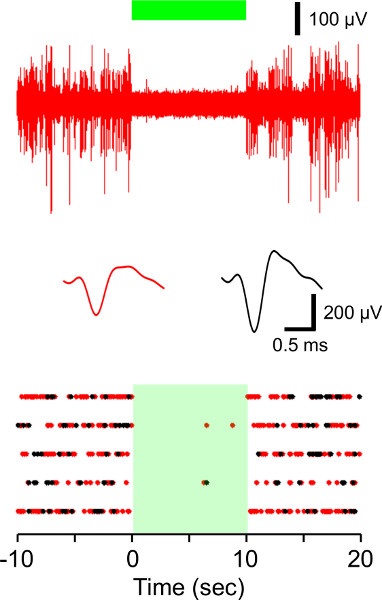
Figure 6. In vivo electrophysiological recordings from NpHR-transduced rat dorsal subiculum. (A) Example trace showing that 10 sec continuous 532 nm illumination (green bar) of the dorsal subiculum expressing NpHR eliminates spontaneous activity. (B) Average waveforms of the two recorded units from the above trace. The amplitude threshold was used for identifying two distinct neurons. (C) Raster plot showing five repetitions of NpHR-induced silencing of these units. Each unit activity is plotted as a dot.
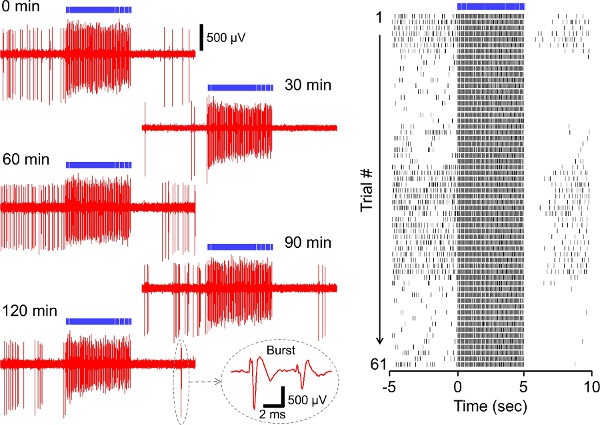
Figure 7. Repetitive 20 Hz CR2-driven spiking of a rat dorsal subicular neuron in vivo. (A) Voltage traces of the light-evoked spiking acquired at the time points 0, 30, 60, 90, 120 min after the beginning of the recordings. Inset: typical bursting activity of this cell. (B) Raster plot showing all 61 repetitions (2 min inter-trial interval, 120 min total) of the light-induced activation. Each unit activity is plotted as a dot. Click here to view larger figure.
Discussion
A wide array of techniques are available for genetically-targeting microbial opsin genes to discrete brain regions in rodents. Viral gene delivery provides a relatively inexpensive and quick approach for mediating ChR2 and NpHR expression with cell-type specificity. AAV vector systems are a common choice for use in optogenetic experiments due to high production titers that remain stable during storage, its lack of pathogenicity, and its ability to produce long-term gene expression 10. Many of the opsin constructs with cell-type specific promoters are commercially available in a variety of AAV serotypes from vector core facilities such as the University of Pennsylvania (http://www.med.upenn.edu/gtp/vectorcore) or the University of North Carolina at Chapel Hill (http://genetherapy.unc.edu). One drawback to AAV technology is the limited packaging capacity (~4.7 kb), which places a restriction on the transgene cassette size that can be used for cell-specific targeting. As an alternative, lentiviral vectors, which have a larger packaging capacity, are able to accommodate opsin targeting under the control of larger promoter sequences.
The use of an optrode allows for reliable detection of electrophysiological signals in combination with temporally-precise light delivery. As described above, however, sufficient care is needed in its construction. Since the cleaved optical fiber is fragile, tying the suture thread too tightly may cause the fiber to break. Even though the optrode can be used for multiple recordings, the electrode and optical fiber should be cleaned (or manually cleaved in the case of the optical fiber bare end) prior to insertion because the impedance of the electrode and quality of the delivered light will degrade with repeated use.
During electrophysiological recordings it is important that the optrode be advanced slowly. The optrode used here has approximately 350 μm thickness (tungsten electrode: 125 μm; core of optic fiber: 200 μm) and lowering it fast could lead to movement of brain tissue. Light-induced artifacts also might be considered with particular recording and light delivery set-ups 11-12. Although we found no light-induced artifact in our experimental condition, recordings outside the transduced brain area can potentially be used as a control for light artifacts. Another consideration during the recording process is the required light intensity for observing changes in ChR2- or NpHR-expressing neurons, especially for long duration recordings. Strong laser intensity and long laser illumination could result in the tissue damage and/or a decreased response to subsequent delivered light 12-13. For behavioral experimentation the use of a control viral vector is required for eliminating any effect due to heat delivered from the fiber. For many of the commercially available optogenetic constructs there are complementary control constructs in which the opsin gene sequence has been removed.
It should be noted that engineered ChR2 variants with improved properties and kinetics have been developed along with other silencing opsins that may be better suited for certain experimental questions 14-15. For instance, a new ChR2 variant established via targeted mutagenesis, termed “ChETA”, can drive high-fidelity neuronal spiking at frequencies (up to at least 200 Hz) above that of the original ChR2 14.
The combination of in vivo light delivery and simultaneous recording of neuronal responses is a critical step in establishing causal relationships between patterned activity in genetically-targeted cell populations and corresponding time-locked behavioral events. The procedures and hardware outlined here provide a straightforward approach for implementing optogenetics for in vivo head-fixed recordings in rodents.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Institute on Drug Abuse (NIDA) grant R01 DA24040 (DCC), University of Colorado Innovative Seed Grant (DCC), and NIDA training grant T32 DA017637 (MVB).
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| Syringe | Hamilton | 7653-01 | 10 μl |
| Removable Needle | Hamilton | 7803-03 | 31 gauge, beveled tip |
| Microinjector Pump | World Precision Instruments | UMP3 | |
| Pump Controller | World Precision Instruments | SYS-MICRO4 | |
| Silicone Oil | Alfa Aesar | A12728 | |
| Laser Protective Eyeware | Kentek | KMT-4501 | |
| Multimode Optical Fiber | Thorlabs | BFL37-200 | 200 μm diameter core, 0.37 NA |
| Fiber Stripping Tool | Thorlabs | T12S21 | for 200 μm diameter core multimode fiber |
| Optical Power Meter | Lumiphy LLC | www.lumiphy.com | |
| Photodetector | Newport | 818-SL/DB | |
| Blue Laser (445-473 nm, 100-200 mW) | Lumiphy LLC | www.lumiphy.com | coupled to a 200 μm multimode fiber with FC/PC adapter |
| Green Laser (532 nm, 100-200 mW) | Lumiphy LLC | www.lumiphy.com | coupled to a 200 μm multimode fiber with FC/PC adapter |
| Tungsten/Fiber Optrode | Lumiphy LLC | www.lumiphy.com | Lumitrode |
| Glass Capillary Tube | Fisher Scientific | 22-362-566 | |
| Hydraulic Micromanipulator | Narishige | MO-22 | |
| Amplifier | Kation Scientific | ExAmp-20K | |
| Data Acquistion Device | National Instruments | NI USB-6009 | |
| Rodent Head Restraint for Recording | Lumiphy LLC | www.lumiphy.com | |
| Small Animal Stereotaxic Unit | David Kopf Instruments | Model 963 |
References
- Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G., Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 8 (9), 1263-1268 (2005).
- Han, X., Boyden, E. S. Multiple-color optical activation, silencing, and desynchronization of neural activity with single-spike temporal resolution. PLoS One. 2 (3), e299 (2007).
- Zhang, F., Wang, L. P., et al. Multimodal fast optical interrogation of neural circuitry. Nature. 446 (7136), 633-639 (2007).
- Nagel, G., Brauner, M., Liewald, J. F., Adeishvili, N., Bamberg, E., Gottschalk, A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 15 (24), 2279-2284 (2005).
- Adamantidis, A. R., Zhang, F., Aravanis, A. M., Deisseroth, K., de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 450 (7168), 420-424 (2007).
- Han, X. Optogenetics in the nonhuman primate. Prog Brain Res. 196, 215-233 (2012).
- Zhao, S., Ting, J. T., et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 8 (9), 745-752 (2011).
- Madisen, L., Mao, T., et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-activation and silencing. Nat Neurosci. 15 (5), 793-802 (2012).
- Benson, D. L., Isackson, P. J., Gall, C. M., Jones, e. g. Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience. 46 (4), 825-849 (1992).
- McCown, T. J. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 5 (3), 333-338 (2005).
- Cardin, J. A., Carlen, M., et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 5 (2), 247-254 (2010).
- Cardin, J. A. Dissecting local circuits in vivo: Integrated optogenetic and electrophysiology approaches for exploring inhibitory regulation of cortical activity. J Physiol Paris. 106 (3-4), 104-111 (2012).
- Tsunematsu, T., Kilduff, T. S., et al. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 31 (29), 10529-10539 (2011).
- Gunaydin, L. A., Yizhar, O., et al. Ultrafast optogenetic control. Nat Neurosci. 13 (3), 387-392 (2010).
- Chow, B. Y., Han, X., et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 463 (7277), 98-102 (2010).

