Force Spectroscopy of Single Protein Molecules Using an Atomic Force Microscope
Summary
We describe the detailed procedures and strategies to measure the mechanical properties and mechanical unfolding pathways of single protein molecules using an atomic force microscope. We also show representative results as a reference for selection and justification of good single protein molecule recordings.
Abstract
The determination of the folding process of proteins from their amino acid sequence to their native 3D structure is an important problem in biology. Atomic force microscopy (AFM) can address this problem by enabling stretching and relaxation of single protein molecules, which gives direct evidence of specific unfolding and refolding characteristics. AFM-based single-molecule force-spectroscopy (AFM-SMFS) provides a means to consistently measure high-energy conformations in proteins that are not possible in traditional bulk (biochemical) measurements. Although numerous papers were published to show principles of AFM-SMFS, it is not easy to conduct SMFS experiments due to a lack of an exhaustively complete protocol. In this study, we briefly illustrate the principles of AFM and extensively detail the protocols, procedures, and data analysis as a guideline to achieve good results from SMFS experiments. We demonstrate representative SMFS results of single protein mechanical unfolding measurements and we provide troubleshooting strategies for some commonly encountered problems.
Introduction
Advances in single molecule force spectroscopy (SMFS) by AFM have enabled mechanical manipulation and precise characterization of single protein molecules. This characterization has produced novel insights about protein mechanics1,2, protein folding3, protein-ligand interactions4, protein-protein interactions5, and protein-based engineered materials6,7,8. SMFS is especially useful for studying protein unfolding, as stretching by AFM allows the chemical and physical bonds within the protein molecule to gradually extend according to their stiffness, which gives rise to a continually increasing contour length. This overstretching of a protein molecule can produce an abrupt transition in the force-extension curve resulting in a rupture event (or force peak). The force peak gives direct information on the unfolding force and structural change of the protein during the mechanical unfolding process. One of the first studies using AFM measured titin1 and found novel aspects of protein unfolding and refolding under physiological conditions without the use of unnatural denaturants like concentrated chemicals or extreme temperatures.
SMFS experiments are conducted on a variety of instruments, though here we consider only the AFM. The AFM is composed of four main elements: the probe, the detector, the sample holder, and the piezoelectric scanner. The probe is a sharp tip on the free-swinging end of a cantilever. After calibration, bending of the cantilever during stretching of an attached molecule is measured using a laser beam that is reflected off the back of the cantilever to precisely determine forces using Hooke's law. The reflected laser beam projects into a quadrant photodiode detector which produces a voltage in proportion to the displacement of the laser beam from the diode center. The substrate with the protein sample in fluid is mounted onto a 3D piezoelectric stage that can be controlled with sub-nanometer precision. A computer reads the voltage from the photodiode detectors and controls the 3D stage through a computer-controlled voltage supply. These piezo actuator stages are usually equipped with capacitive or strain-gauge position sensors to precisely measure piezo displacement and to correct hysteresis through feed-back control system. The sensor signal output from the piezo controller is converted into distance using the voltage constant of the piezo that is factory-calibrated. An example force-extension curve from a pulling experiment is shown in Figure 2.
There are two types of AFM-SMFS experiments: constant velocity and constant force pulling measurements. Constant force SMFS measurements are described in Oberhauser et al.9, while here we focus on constant velocity measurements. A typical AFM constant-velocity pulling experiment is done by providing voltage to a piezo to gently move a substrate relative to a cantilever tip. A typical experiment has the tip initially pressing against the surface. The pulling measurement is begun by moving the substrate away from the tip to bring out of contact. If a protein comes into contact with the tip initially, it will be pulled and the unfolding trace of force against displacement will be measured. The substrate is then brought back into contact with the tip and a relaxing trace is measured where protein folding can be determined from the force displacement.
Protocol
1. Protein Preparation
- DNA cloning.
- Synthesize a DNA sequence of interest, for example, the DNA sequence of NI10C10, or isolate via PCR from the host organism using standard molecular biology techniques11. Flank the gene of interest with restriction sites during synthesis or by placing sites in the 5’- end of the PCR primers to correspond to a module in the plasmid pEMI91 (Addgene #74888)12.
- Separately digest both the plasmid pEMI9112 and the DNA sequence of interest with a pair of restriction sites so that the sequence of interest will be flanked by the tandem I91 repeats (see Figure 1). Follow the standard protocol for the restriction sites.
- Purify the digested product using gel electrophoresis and then ligate the products using T4 DNA ligase, following a standard protocol. After ligation, transform the plasmid into E. coli cells for plasmid purification and purify the plasmid using standard protocols. Sequence using T7 primers or internal pEMI91 primers to verify that the sequence was successfully transformed.
- Transformation.
- Remove protein expression cells, such as C41 (DE3) pLysS cells, from a -80 °C freezer and thaw completely on ice.
- Add 1 µL of plasmid DNA to the cells and stir briefly; pipetting up and down is not advisable as it will introduce air bubbles and warm the cells.
- Incubate the culture tube containing the cells and the plasmid on ice for 30 min.
- Heat shock the cells in a water bath at 42 °C for 45 s.
- Return the tube to ice for 2 min.
- Place 950 µL of LB broth in the cells and shake at 250 rpm for 1 h at 37 °C.
- Place about 200 µL of the transformation onto LB plates containing 100 µg/mL Ampicillin. If using a plasmid other than pEMI91, then use the appropriate antibiotic for that plasmid. Place plate overnight in an incubator at 37 °C. The next day, remove plate from incubator, wrap with parafilm, and store refrigerated up to 1 month.
- Protein expression.
- Inoculate 15 mL of LB broth medium with 100 µg/mL ampicillin in a 50 mL tube at 37 °C for overnight growth by touching a sterile pipette tip to a single bacterial colony on the plate and pipetting up and down in the LB.
- Transfer the 15 mL culture into 1 L of LB broth medium with 100 µg/mL ampicillin and shake for 4-12 h at 37 °C (until OD600 > 0.8).
- Collect 10 mL of the culture for plasmid preparation.
- Add 0.2-1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) and lower the temperature to room temperature for overnight expression.
- Harvest cells the next day by splitting into four tubes and centrifuging at 4,000 × g for 40 min, and then freeze the pellet at -80 °C for several hours.
- Send the plasmids extracted from the cells for sequencing with the primers corresponding to the inserted cassette of the protein12 and verify the fidelity of the inserted DNA.
- Protein purification.
- Thaw one tube of frozen cells for 30 min at room temperature.
- Suspend thawed cells in lysis buffer (38 mL of water, 2 mL of glycerol, 50 mM Tris-HCl pH 7.6, 150 mM NaCl, 1 mM CaCl2, 10 µg/mL DNase, 1 mM PMSF, 1 mM TCEP, 500 µg/mL lysozyme) and shake on ice for 1 h.
- Freeze the lysed cells at -80 °C for several hours and then re-thaw at room temperature.
- Spin the lysate down at 13,100 × g for 30 min at 4 °C.
- Run the supernatant through a gravity flow column for the specific tag (e.g., Strep-tag or His-tag).
- Perform a buffer exchange using a centrifugal filter using the appropriate buffer for Strep-tag or His-tag and store at 4 °C before use.
2. Slides Preparation for Sample Preparation
- Prepare glass slides using Piranha solution.
- Place 10-30 pieces of round glass cover slips (radius of 7.5 mm) into a 40 mL glass beaker.
Note: Perform this step and following steps in a hood. Follow standard procedures for dealing with dangerous chemicals during this step. - Add 30 mL of concentrated (18.4 M) sulfuric acid.
- Add 10 mL of 30% hydrogen peroxide.
- Heat the mixture for 10-30 min to 95 °C.
- Carefully decant the Piranha solution into a separate waste container (to be reused or discarded).
- Rinse the slides with deionized water to remove Piranha solution.
- Suspend the slides in 40 mL of acetone.
- Decant acetone into a waste container and re-suspend slides in 40 mL of ethanol.
- Use clean forceps to carefully extract one slide at a time and dry with argon or purified air.
- Place clean and dried slides under vacuum until the gold evaporation, or under argon for long-term storage.
- Place 10-30 pieces of round glass cover slips (radius of 7.5 mm) into a 40 mL glass beaker.
- Prepare gold coated slides (optional).
Note: This step requires the use of a vacuum (e-beam) evaporator, which is available in many clean room facilities at major universities.- Cut five microscope slides (25 mm x 75 mm rectangular shape) in half using a glasscutter.
- Apply double-sided adhesive tape across the middle of each of the 10 half microscope slides.
- Cut the sticky side of a sticky note and apply to the double-sided tape so that the sticky side of the note is face-up. The sticky note is generally less adhesive, but still firmly attaches to slides to prevent future breaking. Then those half microscope slides can now work as glass slides holder.
- Carefully press four clean glass slides (from section 2.1.10) to each corner of the sticky side of the holder.
- Move the glass slides to an e-beam evaporator. Follow the specifications of the evaporator to apply 70 nm of chromium and 300 nm of gold to the surface.
- Store the gold-coated slides under argon.
3. Sample Preparation
- Prepare slide.
- Select a clean iron disk (15 mm diameter) and attach an adhesive tab to it.
- Unattach the cover piece of the adhesive tab.
- Select a piece of clean glass (from section 2.1.10) or gold (from section 2.2.7) and firmly place it onto the sticky side of the iron disk, this will be the sample slide.
- Deposit protein onto slide.
- Dialyze the polyprotein into the buffer for the experiment (25 mM Tris-HCl, pH 7.6 with 150 mM NaCl) using a buffer exchange column such as a 0.5-mL centrifugal filter. Spin the protein in the column for 10 min at 13,000 rpm and then reverse the column and elute the protein into a new buffer by spinning at 1,000 rpm for 2 min.
- Determine the approximate protein concentration by measuring the absorbance at 280 nm, using a spectrophotometer.
- Dilute the protein to 10-100 µg/mL in a final volume of 100 µL.
- Apply the 60 µL of protein solution onto the center of the slide. Be careful at this stage not to let any liquid enter beneath into the gap between the glass and the iron slide, as this can cause swelling of the adhesive during the experiment and uncontrolled sample movements.
Note: Gold slides are hydrophobic, and a spherical droplet will form, while glass slides are more hydrophilic and the protein solution may spread. - Let the sample sit at room temperature for 10-60 min. During this time, proceed to the next step to begin setting up the atomic force microscope.
4. Atomic Force Microscope (AFM) Setup
Note: The following is a general description for setting up the AFM, and some specific details may differ depending on the specific instrumentation used. The instrumentation used is partially home-built and described in detail in Scholl13.
- Mount the cantilever onto an AFM cell.
- Choose the cantilever with appropriate properties for the application. Use cantilevers with spring constants 4-10 pN/nm for low unfolding forces (~10-50 pN), while use cantilevers with spring constants 15-100 pN/nm for high unfolding forces.
- Carefully pick up the cantilever from the end and place it into the probe holding cell.
- Make sure the holding cell firmly holds the cantilever in place before continuing.
- Place the holding cell into the AFM head for alignment of the laser.
- Align the laser in the AFM head.
- Place the head onto an inverted microscope and connect a battery pack to the AFM head for powering the laser in the head.
- Attach a camera to the microscope detector so that the laser light can be visualized onto a TV or monitor.
- Position the laser so that it is located onto the tip of the cantilever
- Mount head with sample.
- Flush 10 µL of buffer into each of the ports of the probe holding cell.
- Take the sample slide which has been incubating and decant 40 µL of fluid from the incubated solution. Add 40 µL of the buffer on the slide. Place the sample slide onto the magnet above the piezo.
- Make sure that the AFM stage is in the elevated position. Then place the AFM head onto the stage so that the AFM cantilever is above the sample droplet.
- Center the reflected laser beam onto the photodiode.
- Cut a small piece of paper and place it in front of the AFM photodiode.
- Reposition the AFM laser using the knobs so that the laser spot on the paper becomes focused and bright.
- Adjust the AFM head mirror so that the laser hits the photodiode to maximize the total signal in all quadrants (A+B+C+D) and causes the difference signal between top two and bottom two quadrants to be zero (A+B-C-D).
5. Atomic Force Microscope Calibration
- Measure the power spectrum.
- On the filter connected to the AFM, turn the filter setting for the AFM head signal to full bandwidth.
- On the AFM itself, make sure that the piezo is off as this will add noise to the signal.
- Use the AFM software14 to measure the average of 512 calculations of the power spectrum from 1,024 data points per calculation.
- Use the AFM software15 to integrate the power spectral density across the first peak, which corresponds to the main mode of vibration for the cantilever.
- Calculate photodiode sensitivity.
- On the AFM itself, turn the piezo controller on and change the filter setting to 500 Hz (low-pass filter).
- Monitor the difference signal which should be fluctuating around zero in the AFM software. Swiftly move the AFM head down a few hundred micrometers using micropositioners. Repeat moving the head down and monitor the difference signal for consistent jumps. The surface is very nearby when the signal jumps start increasing in height.
- As soon as the deflection signal saturates upon contact with the surface, move the head away from the surface slightly.
- In the AFM software, use the input controls to regulate the piezo voltage to find the surface, by moving up or down 100-5,000 nm. If the surface is still not in reach, continue to reduce the distance between the head and the sample manually.
- In the AFM software, conduct a pulling experiment with a scan size of about 500 nm so that the tip comes in contact for about 100 nm of piezo travel.
- Measure the slope of the linear region of the photodiode signal versus piezo displacement curve where the AFM tip remains in contact with the substrate surface.
- Calculate the spring constant and sensitivity using the slope and the integrated power spectrum (
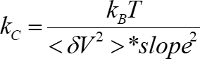 ).
).
6. Data Acquisition
- Preparation.
- On the filters connected to the AFM, set the filter setting so that the sampling frequency is at least twice the bandwidth (Nyquist criterion), which will give an upper bound for the low-pass cutoff.
- Measurements.
- Set the scan size to the total theoretical size of the unfolding polyprotein (number of amino acids × 0.365) plus about 40% to allow for pressing against the substrate. For example, pulling a 9x I91 construct will have a theoretical unfolded length of about 300 nm, so the scan size should be about 420 nm.
- In the AFM software, position the cantilever so that it is 80% of the scan-size away from the surface (in this case, about 340 nm away from the surface).
- In the AFM software, set the scan speed to 300 nm/s initially.
Note: This speed can be modified to be slower, or faster, depending on the application. - Perform a pulling experiment and measure the resulting position of the piezo and the photodiode signal. Use the AFM software to initiate the scan.
- In the AFM software, continue to perform measurements until there are about 10,000 recordings.
Note: Generally, the pickup rate of a positive-controlled molecule is around 0.5%16, so 10,000 are necessary to be able to collect enough data to analyze.
7. Data Analysis
- Normalize data.
- For each recording, compute the extension using extension = displacement – F/kc (kc is from 5.2.7).
- Determine the baseline force level by taking the average of either the beginning or end of the force-extension trace, where no force peaks are present and where the cantilever is not pressing against the surface. The entire force-extension curve can then be shifted by this mean value.
- Translate the data so that the extension is aligned along the zero y-axis. This is because the molecule should be set to zero extension at the beginning of a trace.
- Identifying protein of interest.
- Identify recordings with at least four I91 events that provide a single-molecule fingerprint. These recordings capture true “single-molecule measurements”.
- Save events that are denoted single-molecule events for further analysis.
- Determine contour-length increment.
- For each recording, fit a worm-like chain model to unfolding event,
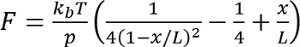 where
where 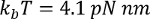 at room temperature, p is the persistence length (typically 0.4 to 1 nm), x is the extension in nanometers, and L is the contour length in nanometers.
at room temperature, p is the persistence length (typically 0.4 to 1 nm), x is the extension in nanometers, and L is the contour length in nanometers.
Note: Form of the worm-like chain is an interpolated solution to the exact, a more exact numerical solution is found in Bouchiat et al.17. Alternate fitting models can be found in Su et al.18. - Calculate the difference between the values for L determined for two consecutive unfolding events, and this difference is coined the “contour-length increment”.
- For each recording, fit a worm-like chain model to unfolding event,
- Determine rupture force.
- Calculate the rupture force for a given unfolding event by taking the highest point before the biggest drop in the unfolding curve.
Representative Results
Representative results from this protocol are shown in Figure 2. Both panels show representative force-extension curves from proteins. The top shows results from a I91 polyprotein, while the bottom shows the I91 protein flanking a protein-of-interest, the NI10C molecule. These recordings show the characteristic force of I91 (200 pN) and contour length increment (28 nm) which indicates that the alignment and calibration of the AFM was successful. These force-extension curves can then be analyzed by worm-like chains (dashed line) which help to determine the force-independent length of the molecule and determine the number of unfolded residues. Once analyzed, the contour-length increments (difference between subsequent contour-lengths) and the unfolding force can be used to determine the protein stability, unfolding rate, and unfolding pathway19.

Figure 1: Plasmid map of polyprotein. This polyprotein plasmid map sequence and physical DNA is available through the Addgene repository (www.addgene.org/74888). It shows the prototypical polyprotein design for AFM, where a polyprotein is composed of 8 identical repeats of I91 protein (gray boxes) which flank a protein of interest (red). Each module contains a unique restriction site which allows customization. Please click here to view a larger version of this figure.
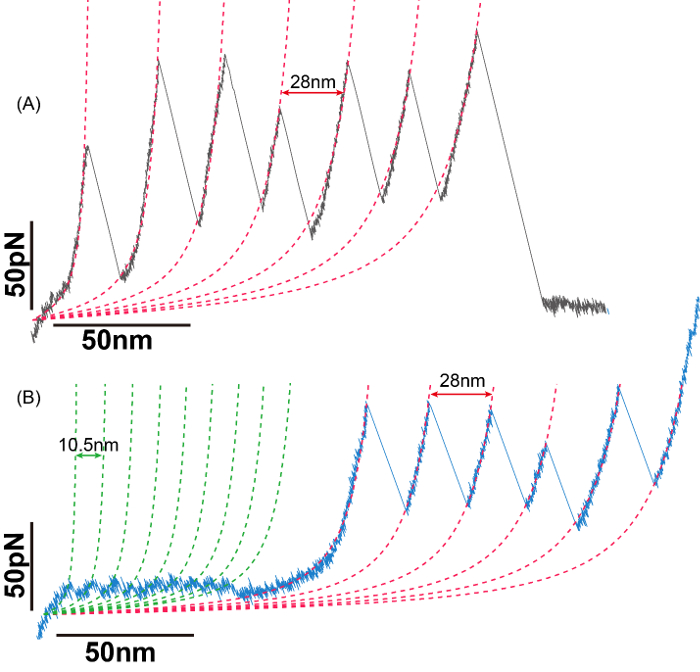
Figure 2: Representative SMFS results. A. Representative force-extension curve for poly I91 protein. Mean contour length increment between peaks is ~28 nm, and unfolding forces are between 100-200 pN. B. Representative force-extension curve for (I91)3-NI10C-(I91)3 protein. Mean contour length increment for ankyrin repeat is ~10.5 nm, and unfolding forces are between 8-25 pN. The regular saw-tooth pattern for ankyrin repeats is followed by I91 unfolding peaks. The pulling speed for both (A) and (B) are 0.02 nm/ms. Please click here to view a larger version of this figure.
Discussion
A critical step in the protocol is the use of a polyprotein, described in step 1.1.2, which serves as a positive control to "fingerprint" single-molecule events. Generally, there must be unfolding events of the polyprotein proteins (for I91, this means an unfolding force of about 200 pN and contour length increment of about 28 nm) to unambiguously conclude that the protein of interest has been unfolded. For example, when the protein of interest is flanked by three I91 domains from either side, then there must be at least four I91 events to conclude that it is positively a single-molecule event. If there is not a positive control through a polyprotein, or other means, then any data is liable to misinterpretation.
The cloning strategy for the plasmid depends on the gene and plasmid of interest. We have made available a polyprotein plasmid which can be used in conjunction with this protocol12. There are plasmids available for this step that contain unique restriction sites for simple cloning20,21. These plasmids only require a unique set of restriction sites to easily clone in the protein of interest into the plasmid. There are also methods available to create this plasmid from scratch using Gibson assembly22 which has the advantage of easily modifying the flaking proteins while inserting the protein of interest. There are also plasmids with modular polyproteins with codon shuffled domains that provide a simple way to efficiently sequence the entire protein of interest, aiding in ensuring the fidelity21.
The choice of gold or glass depends on whether the protein has specific attachment available. Gold substrates allow for forming Au-Cys bonds between the polyprotein and the surface. If a Cys is appended to the end of the polyprotein, then this can serve as a way to specifically attach the protein to the surface. Glass substrates can be used by themselves, which are useful for some proteins which do not adsorb well to gold and are also simpler to generate. Glass slides can also be used as a precursor to using specific attachment with specially made polyproteins23. Mica substrates can also be used, which may be useful for some proteins which have specific charge sites that can bind to the negatively charged surface.
AFM spectroscopy on proteins has several important limitations. Sample preparation may fail if the protein cannot be spliced into a polyprotein. Proteins that are too small to produce measurable contour-length increments or too weak to resist force will also not be able to be resolved by AFM. Typical resolution in AFM experiments only allows resolving forces as low as 10-15 pN due to the typical RMS noise in AFM cantilevers, although recent advances have made available better resolutions with advanced instrumentation24. The speed for constant velocity experiments is also limited to about 4 nm/s25, and the best resolution achieved can typically detect states shorter than 10 µs24. If proteins are less mechanically stable, they are better characterized using optical tweezers which has a lower force range and typically a higher temporal resolution. There are still improvements in the future available to AFM, like combining with FRET26, using AFM imaging to determine protein positions27 and increasing resolution with specially designed cantilevers28.
The AFM technique is significant with respect to existing methods because it allows extracting kinetic parameters, like unfolding rate and transition state distance on a single molecule level29. While other traditional biochemical methods (NMR, chemical denaturation, stopped-flow fluorescence) can also obtain information on kinetic parameters, the results of these methods are functions of a protein ensemble and not a single molecule. This makes an important difference in cases where there are different and distinct ensembles of a single protein. AFM has shown to be able to distinguish the subpopulations, instead of averaging them together30.
The detailed explanation of the SMFS experiment on protein molecules above still does not anticipate possible problems that can be solved with experience. In the following we discuss common problems and troubleshooting associated with operating an AFM for constant-velocity experiments.
Sinusoidal-shaped force baseline. The sinusoidal shape is detrimental to determine correct force peak value since it is difficult to calculate the baseline. The problem is caused by interference of the laser reflected from the cantilever. To alleviate this problem, the position of the laser spot on the cantilever can be slightly shifted as long as the new position still retains a good sum signal from the photodiode. If the problem still persists, consider repositioning the cantilever in the probe holding cell.
Photodiode signal does not saturate in one-step when touching the surface the first time. When touching the surface, the photodiode signal jumps in several steps until it reaches the saturated value. This means that the tip is not the lowest point in the AFM head and some other place on the probe chip may touch the sample surface prior to the tip of the cantilever. This problem has to be fixed in order to continue the SMFS measurements and get correct force-extension curve. To fix the problem, try moving the position of the cantilever in the probe holding cell back and forth and adjust the tightness of the hook which grasps the cantilever in place. If this does not help, try switching to a different cantilever on the same chip or even change to a new cantilever chip.
Many events upon retraction, or no events at all. This is related to finding the correct protein concentration for the experiment. If there's always a single big force peak (> 500 pN) appearing on the stretching trace and no other peaks are displayed while conducting experiments on gold coated surface, the cantilever is actually measuring gold-gold interaction since the sample molecules on the surface are too sparse. In this case, the concentration of the protein sample deposited on the gold surface should be increased. However, if there are multiple irregularly shaped peaks appearing on every stretching trace of each pulling cycle, it indicates that there are too many molecules absorbed to the surface that formed a layer or a complicated network on the surface. In this case, the sample needs to be washed by the buffer used to remove excess protein molecules. Sometimes preparing a new sample with lower concentration of protein is necessary to get rid of this problem. If the new sample also cannot solve the problem, check the frosted annular groove on the AFM holding cell to see if any liquid penetrated into it. The groove should stay dry during the experiment since any liquid in the groove would couple the vibration of the sample into the holding cell and cause the photodiode signal to change abnormally when the cantilever and the surface are in touch.
Spurious modulation of photodiode signal, between ramps. This is indicative of flow that can push the cantilever back and forth, which is usually caused by a small bubble that is transiting through the fluid channels. This type of problem will disrupt measurements with huge signal increases or decreases. To solve this problem, raise the head from the substrate, so that the fluid around the cantilever no longer touches the fluid around the substrate. Then bring them back together, and move the head back down to the surface. The reformation of the fluid column in the AFM cell is usually enough to disrupt the bubble from the fluid channels, but if not, repeating is necessary.
Systematic increase or decrease in photodiode signal. Drifting is a phenomenon that is present throughout each experiment, where the cantilevers and the tip will always drift very fast upon touching the liquid drop on the sample surface. Drift can be caused by temperature equilibration, in which the temperature-induced expansion or contraction of the cantilever causes vibration in the signal. Waiting for more than 10 min before conducting the first experiment will significantly decrease drifting speed. When conducting pulling experiments, if the stretching and relaxing trace do not coincide with each other, then there's a hysteresis between the two halves of the pulling cycle, which corresponds to a fast drifting rate and requires additional time to reach equilibrium. If the force baseline tilts upwards with a small angle, the drifting speed is faster than the pulling speed and it's better to suspend the experiment to wait for less vibration of the tip.
Force-extension curve initial region is not vertical. It is useful to look at the force-extension plot of recordings after they have been acquired from a newly calibrated setup. If these recordings do not show a vertical region when the cantilever presses against the surface, then the calibration may be an issue and it should be repeated from step 5.
Photodiode signal disappears after some time. This could be due to the evaporation of the buffer in the fluid cell. It is important to rehydrate the cell by applying more buffer every hour or so, in order to keep the sample from drying out and making it unusable for further experiments.
Intrinsic noise is high (> 30 pN). A large amount of noise (measured as the RMS of the calibrated photodiode fluctuations) is indicative of external noise. AFM experiments should be done on an air table to reduce the amount of coupling to the building, but external noise can also be caused by insecure stage, auditory noise nearby, or coupling of the air table to nearby static objects (e.g., a chair). The noise may also come from the electronic controlling system of the AFM (e.g., unstable connection in the electronic system).
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Science Foundation grants MCB-1244297 and MCB-1517245 to PEM.
Materials
| AFM Specimen Discs, 15mm diameter | Ted Pella, Inc. | 16218 | Serve as base for glass substrate |
| Round Glass Coverslips, 15mm diamiter No.1 Thick | Ted Pella, Inc. | 26024 | serve as glass substrate and base for gold coating |
| Adhesive Tabs | Ted Pella, Inc. | 16079 | Paste on AFM Specimen Discs to provide a sticky face for attaching glass coverslips |
| STD Multimode head assembly | Bruker Nano Inc. | 1B75C | AFM head |
| Glass probe holder | Bruker Nano Inc. | MTFML-V2 | Glass probe holder for scanning in fluid with the MultiMode AFM. |
| Microlever AFM probes | Bruker Nano Inc. | MLCT | Silicon Nitride cantilevers with Silicon Nitride tips, ideal for contact imaging modes |
| AFM probes with Au coated tips | Bruker Nano Inc. | OBL-10 | Cantilevers for pulling on proteins with low unfolding force |
| Multifunction Data Acquisition (DAQ) Card,16-Bit, 1 MS/s (Multichannel), 1.25 MS/s (1-Channel), 32 Analog Inputs | National Instruments | PCI-6259 | Data Acquisition for signals from AFM head and Piezo Actuators |
| LISA Linear Piezo Stage Actuators | Physik Instrumente LP | P-753.11C | Piezo Actuator to control the position of substrate and perform pulling measurements |
| XY Piezo Stage | Physik Instrumente LP | P-541.2CD | Piezo Actuator to control the position of substrate and scan on substrate surface |
References
- Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J. M., Gaub, H. E. Reversible Unfolding of Individual Titin Immunoglobulin Domains by AFM. Science. 276 (5315), 1109-1112 (1997).
- Fisher, T. E., Oberhauser, A. F., Carrion-Vazquez, M., Marszalek, P. E., Fernandez, J. M. The study of protein mechanics with the atomic force microscope. Trends in Biochemical Sciences. 24 (10), 379-384 (1999).
- Ng, S., Rounsevell, R., Steward, A., Randles, L., Clarke, J. Single molecule studies of protein folding by atomic force microscopy(AFM). Abstracts of Papers of the American Chemical Society. 227, U545-U545 (2004).
- Rico, F., Chu, C., Moy, V. T., Braga, P. C., Ricci, D. . Methods in Molecular Biology. 736, 331-353 (2011).
- Muller, D. J., Dufrene, Y. F. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. Nature Nanotechnology. 3 (5), 261-269 (2008).
- Lv, S., et al. Designed biomaterials to mimic the mechanical properties of muscles. Nature. 465 (7294), 69-73 (2010).
- Kim, M., et al. Nanomechanics of Streptavidin Hubs for Molecular Materials. Advanced Materials. 23 (47), 5684-5688 (2011).
- Gonzalez, M. A., et al. Self-Adhesive Hydrogels from Intrinsically Unstructured Proteins. Advanced Materials. , (2017).
- Oberhauser, A. F., Hansma, P. K., Carrion-Vazquez, M., Fernandez, J. M. Stepwise unfolding of titin under force-clamp atomic force microscopy. Proceedings of the National Academy of Sciences. 98 (2), 468-472 (2001).
- Li, Q., Scholl, Z. N., Marszalek, P. E. Capturing the Mechanical Unfolding Pathway of a Large Protein with Coiled-Coil Probes. Angewandte Chemie International Edition. 53 (49), 13429-13433 (2014).
- Davis, L. . Basic methods in molecular biology. , (2012).
- Scholl, Z. N., Josephs, E. A., Marszalek, P. E. A Modular, Non-Degenerate Polyprotein Scaffold for Atomic Force Spectroscopy. Biomacromolecules. , (2016).
- Scholl, Z. N. . The (Un) Folding of Multidomain Proteins Through the Lens of Single-molecule Force-spectroscopy and Computer Simulation. , (2016).
- Pawlak, K., Strzelecki, J. Nanopuller-open data acquisition platform for AFM force spectroscopy experiments. Ultramicroscopy. 164, 17-23 (2016).
- . Nanopuller Available from: https://sourceforge.net/projects/nanopuller/ (2018)
- Scholl, Z. N., Marszalek, P. E. Improving single molecule force spectroscopy through automated real-time data collection and quantification of experimental conditions. Ultramicroscopy. 136, 7-14 (2014).
- Bouchiat, C., et al. Estimating the persistence length of a worm-like chain molecule from force-extension measurements. Biophysical journal. 76 (1), 409-413 (1999).
- Su, T., Purohit, P. K. Mechanics of forced unfolding of proteins. Acta. 5 (6), 1855-1863 (2009).
- Steward, A., Toca-Herrera, J. L., Clarke, J. Versatile cloning system for construction of multimeric proteins for use in atomic force microscopy. Protein science. 11 (9), 2179-2183 (2002).
- Scholl, Z. N., Josephs, E. A., Marszalek, P. E. Modular, Nondegenerate Polyprotein Scaffolds for Atomic Force Spectroscopy. Biomacromolecules. 17 (7), 2502-2505 (2016).
- Hoffmann, T., et al. Rapid and Robust Polyprotein Production Facilitates Single-Molecule Mechanical Characterization of β-Barrel Assembly Machinery Polypeptide Transport Associated Domains. ACS. 9 (9), 8811-8821 (2015).
- Dudko, O. K., Hummer, G., Szabo, A. Theory, analysis, and interpretation of single-molecule force spectroscopy experiments. Proceedings of the National Academy of Sciences of the United States of America. 105 (41), 15755-15760 (2008).
- Popa, I., Berkovich, R., Alegre-Cebollada, J., Rivas-Pardo, J. A., Fernandez, J. M. Halotag Tethers to Study Titin Folding at the Single Molecule Level. Biophysical journal. 106 (2), 391a (2014).
- Yu, H., Siewny, M. G., Edwards, D. T., Sanders, A. W., Perkins, T. T. Hidden dynamics in the unfolding of individual bacteriorhodopsin proteins. Science. 355 (6328), 945-950 (2017).
- Rico, F., Gonzalez, L., Casuso, I., Puig-Vidal, M., Scheuring, S. High-speed force spectroscopy unfolds titin at the velocity of molecular dynamics simulations. Science. 342 (6159), 741-743 (2013).
- He, Y., Lu, M., Cao, J., Lu, H. P. Manipulating protein conformations by single-molecule AFM-FRET nanoscopy. ACS nano. 6 (2), 1221-1229 (2012).
- Fotiadis, D., Scheuring, S., Müller, S. A., Engel, A., Müller, D. J. Imaging and manipulation of biological structures with the AFM. Micron. 33 (4), 385-397 (2002).
- Edwards, D. T., Faulk, J. K., LeBlanc, M. A., Perkins, T. T. Force Spectroscopy with 9-μs Resolution and Sub-pN Stability by Tailoring AFM Cantilever Geometry. Biophysical journal. 113 (12), 2595-2600 (2017).
- Dudko, O. K., Mathe, J., Szabo, A., Meller, A., Hummer, G. Extracting kinetics from single-molecule force spectroscopy: Nanopore unzipping of DNA hairpins. Biophysical. 92 (12), 4188-4195 (2007).
- Scholl, Z. N., Li, Q., Yang, W., Marszalek, P. E. Single-molecule Force Spectroscopy Reveals the Calcium Dependence of the Alternative Conformations in the Native State of a βγ-Crystallin Protein. Journal of Biological Chemistry. 291 (35), 18263-18275 (2016).

