Brug af en perkutan ventrikulær assist enhed / venstre atrium til lårpulsåren Bypass System for kardiogen shock
Summary
Følgende artikel beskriver den trinvise procedure for placering af en enhed (f.eks Tandemheart) i kardiogen chok (CS), der er en vinkelret venstre ventrikel hjælpe enhed (pLVAD) og en venstre atrie til lårbensarterie bypass (LAFAB) system, der omgår og understøtter venstre ventrikel (LV) i CS.
Abstract
Den venstre atrie til lårpulsåren bypass (LAFAB) system er en mekanisk kredsløbssygdomme støtte (MCS) enhed, der anvendes i kardiogen chok (CS), der omgår venstre ventrikel ved at dræne blod fra venstre atrium (LA) og returnere det til den systemiske arteriel cirkulation via lårpulsåren. Det kan give strømme fra 2,5-5 L/ min afhængigt af kanylens størrelse. Her diskuterer vi virkningsmekanismen af LAFAB, tilgængelige kliniske data, indikationer for dens anvendelse i kardiogen chok, trin til implantation, post-proceduremæssig pleje og komplikationer forbundet med brugen af denne enhed og deres ledelse.
Vi leverer også en kort video af den proceduremæssige komponent i enhedsterapi, herunder forberedelse før placering, perkutan placering af enheden via transseptal punktering under ekkokardiografisk vejledning og postoperativ styring af enhedsparametre.
Introduction
Kardiogen chok (CS) er en tilstand af væv hypoperfusion med eller uden samtidig hypotension, hvor hjertet ikke er i stand til at levere tilstrækkeligt blod og ilt til at opfylde kroppens krav, hvilket resulterer i organsvigt. Det er klassificeret i trin A til E af Society of Cardiovascular Angiography and Interventions (SCAI): fase A – patienter i fare for CS; fase B – patienter i begyndelsen af CS med hypotension eller takykardi uden hypoperfusion; fase C – klassisk CS med kold og våd fænotype, der kræver inotroper / vasopressorer eller mekanisk støtte til at opretholde perfusion; fase D – forværres på nuværende medicinsk eller mekanisk støtte, der kræver eskalering til mere avancerede enheder; og fase E – omfatter patienter med kredsløbskollaps og ildfast arytmier, der aktivt oplever hjertestop med løbende kardiopulmonal genoplivning1. De mest almindelige årsager til CS er akut MI (AMI), der repræsenterer 81% af tilfældene i en nyligt rapporteret analyse2, og akut dekompenseret hjertesvigt (ADHF). CS er klassisk karakteriseret ved overbelastning og nedsat perfusion, manifesteret ved forhøjet påfyldningstryk (lungekapillær kiletryk [PCWP], venstre ventrikulært ende-diastolisk tryk [LVEDP], centralt venøs tryk [CVP] og højre ventrikulært ende-diastolisk tryk [RVEDP]), nedsat hjerteydelse (CO), hjerteindeks (CI), hjerteeffekt (CPO) og end-organ funktionsfejl3 . Tidligere var de eneste tilgængelige behandlinger for AMI, der blev kompliceret af CS, tidlig reascularization og medicinsk ledelse med inotroper og / eller vasopressorer4. For nylig, med fremkomsten af mekaniske kredsløbsstøtte (MCS) enheder og erkendelsen af, at eskalering af vasopressorer er forbundet med øget dødelighed, har der været et paradigmeskift i behandlingen af både AMI og ADHF relaterede CS5,6.
I den nuværende æra af vinkelrette ventrikulære hjælpeanordninger (pVAD) er der en række MCS-enhedsplatforme / konfigurationer tilgængelige, som giver univentrikulær eller biventrikulær kredsløbs- og ventrikulær støtte med og uden iltningskapacitet7. På trods af konstante stigninger i brugen af pVAD’er til behandling af både AMI og ADHF CS er dødeligheden stort set uændret5. Med nye beviser for mulige kliniske fordele for tidlig losning af venstre ventrikel (LV) i AMI8 og tidlig brug af MCS i AMI CS9, fortsætter brugen af MCS med at stige.
Den venstre Atrie til Lårpulsåren Bypass (LAFAB) MCS enhed omgår LV ved at dræne blod fra venstre atrium (LA) og returnere det til den systemiske arteriel omsætning via lårpulsåren (Figur 1). Det understøttes af en ekstern centrifugalpumpe, der tilbyder 2,5-5,0 liter pr. minut (L / m) flow (ny generation pumpe, udpeget som LifeSPARC, der kan bruges til op til 8 L / m flow) afhængigt af kanylernes størrelse. Når blodet er udvundet fra LA via den transseptale venøs kanyle, det passerer gennem den eksterne centrifugalpumpe, som recirkulerer blodet tilbage i patientens krop via arteriel kanyle placeret i lårpulsåren.
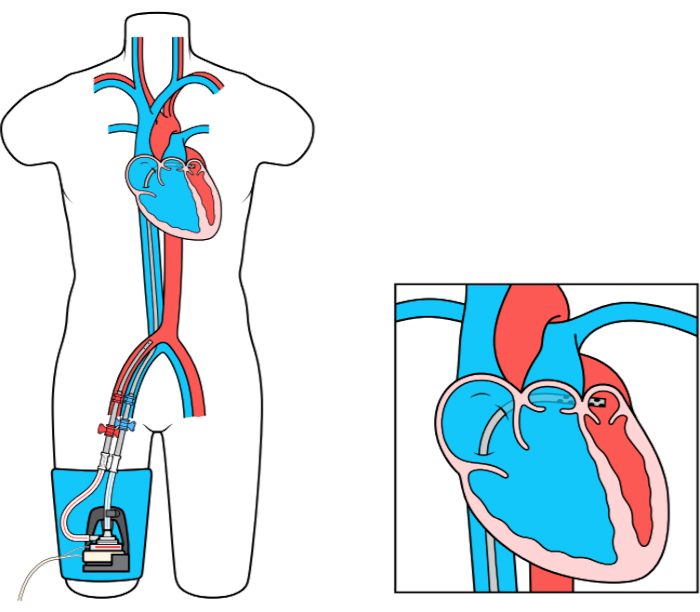
Figur 1: LAFAB-opsætning. Billede venligst udlånt af TandemLife, et helejet datterselskab af LivaNova US Inc. Klik her for at se en større version af dette tal.
Protocol
Representative Results
Discussion
Hæmodynamik af LAFAB enhed:
Lafab-enhedens hæmodynamiske profil adskiller sig fra andre pVAD’er. Ved at dræne blod direkte fra LA og returnere det til lårpulsåren, omgår enheden LV helt. Dermed reducerer det LV ende diastolisk volumen og tryk, bidrager til forbedret LV geometri, og dermed gennemføre et fald i LV slagtilfælde arbejde. Men ved at returnere blodet tilbage i iliaca arterie / faldende aorta, efterbelastning stiger. Dette resulterer i lastning af LV via forhøjet ve…
Disclosures
The authors have nothing to disclose.
Acknowledgements
Til TandemHeart teamet på LifeSparc.
Materials
| For LAFAB (TandemHeart) | |||
| Factory Supplied Equipment for circuit connections. | TandemLife | ||
| ProtekSolo 15 Fr or 17 Fr Arterial Cannula | TandemLife | ||
| ProtekSolo 62 cm or 72 cm Transseptal Cannula | TandemLife | ||
| TandemHeart Controller | TandemLife | For adjusting flows/RPM | |
| TandemHeart Pump | LifeSPARC | Centrifugal pump | |
| For RAPAB (ProtekDuo) | |||
| Factory Supplied Equipment to complete the circuit. | TandemLife | ||
| ProtekDuo 29 Fr or 31 Fr Dual Lumen Cannula | TandemLife | ||
| TandemHeart Controller | TandemLife | For adjusting flows/RPM | |
| TandemHeart Pump | LifeSPARC | Centrifugal pump |
References
- Baran, D. A., et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheterization and Cardiovascular Interventions. 94 (1), 29-37 (2019).
- Harjola, V. -. P., et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. European Journal of Heart Failure. 17 (5), 501-509 (2015).
- Furer, A., Wessler, J., Burkhoff, D. Hemodynamics of Cardiogenic Shock. Interventional Cardiology Clinics. 6 (3), 359-371 (2017).
- Hochman, J. S., et al. Cardiogenic shock complicating acute myocardial infarction–etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK. Journal of the American College of Cardiology. 36 (3), 1063-1070 (2000).
- Shah, M., et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clinical Research in Cardiology. 107 (4), 287-303 (2018).
- van Diepen, S., et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 136 (16), 232-268 (2017).
- Alkhouli, M., et al. Mechanical Circulatory Support in Patients with Cardiogenic Shock. Current Treatment Options in Cardiovascular Medicine. 22 (2), 4 (2020).
- Basir, M. B., et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheterization and Cardiovascular Interventions. 91 (3), 454-461 (2018).
- Basir, M. B., et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheterization and Cardiovascular Interventions. 93 (7), 1173-1183 (2019).
- Alkhouli, M., Rihal, C. S., Holmes, D. R. Transseptal Techniques for Emerging Structural Heart Interventions. JACC: Cardiovascular Interventions. 9 (24), 2465-2480 (2016).
- Dennis, C., et al. Clinical use of a cannula for left heart bypass without thoracotomy: experimental protection against fibrillation by left heart bypass. Annals of Surgery. 156 (4), 623-637 (1962).
- Dennis, C., et al. Left atrial cannulation without thoracotomy for total left heart bypass. Acta Chirurgica Scandinavica. 123, 267-279 (1962).
- Fonger, J. D., et al. Enhanced preservation of acutely ischemic myocardium with transseptal left ventricular assist. Annals of Thoracic Surgery. 57 (3), 570-575 (1994).
- Thiele, H., et al. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 104 (24), 2917-2922 (2001).
- Burkhoff, D., et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. American Heart Journal. 152 (3), 469 (2006).
- Thiele, H., et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. European Heart Journal. 26 (13), 1276-1283 (2005).
- Gregoric, I. D., et al. TandemHeart as a rescue therapy for patients with critical aortic valve stenosis. Annals of Thoracic Surgery. 88 (6), 1822-1826 (2009).
- Kar, B., et al. The percutaneous ventricular assist device in severe refractory cardiogenic shock. Journal of the American College of Cardiology. 57 (6), 688-696 (2011).
- Patel, C. B., Alexander, K. M., Rogers, J. G. Mechanical Circulatory Support for Advanced Heart Failure. Current Treatment Options in Cardiovascular Medicine. 12 (6), 549-565 (2010).
- Tempelhof, M. W., et al. Clinical experience and patient outcomes associated with the TandemHeart percutaneous transseptal assist device among a heterogeneous patient population. Asaio Journal. 57 (4), 254-261 (2011).
- Gregoric, I. D., et al. The TandemHeart as a bridge to a long-term axial-flow left ventricular assist device (bridge to bridge). Texas Heart Institute Journal. 35 (2), 125-129 (2008).
- Bruckner, B. A., et al. Clinical experience with the TandemHeart percutaneous ventricular assist device as a bridge to cardiac transplantation. Texas Heart Institute Journal. 35 (4), 447-450 (2008).
- Agarwal, R., et al. Successful treatment of acute left ventricular assist device thrombosis and cardiogenic shock with intraventricular thrombolysis and a tandem heart. Asaio Journal. 61 (1), 98-101 (2015).
- Vetrovec, G. W. Hemodynamic Support Devices for Shock and High-Risk PCI: When and Which One. Current Cardiology Reports. 19 (10), 100 (2017).
- Al-Husami, W., et al. Single-center experience with the TandemHeart percutaneous ventricular assist device to support patients undergoing high-risk percutaneous coronary intervention. Journal of Invasive Cardiology. 20 (6), 319-322 (2008).
- Vranckx, P., et al. Clinical introduction of the Tandemheart, a percutaneous left ventricular assist device, for circulatory support during high-risk percutaneous coronary intervention. International Journal of Cardiovascular Interventions. 5 (1), 35-39 (2003).
- Vranckx, P., et al. The TandemHeart, percutaneous transseptal left ventricular assist device: a safeguard in high-risk percutaneous coronary interventions. The six-year Rotterdam experience. Euro Intervention. 4 (3), 331-337 (2008).
- Vranckx, P., et al. Assisted circulation using the TandemHeart during very high-risk PCI of the unprotected left main coronary artery in patients declined for CABG. Catheterization and Cardiovascular Interventions. 74 (2), 302-310 (2009).
- Thomas, J. L., et al. Use of a percutaneous left ventricular assist device for high-risk cardiac interventions and cardiogenic shock. Journal of Invasive Cardiology. 22 (8), 360 (2010).
- Vranckx, P., et al. Assisted circulation using the Tandemhear , percutaneous transseptal left ventricular assist device, during percutaneous aortic valve implantation: the Rotterdam experience. Euro Intervention. 5 (4), 465-469 (2009).
- Pitsis, A. A., et al. Temporary assist device for postcardiotomy cardiac failure. The Annals of Thoracic Surgery. 77 (4), 1431-1433 (2004).
- Singh, G. D., Smith, T. W., Rogers, J. H. Targeted Transseptal Access for MitraClip Percutaneous Mitral Valve Repair. Interventional Cardiology Clinics. 5 (1), 55-69 (2016).
- Subramaniam, A. V., et al. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiology and Therapy. 8 (2), 211-228 (2019).
- Morley, D., et al. Hemodynamic effects of partial ventricular support in chronic heart failure: Results of simulation validated with in vivo data. The Journal of Thoracic and Cardiovascular Surgery. 133 (1), 21-28 (2007).
- Naidu, S. S. Novel Percutaneous Cardiac Assist Devices. Circulation. 123 (5), 533-543 (2011).
- Kapur, N. K., et al. Hemodynamic Effects of Left Atrial or Left Ventricular Cannulation for Acute Circulatory Support in a Bovine Model of Left Heart Injury. ASAIO Journal. 61 (3), 301-306 (2015).
- Smith, L., et al. Outcomes of patients with cardiogenic shock treated with TandemHeart percutaneous ventricular assist device: Importance of support indication and definitive therapies as determinants of prognosis. Catheterization and Cardiovascular Interventions. 92 (6), 1173-1181 (2018).
- Ergle, K., Parto, P., Krim, S. R. Percutaneous Ventricular Assist Devices: A Novel Approach in the Management of Patients With Acute Cardiogenic Shock. The Ochsner Journal. 16 (3), 243-249 (2016).
- Sultan, I., Kilic, A., Kilic, A.Short-Term Circulatory and Right Ventricle Support in Cardiogenic Shock: Extracorporeal Membrane Oxygenation, Tandem Heart, CentriMag, and Impella. Heart Failure Clinics. 14 (4), 579-583 (2018).
- Bermudez, C., et al. . Percutaneous right ventricular support: Initial experience from the tandemheart experiences and methods (THEME) registry. , (2018).
- Aggarwal, V., Einhorn, B. N., Cohen, H. A. Current status of percutaneous right ventricular assist devices: First-in-man use of a novel dual lumen cannula. Catheterization and Cardiovascular Interventions. 88 (3), 390-396 (2016).
- Kapur, N. K., et al. Mechanical circulatory support devices for acute right ventricular failure. Circulation. 136 (3), 314-326 (2017).
- Kapur, N. K., et al. Mechanical Circulatory Support for Right Ventricular Failure. JACC: Heart Failure. 1 (2), 127-134 (2013).
- Geller, B. J., Morrow, D. A., Sobieszczyk, P. Percutaneous Right Ventricular Assist Device for Massive Pulmonary Embolism. Circulation: Cardiovascular Interventions. 5 (6), 74-75 (2013).
- Bhama, J., et al. Initial Experience with a Percutaneous Dual Lumen Single Cannula Strategy for Temporary Right Ventricular Assist Device Support Following Durable LVAD Therapy. The Journal of Heart and Lung Transplantation. 35 (4), 323 (2013).
- O’Neill, B., et al. Right ventricular hemodynamic support with the PROTEKDuo Cannula. Initial experience from the tandemheart experiences and methods (THEME) registry category. Miscellaneous. , (2018).
- O’Brien, B., et al. Fluoroscopy-free AF ablation using transesophageal echocardiography and electroanatomical mapping technology. Journal of Interventional Cardiac Electrophysiology. 50 (3), 235-244 (2017).
- O’Brien, B., et al. Transseptal puncture — Review of anatomy, techniques, complications and challenges. International Journal of Cardiology. 233, 12-22 (2017).

