用于在细胞水平上探测真菌 - 微生物相互作用的微流体工具
Summary
由于土壤的不透明度,其组成微生物之间的相互作用不能用细胞分辨率轻松可视化。在这里,介绍了两种微流体工具,它们为研究真菌 – 微生物相互作用提供了新的机会。这些器件用途广泛且易于使用,可在细胞水平上实现高时空控制和高分辨率成像。
Abstract
丝状真菌是土壤的成功居民,在土壤生态系统中起着重要作用,例如有机和无机物质的分解以及养分水平的调节。在那里,他们还发现了许多与各种其他微生物(如细菌或其他真菌)相互作用的机会。然而,由于土壤的黑匣子性质,在细胞水平上研究真菌相互作用可能具有挑战性。正在开发新的微流体工具,用于研究真菌相互作用;突出显示了两个旨在研究细菌 – 真菌和真菌 – 真菌相互作用的平台。在这些微通道中,可以在受控的物理化学环境中以比以前更高的时间和空间分辨率监测真菌 – 微生物相互作用。这些工具的应用产生了许多新的生物学见解,例如观察细菌对菌丝的极性附着或揭示未表征的真菌 – 真菌拮抗作用。这些方法的一个关键特征是非专家易于使用该工具,从而产生了用于微生物实验室的高度可翻译的技术。
Introduction
土壤是一个非常多样化的环境,含有丰富的微生物,有助于碳和磷循环1,2。丝状真菌是许多生态系统的主要组成部分,是有机和无机物质的分解者,可以通过形成共生关系来增强植物的营养3,4。在土壤中,真菌与多种微生物动态相互作用,例如其他真菌5,细菌6,病毒7 和线虫8。这些相互作用对土壤和植物健康具有重大影响。然而,由于缺乏能够对具有高分辨率的相互作用微生物进行成像的适当实验系统,许多系统仍未定义。
关于细菌-真菌相互作用(BFI)和真菌-真菌相互作用(FFI)的研究在一系列领域具有有价值的应用,包括医学中的抗菌剂和农业中的生物控制剂。例如,真菌 Coprinopsis cinerea 产生肽copsin,其已被证明对人病原体 李斯特菌单核细胞增多性9表现出抗菌活性。类似地,真菌衍生的化合物灰黄霉素被广泛用作治疗人类真菌感染的药物,并且还能够抑制植物病原真菌 Alternaria solani10,11的生长。土壤居住细菌 枯草芽孢 杆菌的几种菌株也被证明是真菌植物病原体 根瘤菌Solani 12,13的有效生物防治剂。然而,由于与传统方法相关的局限性,BFI和FFI在单细胞水平上知之甚少。
常规研究通常在宏观尺度上探索BFI和FFI,使用具有两个或更多物种对抗的琼脂平板。通过测量对抗物种14,15,16的生长速率和代谢物产生来评估它们的相互作用;然而,这种方法只能解决到殖民地水平。为了在细胞水平上研究相互作用,可以在涂有琼脂的玻璃显微镜载玻片上培养细菌和真菌接种剂,然后在显微镜下成像17。然而,由于缺乏限制,使用显微镜载玻片跟踪单个菌丝可能很困难,这意味着延时图像更难获得。此外,在这种设置中,不可能有机会在空间上将其他微生物限制在真菌菌丝体的已定义区域内或创建可以扰动的已定义化学环境。土壤的“黑匣子”性质也增加了在单细胞18水平上研究真菌 – 微生物相互作用的复杂性。通过观察远离土壤微生物组令人难以置信的多样性的相互作用物种,可以评估单个成员相互作用的确切方式。因此,对多功能平台的持续需求,以实现BFI和FFI的高分辨率单细胞成像。
微流体技术,即所谓的芯片实验室系统,为在单细胞水平上研究BFI和FFI提供了理想的平台。微流体领域起源于为化学分析和微电子学开发的技术,已被生物科学19采用。微流体技术在定制的微型通道网络中调节少量流体,在微米尺度上至少具有一个维度,并且它们在生物学研究中的应用正在扩大20。特别是,已经开发了微流体装置来检查丝状真菌21,22,23,24,25,26,27,28,29,30的生长。使用该技术的一个好处是,与传统的琼脂方法31相比,菌丝的局限性和微通道内养分的分布更接近土壤环境的结构。最近,微流体平台已被用于研究人类嗜中性粒细胞与真菌病原体32,细菌和植物根33以及真菌和线虫34,35之间的相互作用。
使用微流体研究微生物相互作用的众多优点之一包括对微通道环境的特定控制。例如,可以利用层流状态来产生定义的浓度梯度,这在检查细菌趋化性36时特别有用。另一个优点是聚(二甲基硅氧烷)(PDMS)(一种廉价的,生物相容性的弹性聚合物,通常用于制造微流体器件)的透明性质,有利于使用明场和荧光显微镜37对单细胞进行高分辨率成像。同样,微生物在微通道内的局限性意味着可以进行跟踪单个细胞的延时实验,从而可以记录和量化单个细胞的反应37。最后,由于微流体装置可以设计为用户友好,因此它们可以很容易地被非专家使用38。
进一步了解土壤微生物之间的相互作用,对于改进可持续生态系统管理做法,以维持生物多样性和减轻气候变化对陆地环境的影响十分重要39.因此,新型微流体工具的开发是在细胞水平上扩展对真菌及其相互作用的理解的基础。此处的协议将重点介绍为研究BFI40和FFI41而生产的两种微流体装置,如图 1所示。
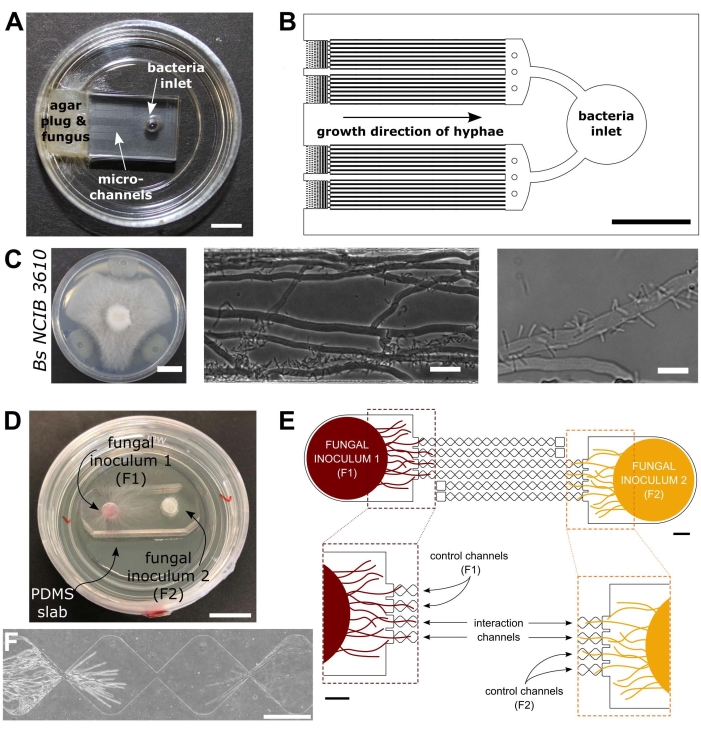
图 1:细菌-真菌相互作用 (BFI) 和真菌-真菌相互作用 (FFI) 装置的视觉和示意图。 (A) BFI 装置的图像。在微通道一端的入口处放置一个菌丝塞,以允许菌丝生长进入设备。细菌入口位于另一端。比例尺= 5 mm. (B) BFI装置的示意图概述,描绘了细菌入口的位置和通过相互作用微通道的菌丝生长方向。通道深度为10 μm,宽100 μm,长7 mm,共有28个观察通道。(C)在 栉 水母和 枯草芽孢杆菌 NCIB 3610之间的琼脂平板上进行对抗试验,比例尺= 20 mm(左)。显微镜图像显示C . cinerea 和 枯草芽孢杆菌 NCIB 3610在微通道(中间和右侧)内的相互作用,即细菌与真菌菌丝的极性附着。比例尺 = 25 μm(中)和 10 μm(右)。(D)FFI装置粘合到玻璃底培养皿上的图像,用菌丝塞双重接种。比例尺 = 1 cm. (E) FFI 设备的原理图概述。两个真菌孕育剂塞子被引入设备两端的入口,允许对微通道进行菌丝探索。控制通道仅连接到一个真菌入口,并具有死端通道,从而防止测试真菌之间的相互作用。相互作用通道连接两个真菌入口,并允许微通道内测试对象之间的菌丝相互作用。每个相互作用通道由18个菱形部分组成,总长度为8.8毫米(每颗钻石490 x 430μm),深10μm,每颗金刚石之间有一个20μm的连接区域。通道类型是重复的,比例尺 = 1 mm.(F) 两个接近的菌丝前沿之间的相互作用区,从相互连接的相互作用通道的两端增长。相差显微镜图像,比例尺= 250μm。该图中的面板已从Stanley等人,2014(A-C)40 和Gimeno等人,2021(D-F)41进行了修改。 请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
本文介绍了一种使用通道微流体研究真菌 – 微生物相互作用的方案。作者旨在证明这些设备的多功能性,并鼓励适应研究人员的兴趣。使用示例性BFI和FFI设备,可以比以前更详细地研究真菌 – 微生物相互作用。通过消除土壤的背景复杂性和异质性,减缓菌丝的生长,使它们局限于单个单层,并严格调节环境参数,随着时间的推移,可以捕获这些生物事件的高分辨率图像。使用这些设备,已经使用?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
我们感谢伦敦帝国理工学院生物工程系和Leverhulme Trust的财政支持(研究资助参考:RPG-2020-352)。
Materials
| Agar | Difco Laboratories | 214010 | Used to solidify culture medium for bacterial and fungal cultivation within Petri dishes |
| Aluminum foil | Fisher Scientific Ltd | 11759408 | |
| AutoCAD 2021 | Autodesk, USA | ||
| Autoclave (VX-75) | Systec | ||
| Centrifuge (5810R) | Eppendorf | ||
| Chlorotrimethysilane | Merck Life Sciences | 386529 | CAUTION: Chlorotrimethylsilane is a hazardous substance. Wear appropriate PPE and handle with care. Avoid contact with skin and eyes and prevent inhalation. Keep away from sources of ignition and use in a well-ventilated area. |
| Cork borer | SLS | COR1000 | |
| Developer solution (mr-Dev 600) | Microresist Technologies | CAUTION: mr-Dev 600 developer solution is flammable | |
| Erlenmeyer flasks | VWR | 214-1108 | e.g. 200 mL; choose size to suit your exact needs |
| Ethanol (70% v/v) | Fisher Scientific Ltd | E/0650DF/15 | Diluted from 99.8% (Analytical Reagent Grade) |
| Fiji | ImageJ | Exemplar software package for imaging processing | |
| Filtered, compressed air | Available as standard in most labs. Altervatively, an oil-free compressor with air regulator can be used. | ||
| Flat-headed wafer tweezers | SLS | INS5026 | |
| Forceps | Fisher Scientific Ltd | 10008051 | Bent, sharp |
| Glass bottom petri dish | World Precision Instruments | FD35-100 | 35 mm |
| Glass bottom petri dish | World Precision Instruments | FD5040-100 | 50 mm |
| Glass crystallisation dishes | VWR | 216-1865 | Used for washing of PDMS slabs |
| Glass crystallisation dishes | VWR | 216-1866 | Used in the development of master moulds |
| Glass media bottles | Fisher Scientific Ltd | 15456113 | e.g. 250 mL; choose size to suit your exact needs |
| Glass syringe (Hamilton) | Fisher Scientific Ltd | 10625251 | Used for dispensing chlorotrimethylsilane |
| Hot plate (HP 160 III BM) | SAWATEC | ||
| Inoculation loop | VWR | COPA175CS01 | |
| Isopropyl alcohol | Sigma-Aldrich | W292907 | |
| Laminar flow hood | Air Science (PCR) | Exemplar laminar flow hood used for device fabrication | |
| LB medium | Fisher Scientific Ltd | BP9723-500 | Exemplar nutrient broth for bacterial overnight culture |
| Light emitting diode light engine (LedHUB) | Omicron-Laserage Laserprodukte GmbH | Exemplar light source that can be used for imaging fungal-microbial interactions (fluorescence) | |
| MA6 Ultraviolet mask aligner | Suss Microtec | ||
| Malt extract | VWR | 84618 | Used to make exemplar fungal culture medium (Malt extract agar) |
| Mask Writer | Applied Materials | 4700DP | Example of a mask writer which can be used to print photo-mask for photolithography |
| Master mould plastic mount | 3D-printed bespoke holder manufactured in-house | ||
| Microbiological safety cabinet (BioMat2) | Contained Air Solutions | Exemplar MSC used for microbial culture and device inoculation | |
| Milli-Q purified water | Available as standard in biology labs. | ||
| NaOH | Fisher Scientific Ltd | BP359-500 | |
| NIS-Elements Advanced Research imaging software | Nikon | Exemplar software package for image acquisition | |
| NIS-Elements Free Viewer | Nikon | Exemplar software package for viewing acquired images | |
| Oven (Binder BD115) | Fisher Scientific Ltd | 15602126 | Used for curing poly(dimethylsiloxane)(PDMS) |
| Oven (CLO-2AH-S) | KOYO | Used for preparing silicon wafers | |
| Parafilm | Bemis | HS234526B | transparent film |
| Petri dishes, square sterile | Fisher Scientific Ltd | 11708573 | 120.5 mm |
| Petri dishes, sterile | Fisher Scientific Ltd | 15370366 | 90 mm |
| Photolithography mask | Micro Lithography Services Ltd. UK | ||
| Plasma cleaner (Zepto) | Diener Electronic | 100012601 | |
| Plastic cup | Semadeni | 8323 | |
| Plastic spatula | Semadeni | 3340 | |
| Portable precision balance (OHAUS Scout) | Fisher Scientific Ltd | 15519631 | Used for weighing PDMS, media components etc. |
| Precision cutter | Syneo | HS1251135P1183 | Cutting edge diameter: 3.18 mm |
| Precision cutter | Syneo | HS1871730P1183S | Cutting edge diameter: 4.75 mm |
| Profilometer | Bruker | Dektak XT-stylus | |
| Razor blades | Häberle Labortechnik | 9156110 | |
| Refridgerator | Haden | 4-6 °C | |
| Retiga R1 CCD camera | Qimaging | Exemplar camera that can be used for imaging fungal-microbial interactions | |
| Scotch magic tape | Office Depot | 3969954 | 19 mm invisible tape; clear tape |
| Shaking incubator (Cole-Parmer SI500) | Fisher Scientific Ltd | 10257954 | |
| Silicon wafer | Inseto | 100 mm | |
| Soda lime glass plate | Inseto | 125 mm x 125 mm x 2 mm. Used to hold photolithography mask in mask aligner | |
| Sodium chloride | Sigma-Aldrich | S7653 | |
| Spincoater | SAWATEC | SM-180-BM | |
| SU-8 2010 photoresist | MicroChem | CAUTION: SU-8 photoresist is hazardous, take care when handling and prevent inhalation and contact with skin. Flammable, potentially carcinogenic and toxic to the environment. | |
| Sylgard 184 elastomer kit | VWR | 634165S | Used for the preparation of poly(dimethylsiloxane)(PDMS) devices |
| Temperature controlled incubator | Okolab | Exemplar incubator that can be used for imaging fungal-microbial interactions | |
| Ti2-E inverted epifluorescence microscope | Nikon | MEA54000 | Exemplar microscope that can be used for imaging fungal-microbial interactions |
| Ultrasonic cleaner S-Line | Fisher Scientific Ltd | FB15050 | |
| Vacuum desiccator | Fisher Scientific Ltd | 10528861 | Silianisation and PDMS degassing should be conducted in separate desiccators |
| x10/0.3 NA CFI Plan Fluor DL objective lens | Nikon | MRH20105 | Exemplar objective lens that can be used for imaging fungal-microbial interactions |
| x20/0.45 NA CFI Plan Fluor DL objective lens | Nikon | MRH48230 | Exemplar objective lens that can be used for imaging fungal-microbial interactions |
References
- Zhu, Y. -. G., Miller, R. M. Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends in Plant Science. 8 (9), 407-409 (2003).
- Dai, Z., et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. The ISME Journal. 14 (3), 757-770 (2020).
- Op De Beeck, M., et al. Regulation of fungal decomposition at single-cell level. The ISME Journal. 14 (4), 896-905 (2020).
- Bender, S. F., et al. Symbiotic relationships between soil fungi and plants reduce N2O emissions from soil. The ISME Journal. 8 (6), 1336-1345 (2014).
- Dullah, S., et al. Melanin production and laccase mediated oxidative stress alleviation during fungal-fungal interaction among basidiomycete fungi. IMA Fungus. 12 (1), 33 (2021).
- Deveau, A., et al. Bacterial-fungal interactions: ecology, mechanisms and challenges. FEMS Microbiology Reviews. 42 (3), 335-352 (2018).
- Bian, R., et al. Facilitative and synergistic interactions between fungal and plant viruses. Proceedings of the National Academy of Sciences of the United States of America. 117 (7), 3779-3788 (2020).
- Jiang, X., Xiang, M., Liu, X. Nematode-trapping fungi. Microbiology Spectrum. 5 (1), (2017).
- Essig, A., et al. a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. Journal of Biological Chemistry. 289 (50), 34953-34964 (2014).
- Tang, H. -. Y., Zhang, Q., Li, H., Gao, J. -. M. Antimicrobial and allelopathic metabolites produced by Penicillium brasilianum. Natural Product Research. 29 (4), 345-348 (2015).
- Bai, Y. -. B., et al. Antifungal activity of griseofulvin derivatives against phytopathogenic fungi In vitro and In vivo and three-dimensional quantitative structure-activity relationship analysis. Journal of Agricultural and Food Chemistry. 67 (22), 6125-6132 (2019).
- Solanki, M. K., et al. Characterization of antagonistic-potential of two Bacillus strains and their biocontrol activity against Rhizoctonia solani in tomato. Journal of Basic Microbiology. 55 (1), 82-90 (2015).
- Jamali, H., Sharma, A., Srivastava, A. K. Biocontrol potential of Bacillus subtilis RH5 against sheath blight of rice caused by Rhizoctonia solani. Journal of Basic Microbiology. 60 (3), 268-280 (2020).
- Válková, H., Novotný, &. #. 2. 6. 8. ;., Malachová, K., Šlosarčíková, P., Fojtík, J. Effect of bacteria on the degradation ability of Pleurotus ostreatus. Science of The Total Environment. 584-585, 1114-1120 (2017).
- Leyva-Rojas, J. A., Coy-Barrera, E., Hampp, R. Interaction with soil bacteria affects the growth and amino acid content of Piriformospora indica. Molecules. 25 (3), 572 (2020).
- Dullah, S., et al. Fungal interactions induce changes in hyphal morphology and enzyme production. Mycology. 12 (4), 279-295 (2021).
- Marfetán, J. A., Romero, A. I., Folgarait, P. J. Pathogenic interaction between Escovopsis weberi and Leucoagaricus sp.: mechanisms involved and virulence levels. Fungal Ecology. 17, 52-61 (2015).
- Cortois, R., De Deyn, G. B. The curse of the black box. Plant and Soil. 350 (1), 27-33 (2012).
- Whitesides, G. M. The origins and the future of microfluidics. Nature. 442 (7101), 368-373 (2006).
- Sackmann, E. K., Fulton, A. L., Beebe, D. J. The present and future role of microfluidics in biomedical research. Nature. 507 (7491), 181-189 (2014).
- Hanson, K. L., et al. Fungi use efficient algorithms for the exploration of microfluidic networks. Small. 2 (10), 1212-1220 (2006).
- Held, M., Edwards, C., Nicolau, D. V. Probing the growth dynamics of Neurospora crassa with microfluidic structures. Fungal Biology. 115 (6), 493-505 (2011).
- Thomson, D. D., et al. Contact-induced apical asymmetry drives the thigmotropic responses of Candida albicans hyphae. Cellular Microbiology. 17 (3), 342-354 (2015).
- Lee, K. K., Labiscsak, L., Ahn, C. H., Hong, C. I. Spiral-based microfluidic device for long-term time course imaging of Neurospora crassa with single nucleus resolution. Fungal Genetics and Biology. 94, 11-14 (2016).
- Asenova, E., Lin, H. Y., Fu, E., Nicolau, D. V., Nicolau, D. V. Optimal fungal space searching algorithms. IEEE Transactions on NanoBioscience. 15 (7), 613-618 (2016).
- Soufan, R., et al. Pore-scale monitoring of the effect of microarchitecture on fungal growth in a two-dimensional soil-like micromodel. Frontiers in Environmental Science. 6, (2018).
- Uehling, J. K., et al. Microfluidics and metabolomics reveal symbiotic bacterial-fungal interactions between Mortierella elongata and Burkholderia include metabolite exchange. Frontiers in Microbiology. 10, 2163 (2019).
- Millet, L. J., et al. Increasing access to microfluidics for studying fungi and other branched biological structures. Fungal Biology and Biotechnology. 6 (8), 1-14 (2019).
- Baranger, C., Fayeulle, A., Le Goff, A. Microfluidic monitoring of the growth of individual hyphae in confined environments. Royal Society Open Science. 7 (8), 191535 (2020).
- Aleklett, K., Ohlsson, P., Bengtsson, M., Hammer, E. C. Fungal foraging behaviour and hyphal space exploration in micro-structured Soil Chips. The ISME Journal. 15 (6), 1782-1793 (2021).
- Aleklett, K., et al. Build your own soil: exploring microfluidics to create microbial habitat structures. The ISME Journal. 12 (2), 312-319 (2018).
- Ellett, F., Jorgensen, J., Frydman, G. H., Jones, C. N., Irimia, D. Neutrophil interactions stimulate evasive hyphal branching by Aspergillus fumigatus. PLOS Pathogens. 13 (1), 1006154 (2017).
- Massalha, H., Korenblum, E., Malitsky, S., Shapiro, O. H., Aharoni, A. Live imaging of root-bacteria interactions in a microfluidics setup. Proceedings of the National Academy of Sciences of the United States of America. 114 (17), 4549-4554 (2017).
- Schmieder, S. S., et al. Bidirectional propagation of signals and nutrients in fungal networks via specialized hyphae. Current Biology. 29 (2), 217-228 (2019).
- Tayyrov, A., Stanley, C. E., Azevedo, S., Künzler, M. Combining microfluidics and RNA-sequencing to assess the inducible defensome of a mushroom against nematodes. BMC Genomics. 20 (1), 243 (2019).
- Stanley, C. E., Grossmann, G., Casadevall i Solvas, X., deMello, A. J. Soil-on-a-Chip: microfluidic platforms for environmental organismal studies. Lab on a Chip. 16 (2), 228-241 (2016).
- Stanley, C. E., vander Heijden, M. G. A. Microbiome-on-a-Chip: new frontiers in plant-microbiota research. Trends in Microbiology. 25 (8), 610-613 (2017).
- Ortseifen, V., Viefhues, M., Wobbe, L., Grünberger, A. Microfluidics for biotechnology: bridging gaps to foster microfluidic applications. Frontiers in Bioengineering & Biotechnology. 8, 589074 (2020).
- Jansson, J. K., Hofmockel, K. S. The soil microbiome-from metagenomics to metaphenomics. Current Opinion in Microbiology. 43, 162-168 (2018).
- Stanley, C. E., et al. Probing bacterial-fungal interactions at the single cell level. Integrative Biology (Camb). 6 (10), 935-945 (2014).
- Gimeno, A., et al. A versatile microfluidic platform measures hyphal interactions between Fusarium graminearum and Clonostachys rosea in real-time. Communications Biology. 4 (1), 262 (2021).
- Duffy, D. C., McDonald, J. C., Schueller, O. J. A., Whitesides, G. M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Analytical Chemistry. 70 (23), 4974-4984 (1998).
- Stanley, C. E., et al. Fabrication and use of the dual-flow-RootChip for the imaging of Arabidopsis roots in asymmetric microenvironments. Bio-protocol. 8 (18), 3010 (2018).
- Choi, C. -. H., Lee, H., Weitz, D. A. Rapid patterning of PDMS microfluidic device wettability using syringe-vacuum-induced segmented flow in nonplanar geometry. ACS Applied Materials & Interfaces. 10 (4), 3170-3174 (2018).
- Sanders, E. R. Aseptic laboratory techniques: plating methods. Journal of Visualized Experiments. (63), e3064 (2012).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Harting, R., et al. Pseudomonas strains induce transcriptional and morphological changes and reduce root colonization of Verticillium spp. Frontiers in Microbiology. 12, 652468 (2021).
- Boenisch, M. J. . Structural and molecular characterisation of the penetration process of Fusarium graminearum during Fusarium head blight infection. , (2013).
- Eynck, C., Koopmann, B., Grunewaldt-Stoecker, G., Karlovsky, P., von Tiedemann, A. Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. European Journal of Plant Pathology. 118 (3), 259-274 (2007).
- Ghanem, N., Stanley, C. E., Harms, H., Chatzinotas, A., Wick, L. Y. Mycelial effects on phage retention during transport in a microfluidic platform. Environmental Science & Technology. 53 (20), 11755-11763 (2019).
- Alrifaiy, A., Lindahl, O. A., Ramser, K. Polymer-based microfluidic devices for pharmacy, biology and tissue engineering. Polymers. 4 (3), 1349-1398 (2012).
- Duncombe, T. A., Tentori, A. M., Herr, A. E. Microfluidics: reframing biological enquiry. Nature Reviews Molecular Cell Biology. 16 (9), 554-567 (2015).
- Hoelzle, D., et al. Microfluidic device design, fabrication, and testing protocols. Protocol Exchange. , (2015).

