Techniques of Sleeve Gastrectomy and Modified Roux-en-Y Gastric Bypass in Mice
Summary
Bariatric surgery is the most efficient way to reduce body weight and the deadly metabolic complications (diabetes, obesity, and dyslipidemia) frequently associated with morbid obesity. Mouse models of bariatric surgery represent a unique asset for deciphering molecular mechanisms behind the beneficial effects of these surgeries on diabetes, hypertension, and dyslipidemia.
Abstract
Obesity is a major public health issue, with a prevalence of 4 to 28% for men and 6.2 to 36.5% for women in Europe (from 2003 to 2008). Morbid obesity is frequently associated with metabolic complications, such as type 2 diabetes, hypertension, and dyslipidemia, reducing life expectancy and quality. In the absence of any effective noninvasive treatments, bariatric surgery is a valuable therapeutic option for patients with morbid obesity (body mass index (BMI) >40 kg/m2), leading to long-term, sustained weight loss and improvements in metabolic complications. However, the underlying cellular and molecular mechanisms sustaining the beneficial effects of bariatric surgery are not yet fully understood. Due to the numerous genetically-modified strains available, the mouse model is the most convenient animal model to explore the molecular mechanisms behind the pleiotropic beneficial effects of bariatric surgeries. Here, we detailed the optimized healthcare methods and surgical protocols in mice for the two most widely-used bariatric surgeries: the sleeve gastrectomy and the modified Roux-en-Y gastric bypass. Deciphering the molecular mechanisms underlying the therapeutic effects of bariatric surgeries offers the promise of identifying new therapeutics targets.
Introduction
The worldwide pandemic of obesity and diabetes is devastating in severity. Over two billion adults worldwide (30% of the population) are either overweight (BMI >25 kg/m2) or obese (BMI >30 kg/m2)1. This can come along with metabolic complications, such as type 2 diabetes, hypertension, and dyslipidemia, leading to increased morbidity and mortality. Obesity increases the overall mortality and the prevalence of cancer2. Due to the lack of any effective noninvasive treatments, bariatric surgery represents the only option that can lead to long-term, sustained weight loss3,4. A number of different surgical methods have been developed, but the sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) are the two procedures most commonly used in clinical practice. During the SG procedure, 80% of the initial volume of the stomach is removed; thus, this technique is one of the restrictive surgeries that improves satiety. The RYGB is one of the restrictive-malabsorptive techniques. During RYGB, a small gastric pouch (1-2% of the total gastric volume) is created and the intestine is rearranged into a Y-shape, which delays digestion and the absorption of nutrients. These two techniques lead to significant body weight reductions and general improvements in frequently-associated comorbidities (e.g., hypertension, type 2 diabetes, and dyslipidemia)3, with a higher efficiency seen in RYGB. However, the molecular mechanisms behind the pleiotropic beneficial effects of the bariatric surgeries are often not fully elucidated. Due to the numerous genetically-modified strains available, a mouse model is the most convenient animal model to explore these molecular mechanisms.
However, bariatric procedures are difficult to directly adapt to small animal models and require high surgical dexterity. While SG can be easily performed in rodents with a very good survival rate, RYGB is lethal in mice due to severe bowel obstructions5. Different modified RYGB techniques have been proposed to counteract this problem, notably the oesojejunostomy5. Here, we present another alternative: the gastrojejunostomy without a stomach excision. This modified RYGB reproduces most of the beneficial effects observed in humans (i.e., a significant body weight reduction and an improvement in glucose and lipid homeostasis).
This manuscript aims to summarize and discuss the technical and experimental details of SG and RYGB in mice and to facilitate these procedures with the help of videos. A specific highlight will be made regarding the optimization of the preoperative and postoperative healthcare protocols that allow the reduction of vitamin and iron deficiencies.
Protocol
Animal and housing: Obtain 8-week-old C57Bl/6 male mice. At 10 weeks old, give the C57Bl/6 mice free access to water and a high-fat diet (DIO diet: 35% kcal from fat, 25.8% caseine, 1.30% mineral AIN, 1.30% vitamins, 1.70% dicalcium phosphate, 0.7% calcium carbonate, 2.10% citrate potassium, 0.026% choline bitartrate, 8.9% sucrose, 0.384% cystine, 6.5% cellulose, 31.7% lard, 3.3% soybean oil, and 16.29% maltodextrine) for 8 (SG) or 14 (RYGB) weeks prior to the bariatric surgeries. To promote weight gain, give the mice dedicated to the RYGB surgery (sham & RYGB) water containing 20% fructose (w/v) in addition to the high-fat diet.
Ethics Statement: All protocol steps described below follow the guidelines of the Ethics Committee for Animal Experimentation of Pays de la Loire under the approval number 01953.01.
1. General Preoperative Preparation
- Fast the mice for 6 h before the surgery. Gel diet food was given 3 days before surgery and exclusively one day before surgery. Induce anesthesia in a chamber with 5% isoflurane (0.4 L/min) and O2 (dioxygen; 0.4 L/min). Perform a toe-pinch test to confirm that the anesthetization is effective. Administer analgesics (0.1 mg/kg buprenorphine), antibiotics (10 mg/kg marbofloxacine), and pro-kinetics (1 mg/kg metoclopramide) via subcutaneous injections.
- Allocate a specific work area for surgical procedures (distant from laboratory traffic and ventilation fans). Clean the surgical area with dedicated hard surface disinfectants. Place a heating pad on the working space area to maintain mouse homeothermy during the surgery. Apply a clean, absorbent underpad over the heating pad.
- Shave the abdomen from sternum to pelvis using an electric razor. Wrap the mouse abdomen in sterilized plastic wrap (Figures 1A and 2A) and place the mouse in supine position on the heating pad.
- Apply vet ointment on the eyes to prevent dryness while under anesthesia. Place the mouse in the nose cone and maintain anesthesia with 2% isoflurane (0.4 L/min) and O2 (0.4 L/min).
- Before beginning the surgery, put on a sterile examination gown, a disposable scrubs cap, a facemask, and sterile gloves. Open an autoclaved package containing a sterilized surgical instrument set, which is required for all steps of the surgery. Cut a window in the sterilized plastic wrap and disinfect the skin with 2 successive povidone-iodine solutions.
2. Sleeve Gastrectomy: Surgical Procedure
- Median laparotomy (Figure 1B)
- Under a binocular microscope and with a scalpel, perform a midline incision from the sternum to the middle of the abdomen to open the abdominal cavity (do not damage the abdominal muscles). Protect the skin with a sterile compress soaked with 37 °C saline solution.
- Stomach exposure (Figure 1C)
- Gently mobilize the stomach using moistened-cotton swabs. Free the stomach from its lateral close connective tissue attachments using moistened cotton swabs or micro scissors.
- Gently, externalize the stomach fully and place a hemostatic collagen compress behind the stomach. Suture the pylorus and esophagus vessels along the stomach greater curvature with 8.0 non-absorbable sutures to avoid future bleeding (Figure 1D and E). Note: Hemostat use can induce tissue damage, make sure to only use it on tissue that aim to be resected.
- Resection of the cardiac region of stomach (fundus)
- Perform a gastrotomy on the anatomical line present between the pyloric region (corpus) and the cardiac region of stomach (fundus) (Figure 1F).
- Remove residual food with 2 moistened cotton swabs towards each side of the stomach. Using micro scissors, cut the stomach along the boundary between the fundus and the corpus of the stomach (Figure 1G).
- Press a hemostatic collagen compress for 2 min in case of major bleeding. Close the stomach opening with 8.0 non-absorbable sutures from the gastro-oesophagal junction to the end of the incision (Figure 1H).
- Resection of the pyloric region of stomach (corpus)
- Complete the stomach resection along the greater curvature so as to remove approximately 80% of the stomach (Figure 1I). Standardize the width of the SG to 3 mm. Press a hemostatic collagen compress for 2 min in case of major bleeding.
- Close the opening of the pyloric region of the stomach with 8.0 non-absorbable suture (Figure 1J). Gently roll 2 moistened cotton swabs towards each side of the stomach to ensure that the suture is leak-proof.
- Complete with 8.0 non-absorbable sutures in case of leakage. Remove the hemostatic collagen compresses and return the stomach to the abdominal cavity. Leave a hemostatic collagen compress against the suture.
- Abdominal closure
- Close the muscle layer of the abdominal wall with 5.0 non-absorbable sutures (Figure 1K). Reduce anesthesia by reducing the isoflurane concentration to 1%. Close the skin using 5.0 non-absorbable sutures (Figure 1L).
3. Roux-en-Y Gastric Bypass: Surgical Procedure
- Median laparotomy
- Under a binocular microscope and with a scalpel, perform a midline incision from the sternum to the middle of the abdomen to open the abdominal cavity (do not damage the abdominal muscles). Protect the skin with a sterile compress soaked with 37 °C saline solution (Figure 2B).
- Biliopancreatic limb and alimentary limb
- Externalize the intestine (Figure 2C). Measure 8 cm from the pylorus and perform two ligatures of the intestine with 5.0 non-absorbable sutures (Figure 2D).
- Cut the intestine between the two ligatures (Figure 2E). Place the proximal limb of the two ends in the upper-left quadrant of the abdomen. Note: This will be used as the alimentary limb (Figure 2F).
- Place the distal limb of the two ends facing the alimentary limb 6 cm below the proximal limb (Figure 2F).
NOTE: This will be used as the biliary limb.
- Jejuno-jejunostomy
- Cut both the proximal limb and the intestine loop using micro scissors and perform two antimesenteric incisions of the same length (Figure 2G and 2H).
- Perform side-to-side anastomosis with two 8.0 non-absorbable sutures. Perform the dorsal side anastomosis first (Figure 2I), followed by the ventral side anastomosis (Figure 2J).
- Gently roll 2 moistened cotton swabs towards each side of the anastomosis to ensure that the suture is leak-proof. Complete with 8.0 non-absorbable sutures in case of leakage.
- Gastro-jejunostomy
- Gently mobilize the stomach using moistened cotton swabs. Free the stomach from its lateral close connective tissue attachments using moistened cotton swabs or micro scissors.
- Gently, externalize the stomach fully, placing a re-absorbable hemostatic collagen compress behind the stomach. Perform a ligature of the pylorus using a 5.0 non-absorbable suture passed through the omentum using curved micro forceps (Figure 2K and 2L).
- Cut both the ventral side of the stomach, 1.5 cm from the pylorus, and the distal limb using micro scissors, creating two incisions of the same length (Figure 2M).
- Perform side-to-side anastomosis with two 8.0 non-absorbable sutures. Start with the dorsal side anastomosis (Figure 2N), and then perform the ventral side anastomosis (Figure 2O). Gently roll 2 moistened-cotton swabs towards each side of the anastomosis to ensure that the suture is leak-proof. Complete with 8.0 non-absorbable sutures in case of leakage.
- Abdominal closure
- Close the muscle layer of the abdominal wall using 5.0 non-absorbable sutures (Figure 2P). Reduce the anesthesia by reducing the isoflurane concentration to 1%. Close the skin using 5.0 non-absorbable sutures (Figure 2Q).Administer 25 mL/kg of warm saline solution via subcutaneous injection.
4. General Postoperative Care
- Stop isoflurane and continue with an O2 flow of 0.8 L/min until the mouse is fully awake. Do not leave the mouse unattended until it regains motor control, indicated by the animal starting to move around the cage and being able to stand and walk without falling.
- Place the mouse (only one mouse per cage) in an incubator under 30 °C temperature condition for 5 days.
- Maintain iron (0.5 mg/kg/day; subcutaneous injection) and vitamin (800 mg/180 mL in water) supplementations in the RYGB mice until the end of the protocol.
- Return free access to gel diet food (high-fat gel diet: 10% lard, 10% liquid sugar, 57% water) for 5 days after surgery. Reintroduce a solid diet 3 days after surgery.
- Subcutaneously inject buprenorphine (0.1 mg/kg, twice daily, from day 0 to day 3 after surgery), meloxicam (1 mg/kg, from day 0 to day 3 after surgery), metoclopramide (1 mg/kg, from day 0 to day 5 after surgery), and marbofloxacine (10 mg/kg, from day 0 to day 3 after surgery).
5. Postoperative Metabolic Parameters Assessment
- Food intake measurement
- Allow the mice to recover for 1 week after surgery. House the mice one per cage. Measure the amount of solid diet placed in the cage. Weight the remaining food 24 h later. Repeat this step as long as required.
- Oral glucose tolerance test
- Fast the mice for 6 h before the oral bolus. Harvest a blood drop from the tail tip and apply it on a glucose strip inserted in a glucometer to determine the time 0 blood glucose value.
- Administer an oral bolus of 20% D-glucose solution (2 g/kg). Measure the blood glucose levels at 15, 30, 60, and 120 min post gavage.
- Hemoglobin level analysis
- Using a hematology system, measure 20 µL of fresh blood to sample the hemoglobin level.
6. Euthanasia
- Anesthetize the mice by intraperitoneally injecting a xylazine/ketamine solution (10/80 mg/kg). Perform a cervical dislocation. Verify complete euthanasia by ensuring that the heart is not beating and the eyeball blink reflex is lost.
Representative Results
General conditions
The mean operative time for the SG procedure was 49.3 ± 1.5 min. We removed 62.8 ± 5.0 mg of stomach, which represents about 80% percent of the stomach. No mice died during the surgery or during the following seven days. One mouse (7.1%) died on the 11th postoperative day because of a gastric obstruction caused by a bezoar.
The mean operative time for the RYGB procedure was 89.1 ± 2.8 min. The mortality rate was significantly higher after RYGB (sham: 25% versus RYGB: 66.6%; p = 0.038). The main causes of postoperative deaths were anastomotic leaks (n = 8), anastomotic stenosis (n = 5), anesthesia complications (n = 4), and postoperative bleeding (n = 2). We also observed two long-term deaths due to gastrointestinal anastomosis stenosis with chronic obstruction and malnutrition. As shown in Figure 3, we noticed a progressive improvement of the post-operative survival rate throughout the protocol, indicating that intensive surgical training is required to master the RYGB surgical technique.
Body weight, food intake, and glucose homeostasis
The mice had similar body weights before surgery. SG demonstrated significant weight loss 14 days after surgery compared to sham control mice (sham: -6.6% versus SG: -16.0%; p <0.01; Figure 4A). RYGB was associated with significant weight loss from postoperative days 14 (sham: -7.9% on day 14 post-surgery and -5.1% on day 28 post-surgery versus RYGB: -22.9% on day 14 post-surgery and -25.5% on day 28 post-surgery; Figure 4B). The daily food intake was reduced by 35% during the 14-day period following the SG (Figure 4C). In contrast, we did not observe such an anorexigenic effect between the sham and RYGB mice (Figure 4D). We also reported an improvement of glucose tolerance following SG and RYGB surgeries (Figure 4E, 4F).
Beneficial effect of iron supplementation on RYGB-induced anemia
We did not observe a significant decrease of hemoglobinemia 14 days after SG surgery (data not shown). In contrast, severe anemia was measured in RYGB-operated mice, but it did benefit from specific iron supplementation (Figure 5A). Daily iron supplementation was indeed crucial and prevented significant anemia 28 days after RYGB surgery (Figure 5B).
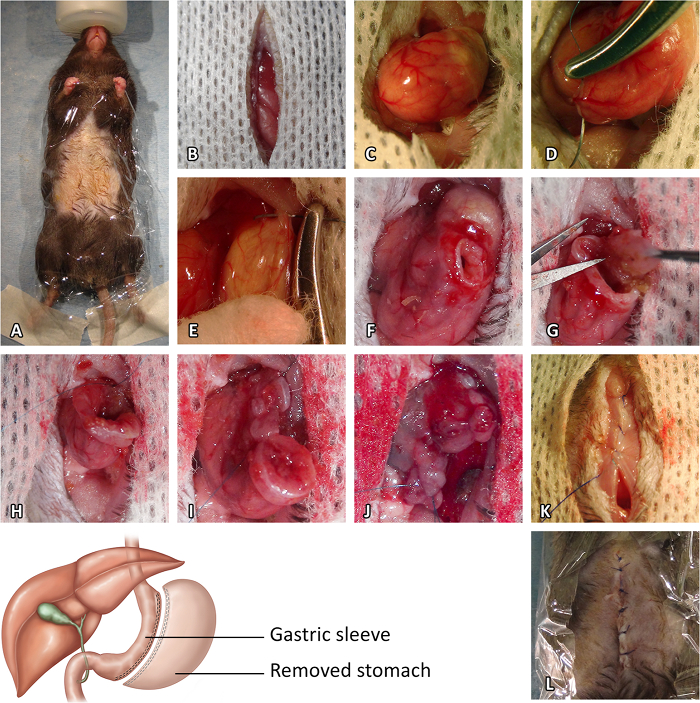
Figure 1: Sleeve Gastrectomy Procedure. (A) Preoperative preparation. (B) Median laparotomy. (C) Stomach exposure. (D) Pylorus vessel suture. (E) Esophagus vessel suture. (F) Initial gastrotomy. (G) Resection of the cardiac region of the stomach. (H) Closure of the stomach opening from the gastroesophageal junction to the end of the incision. (I) Resection of the pyloric region of the stomach. (J) Closure of the opening of the pyloric region. (K) Closure of the muscle layer of the abdominal wall. (L) Closure of the skin. Please click here to view a larger version of this figure.
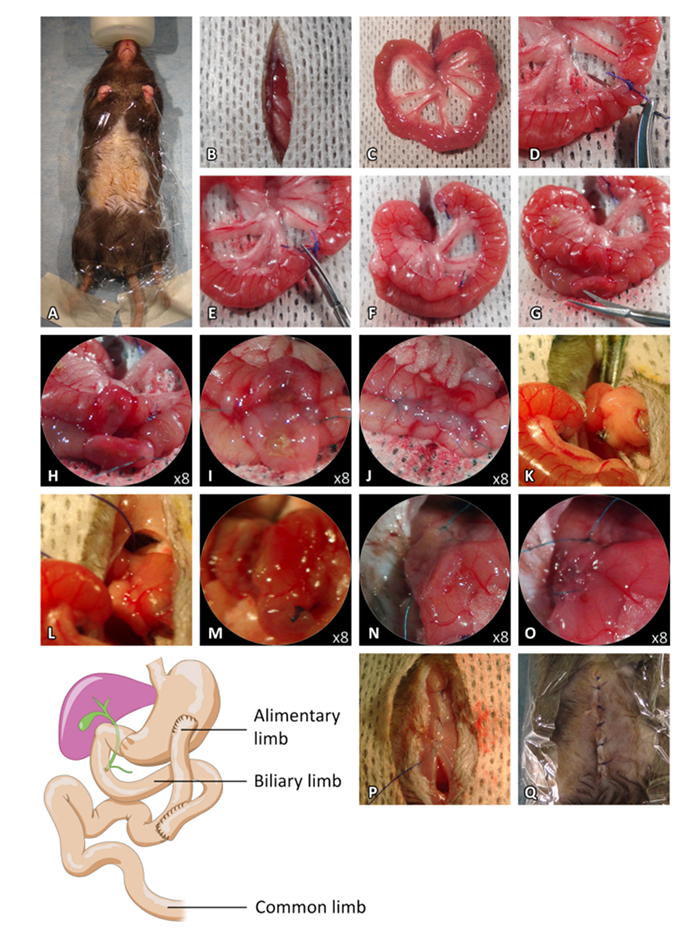
Figure 2: Roux-en-Y bypass Procedure. (A) Preoperative preparation. (B) Median laparotomy. (C) Externalization of the small intestine. (D) Biliopancreatic and alimentary limbs ligatures. (E) Small intestine section between the ligature. (F) Intestinal limb positioning for the jejuno-jejunostomy. (G–H) Antimesenteric incisions of the proximal limb and the intestine loop. (I) Dorsal side-to-side anastomosis. (J) Ventral side-to-side anastomosis. (K–L) Pylorus ligature. (M) Incision of the ventral side of the stomach. (N) Incision of the distal limb and dorsal side-to-side anastomosis. (O) Ventral side-to-side anastomosis. (P) Closure of the muscle layer of the abdominal wall. (Q) Closure of the skin. Please click here to view a larger version of this figure.
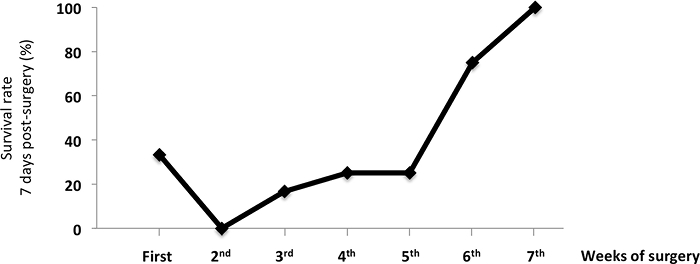
Figure 3: Survival Rate of Mice 7 Days after the RYGB Surgery. The data show the benefit of surgical training on the survival rate of RYGB-operated mice. The values are shown as the mean value ± SEM. Please click here to view a larger version of this figure.
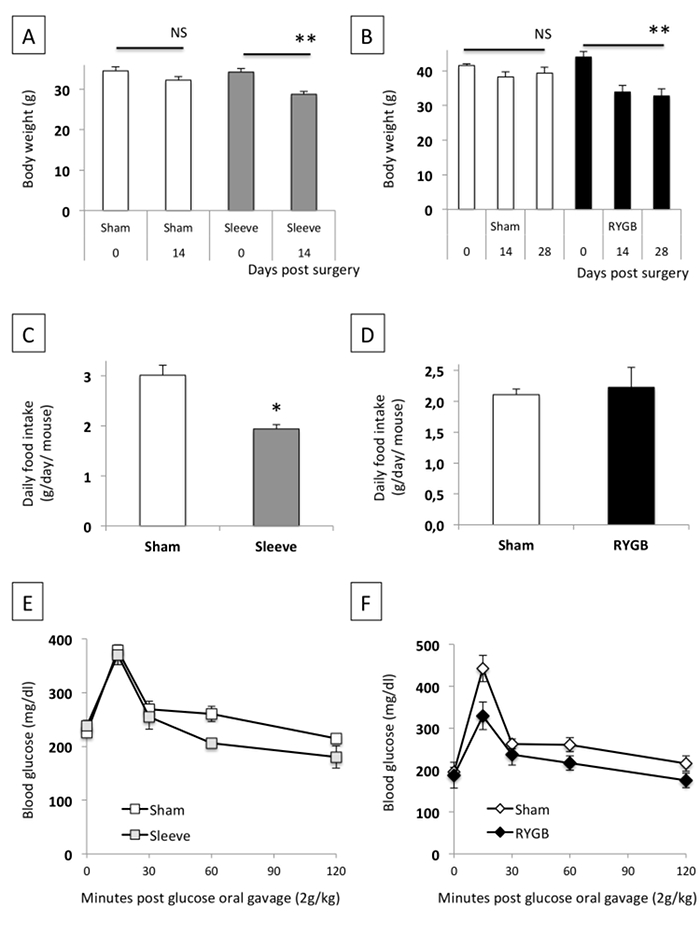
Figure 4: Effect of SG (A, C, D) and RYGB (B, D, F) on Bodyweight, Daily Food Intake, and Glucose Tolerance. The data are shown as the mean value ± SEM. NS: not significant; * P <0.05; ** P <0.01 (Mann-Whitney test, ANOVA test). Please click here to view a larger version of this figure.
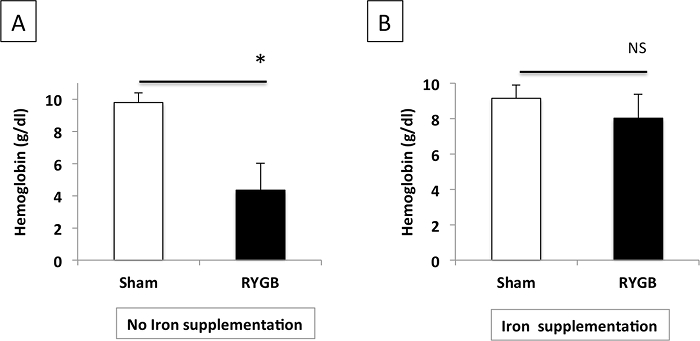
Figure 5: Plasma Hemoglobin Levels after RYGB in Mice Without (A) or With (B) Iron Supplementation. The data are shown as the mean value ± SEM. NS: not significant; * P <0.05 (Mann-Whitney test). Please click here to view a larger version of this figure.
Discussion
To overcome the growing epidemic of obesity, the first bariatric surgery procedures emerged in the 1960s in the United States. Since then, the number of procedures performed worldwide each year still increase, and today, these techniques represent the best therapeutic option for the management of morbid obesity6. Among the procedures developed, SG and RYGB are the two most popular methods used in clinical practice4. Animal models, notably rodents, have been used to decipher the mechanisms behind the general health improvement observed after bariatric surgery.
To decipher the molecular mechanisms sustaining the beneficial metabolic effects of bariatric surgeries, we developed two mouse models of bariatric surgery: the SG, which is often defined as a restrictive technique, and the RYGB, which is described as a restrictive and malabsorptive surgery. The realization of these mouse bariatric procedures requires experience in microsurgery. These models were developed using a technician specialized in animal surgery and a human visceral surgeon. From experience, surgical training was necessary to reach over 75% survival after RYGB. Mastering SG does not require as much time, and the learning curve is shorter, with mortality at about 95%. By contrast, RYGB is a more complex technique and requires 6 to 8 weeks of training to reach an acceptable postoperative survival rate.
In the literature, there are a variety of rodent bariatric procedures that have been previously described7,8,9. In this SG technique, we removed the cardiac region of the stomach (also called the fundus), not present in humans. The objective was to remove about 80% of the stomach. The pouch size of RYGB models in rats ranges from <5% to >20% of the initial stomach size. Several studies used mouse RYGB models, presenting either a complete preservation of the stomach10, a small gastric pouch5, or a total stomach diversion5. In a very comprehensive study, Deng Ping Yin and colleagues have evaluated the different mouse models of RYGB and reported that the murine stomach was not anatomically adapted to the creation of a gastric pouch5. For our RYGB procedure, we choose to perform a Roux-en-Y without cutting the stomach. As a limitation, our model did not induce a reduction in food intake, which contrasts what it observed in human models of RYGB.
The survival rate after RYGB operations in rats is rarely mentioned by the authors in the literature, but it seems to reach about 30 to 35% on average11. In our hands, mortality was mainly due to anastomotic leakage or stenosis or to late postoperative gastrojejunal anastomosis stenosis, leading to chronic obstruction with persistent excessive weight loss, which compromised animal welfare. RYGB requires specific perioperative management, including a gel diet during the first three postoperative days and a pain management regime. To improve long-term survival after surgery, we added vitamins postoperatively, particularly fat-soluble vitamins and iron, to prevent anemia. Conversely, mice do not need liposoluble vitamin supplementation after SG, since it was only a restrictive procedure. In the previous papers, the authors did not describe the health condition of operated animals during the postoperative course. Because it is a major surgery, we cannot assume the systematic well-being of the animals in these studies.
In conclusion, mouse models of either restrictive (SG) or restrictive-malabsorptive (RYGB) bariatric surgeries are feasible but require microsurgical training with a learning curve, especially for the RYGB procedure. To keep the animals in good health, perioperative management is very important in the RYGB procedure, particularly in terms of pain, but also in terms of vitamin supplementation. By achieving a faster, sterile surgery and by using microsurgical training, we can greatly improve the postoperative survival rate. These models are useful and essential tools to understand the metabolic effects of bariatric surgery.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Gilles Mithieux and Aude Barataud (INSERM U1213, Lyon, France) and Marie Liabeuf and Stephanie Lemarchand-Minde (Animal facility, l’Institut du Thorax, Nantes, France) for their help with the animal care protocol. This work was supported by grants from La région des Pays de la Loire, the Fondation d’Avenir, and the Casden Bank. We would like to thank Catherine Postic, Fadila Benhamed and Michelle Caüzac from l’institut Cochin for their hospitality and their help during the filming process.
Materials
| Drugs | |||
| High Fat diet | DIO diet | Safe | |
| Isoflurane | Forane | Baxter | |
| Buprenorphin | Buprecare | Animalcare | |
| Marbofloxacine | Marbocyl | Vetoquinol | |
| Ammonium iron citrate, vitamins PP-B12 | Fercobsang | Vetoquinol | |
| Vitamins A-D3-E-K-B | Vita Rongeur | Virbac | |
| NaCl 0,9% | NaCl 0,9% | ||
| Povidone solution | Betadine Scrub | Betadine | |
| Povidone solution | Betadine Solution | Betadine | |
| Carboptol 980 NF | Ocrygel | TVM | |
| Name | References | Company | Comments |
| Sutures | |||
| Prolene® | 8.0, 6,5 mm | Ethicon | |
| Prolene® | 5.0, 13 mm | Ethicon | |
| Name | References | Company | Comments |
| Surgical equipments | |||
| Scissors | FST | ||
| Needle holder | Olsen-Hegar | FST | |
| Micro scissors | Vannas | FST | |
| Micro forceps | Graefe | FST | |
| Micro forceps curved | Graefe | FST | |
| Curved micro needle holder | Castroviejo | FST | |
| Hemostatic collagen compress | Pangen | Urgo | |
| Absorbent underpads | VWR | ||
| Name | References | Company | Comments |
| Specific equipments | |||
| Hematology system | Hemavet 950FS | Hemavet | |
| Glucose strips and glucometer | One touch Verio | Life scan | |
| Stereo microscope | MZ6 | Leica |
References
- Ng, M., Fleming, T., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 384 (9945), 766-781 (2014).
- Calle, E. E., Thun, M. J., Petrelli, J. M., Rodriguez, C., Heath, C. W. Body-mass index and mortality in a prospective cohort of U.S. adults. The New England Journal of Medicine. 341 (15), 1097-1105 (1999).
- Sjöström, L., Lindroos, A. K., et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. The New England Journal of Medicine. 351 (26), 2683-2693 (2004).
- Buchwald, H., Avidor, Y., et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 292 (14), 1724-1737 (2004).
- Yin, D. P., Gao, Q., et al. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Annals of surgery. 254 (1), 73-82 (2011).
- Buchwald, H., Oien, D. M. Metabolic/Bariatric Surgery Worldwide 2008. Obesity Surgery. 19 (12), 1605-1611 (2009).
- Liu, W., Zassoko, R., et al. Establishment of duodenojejunal bypass surgery in mice: A model designed for diabetic research. Microsurgery. 28 (3), 197-202 (2008).
- Lan, Z., Zassoko, R., et al. Development of techniques for gastrojejunal bypass surgery in obese mice. Microsurgery. , (2010).
- Schlager, A., Khalaileh, A., et al. A mouse model for sleeve gastrectomy: Applications for diabetes research. Microsurgery. 31 (1), 66-71 (2011).
- Troy, S., Soty, M., et al. Intestinal Gluconeogenesis Is a Key Factor for Early Metabolic Changes after Gastric Bypass but Not after Gastric Lap-Band in Mice. Cell Metabolism. 8 (3), 201-211 (2008).
- Seyfried, F., Lannoo, M., et al. Roux-en-Y gastric bypass in mice–surgical technique and characterisation. Obesity surgery. 22 (7), 1117-1125 (2012).

