A High-throughput Compatible Assay to Evaluate Drug Efficacy against Macrophage Passaged Mycobacterium tuberculosis
Summary
New models and assays that would improve the early drug development process for next-generation anti-tuberculosis drugs are highly desirable. Here, we describe a quick, inexpensive, and BSL-2 compatible assay to evaluate drug efficacy against Mycobacterium tuberculosis that can be easily adapted for high-throughput screening.
Abstract
The early drug development process for anti-tuberculosis drugs is hindered by the inefficient translation of compounds with in vitro activity to effectiveness in the clinical setting. This is likely due to a lack of consideration for the physiologically relevant cellular penetration barriers that exist in the infected host. We recently established an alternative infection model that generates large macrophage aggregate structures containing densely packed M. tuberculosis (Mtb) at its core, which was suitable for drug susceptibility testing. This infection model is inexpensive, rapid, and most importantly BSL-2 compatible. Here, we describe the experimental procedures to generate Mtb/macrophage aggregate structures that would produce macrophage-passaged Mtb for drug susceptibility testing. In particular, we demonstrate how this infection system could be directly adapted to the 96-well plate format showing throughput capability for the screening of compound libraries against Mtb. Overall, this assay is a valuable addition to the currently available Mtb drug discovery toolbox due to its simplicity, cost effectiveness, and scalability.
Introduction
Tuberculosis (TB) remains a serious global health threat despite the availability of anti-TB chemotherapy regimens for over 40 years1. This is due in part to the requirement for long treatment periods of over 6 months using multiple drug combinations, which leads to patient non-compliance2. The emergence of drug-resistant TB in recent years has further compounded problems in a field where successful development of clinically approved drugs is virtually non-existent3. Indeed, despite exhaustive anti-TB drug development, only a single drug has been FDA approved for clinical use in the past 40 years4. Thus, new generations of anti-TB drugs are urgently needed to address this problem.
A key problem in TB drug discovery is the lack of successful transfer from compounds with in vitro activity to efficacy in the clinical setting5,6,7. Initially, target based approaches were used to screen for anti-Mtb drugs5, which failed to translate into whole bacterial cells. Even when Mtb cells are used, it is often performed using broth grown cultures, which do not accurately predict drug efficacy in vivo8,9. These problems have been recognized and drug screening assays against macrophages containing Mtb or latent Mtb have been successfully established8,10,11,12. However, even these more advanced assays do not give sufficient consideration to the penetration barriers that drugs encounter in the non-vascularized pulmonary lesions, and in the necrotic foci at the site of infection. Indeed, even for the first-line TB drug rifampicin, sub-optimal dosing has been questioned due to inadequate in vivo tissue and cerebral spinal fluid (CSF) penetration13,14,15 as well as decreased efficacy against intracellular Mtb8,9. As such, new models and assays that would take into account these parameters during the early lead development process would undoubtedly improve TB drug discovery efforts.
To address this need, we recently established an inexpensive, rapid, and BSL-2 compatible alternative infection model for Mtb drug efficacy testing16. This infection model produced densely packed Mtb within large macrophage aggregate structures, which recapitulated physiologically relevant cellular penetration barriers and generated macrophage-passaged Mtb with an altered physiological status resembling latent Mtb. Mtb derived from this infection model was combined with the resazurin microtiter assay (REMA) to evaluate drug efficacy, which produced results consistent with other intracellular infection models and correlated well with the reported ability of common TB drugs to achieve high CSF concentrations relative to serum concentrations16.
Here we describe in detail the generation of Mtb/macrophage aggregate structures to produce macrophage-passaged Mtb suitable for drug susceptibility testing using REMA. In particular, we show how this infection system could be adapted to a 96-well format for compatibility with throughput screening of candidate anti-TB drugs.
Protocol
NOTE: As M. tuberculosis mc26206 is an avirulent strain17,18, all work in this protocol can be performed in a Biosafety Level 2 facility (BSL-2).
1. Culture Conditions for Green Fluorescent Protein Expressing M. tuberculosis mc26206 (Mtb-GFP)
NOTE: The M. tuberculosis H37Rv derived auxotroph strain mc26206 (ΔpanCD, ΔleuCD) transformed with the gfp expressing plasmid pMN437 is used throughout this protocol16. It is possible to substitute the Mtb-GFP strain with non-gfp expressing wild-type strain to enable easier accessibility of the strain for researchers. However, gfp expression is desirable to enable visual confirmation of phagocytosis and formation of Mtb/macrophage aggregates. For long term storage, Mtb-GFP were frozen at -80 °C in complete 7H9 media (described in step 1.1) supplemented with 20% glycerol.
- Prepare complete Mtb-GFP media (7H9-C) by supplementing 7H9 medium with 10% Middlebrook OADC, 0.02% tyloxapol, 24 µg/mL D-pantothenic acid, 50 µg/mL L-leucine and 50 µg/mL Hygromycin B.
- Thaw one vial of Mtb-GFP and add to 10 mL of 7H9-C in a 30 mL square bottom PETG flask.
- Incubate at 37 °C on rotary shaker (50 rpm). Take optical density measurements (OD600) every few days until OD600 reaches 1.0 (approximately 5-8 days).
- Dilute and passage culture as needed to maintain an OD600 between 0.2-1.0.
- For infection, use an established culture of Mtb-GFP within the logarithmic growth phase (OD600 of 0.6-1). To avoid clumpy cultures, sonicate the Mtb culture flask in a water bath (130 W; 3 x 5 s pulses) once or twice a week
2. Culture Conditions for THP-1 Cells
- Prepare complete THP-1 cell media (RPMI-C) by supplementing RPMI 1640 medium with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, and 10 mM HEPES.
- Incubate cells in a T-75 flask at 37 °C in a humidified atmosphere of 5% CO2.
- Count cells using a hemocytometer or flow cytometry every other day and maintain THP-1 cells at a density between 0.1 to 0.6 million per mL.
3. Infection Protocol to Generate Mtb/Macrophage Aggregate Structures
- THP-1 cell preparation (per 96-well plate)
- Centrifuge 7 x 106 THP-1 cells at 250 x g for 5 min. Resuspend in 7 mL RPMI-C.
- Mtb-GFP preparation
- Centrifuge 2.8 x 108 Mtb-GFP at 3,200 x g for 5 min in a swinging-bucket centrifuge using a conversion of OD600 1.0 = 3 x 108 bacteria/mL.
- Wash once with RPMI-C and centrifuge as in step 3.2.1.
- Resuspend in 7 mL RPMI-C and vortex for 10 s.
- Infection
NOTE: See Figure 1 for template.- Prepare a 96-well plate by adding 200 µL of sterile water in rows A and H and columns 1 and 12 for a water rim to prevent evaporation of culture medium.
- Add 200 µL RPMI-C to column 2 (B2 to G2) for the background control (Blank).
- To infect, add Mtb-GFP suspension (step 3.2) to THP-1 cell suspension (step 3.1) and mix well by pipetting. The final THP-1 cell density is 5 x 105 per mL and the corresponding multiplicity of infection is 40.
- Pour the THP-1/Mtb-GFP suspension into a 25 mL reservoir.
- Add 200 µL of THP-1/Mtb-GFP suspension to all remaining 96-wells (B3 through G11) using a multi-channel pipette.
NOTE: If working with multiple 96-well plates, regularly resuspend the remaining THP-1/Mtb suspension in the reservoir to ensure an even mixture is added to each well. - Incubate at 37 °C with 5% CO2 for 7-10 days.
- Change media every 2 days by slowly removing 100 µL spent media from the top of each well and gently adding 100 µL pre-warmed RPMI-C using a multi-channel pipet. Do not resuspend wells and disturb the Mtb/macrophage aggregates on the bottom of the wells.
- Visually examine the wells by fluorescence microscopy (4-10X objective) daily, taking note of the size of Mtb/macrophage aggregates. By day 7-10, Mtb/macrophage aggregates should be sufficiently large (refer to Figure 2 for reference) to proceed for drug efficacy testing (Section 4).
- If desired, capture images of the 96-wells using an automated cell imaging system fitted with bright field and GFP filter sets to document Mtb/macrophage aggregate formation.
NOTE: If users do not have access to an automated imaging system, images of representative wells can be taken using any appropriate microscope with GFP and bright field capabilities.
4. Growth Inhibition Assay to Assess Drug Efficacy Against Mtb Derived from Mtb/Macrophage Aggregates
- Drug preparation (triplicate conditions for two drugs)
NOTE: See Figure 1A for template.- In a separate 96-well plate, add 125 µL of 7H9-C media to B2 through to G10.
- Prepare two drugs at double the highest desired final concentration in 1 mL of 7H9-C to account for the dilution in step 4.2.4.
- Add 250 µL of each drug to wells B11-C11-D11 and E11-F11-G11, respectively for triplicate treatments.
- Using a multi-channel pipet, serially dilute the test drugs two-fold by moving 125 µL from B11-G11 into B10-G10. Mix by pipetting 5 times at each step.
- Continue to move 125 µL from column to column (right to left) across the plate, and stop after column 4.
- After mixing column 4, discard 125 µL into a waste container. Columns 2 and 3 should not contain any drugs to allow for use as a background (Blank) and positive growth controls.
NOTE: Alternatively, follow template in Figure 1B for testing drug libraries. Each 96-well plate can accommodate 58 drugs at a single concentration. Prepare this drug plate using double the desired final concentration since it will be diluted in half in step 4.2.4.
- Drug treatment:
- Retrieve the 96-well plate containing the Mtb-infected macrophages (Mtb/macrophage aggregates).
- Carefully decant all the relevant wells (B2 through G11) with a multi-channel pipet. Perform this in a two-step manner: first remove 150 µL without tilting the plate, and then remove remaining media (~50 µL) by tilting the plate and inserting the pipette tip to the bottom edge of the well. As Mtb/macrophage aggregates are adherent to the bottom of the well, no material should be lost.
- Gently add 100 µL of 7H9-C media to all relevant wells (B2 through G11) of the plate containing the Mtb/macrophage aggregates.
- Using a multi-channel pipet, transfer 100 µL from the drug containing 96-well plate (step 4.1) to the corresponding wells of the infection plate.
- Place in sealed bag and incubate for 3 days at 37 °C.
5. Quantification of Drug Efficacy Using the Resazurin Microtiter Assay
- Prepare resazurin stock solution at a final concentration of 0.8 mg/mL in H2O. Filter through 0.22 µm pore size PVDF membrane for sterilization.
- Prepare resazurin working solution by mixing resazurin stock solution, H2O and Tween-80 (20% solution in H2O) in a 2:1:1 ratio. Final concentrations are 0.4 mg/mL resazurin and 5% Tween-80.
- Using a plate reader, setup a program to read fluorescence at 530 nm excitation and 590 nm emission every 30 min for 24 h at 37 °C. Pre-warm plate reader to 37 °C.
- Add 20 µL of resazurin working solution to all relevant wells (B2 through G11) of the drug treated plate (step 4.11) using a multi-channel pipet.
- Place plate on the plate reader and start the program set up in step 5.3.
NOTE: To facilitate the processing of high volumes of assay plates, it is sufficient to develop the assay in a 37 °C incubator and perform single fluorescence reads every 24 h. This is also applicable to users who do not have access to a plate reader with kinetic and incubation capabilities.
Representative Results
To confirm the robustness of adapting this infection model to 96-well plate format, we here examined the drug susceptibility of Mtb derived from our 96-well adapted infection model to rifampicin (RIF) and moxifloxacin (MOXI) according to the template given in Figure 1A. We demonstrate that the generation of Mtb/macrophage aggregate structures key to this assay can be reliably produced in a 96-well plate format (Figure 2), thereby enabling throughput compatibility (Figure 1B). Macrophage passaged Mtb produced in this manner can be directly used for drug efficacy testing using the well characterized resazurin microtiter assay (Figure 3).
As the resazurin assay relies on the oxidative species produced by metabolically active Mtb to convert the blue resazurin to the fluorescent pink resorufin, the change in color and fluorescence can be used to as a surrogate marker to determine the amount of bacterial growth. In Figure 3A, we show that there is enough sensitivity within the resazurin assay to reliably detect viable Mtb bacteria capable of replicating in the absence of drugs after only 3 days of incubation. While visual confirmation of endpoint color change is only an approximate evaluation of growth, we can accurately quantify this by kinetically measuring the conversion of resazurin to its fluorescent metabolite resorufin using a plate reader (Figure 3B). Using these data, normalizing to the positive growth control (absence of drugs) allows for the computation of susceptibility killing curves to visualize drug efficacy against macrophage-passaged Mtb (Figure 4).
Here, the representative results in Figures 3 and 4 show that the minimal inhibitory concentration (MIC), defined as the lowest concentration of the antibiotic at which 90% growth inhibition is observed, is greater than 2 µg/mL for both rifampicin and moxifloxacin against Mtb derived from our infection model. This demonstrates that our infection model predicts efficacy of anti-Mtb drugs that are more in line with MIC values determined against intracellular Mtb8, and thus reflects the diffusion barrier properties that are neglected in most Mtb drug susceptibility assays using broth grown bacterial cells.
Importantly, the data obtained using the described assay in 96-well format showed highly comparable results to those we had previously determined for rifampicin and moxifloxacin against macrophage-passaged Mtb16.
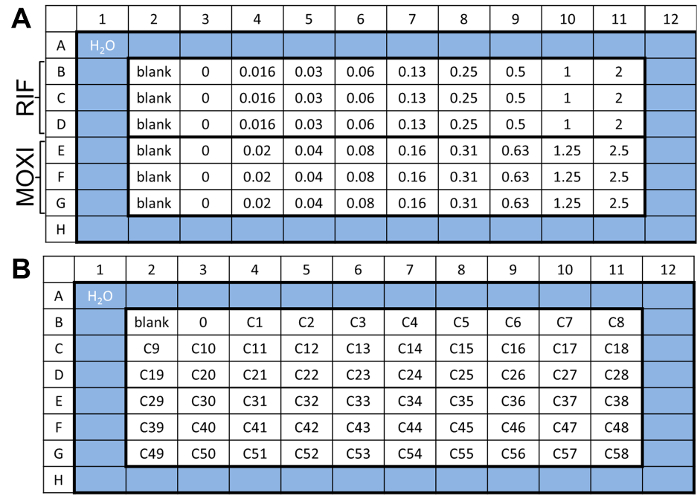
Figure 1: Drug plate layout. (A) Example template of the drug challenge plate used in step 4. This allows for the parallel testing of two drugs in triplicate wells at 8 defined concentrations. As a representative experiment, we used rifampicin (RIF) and moxifloxacin (MOXI) starting at 2 µM and 2.5 µM, respectively. (B) An alternative template for throughput screening is also provided to show the possibility of testing of 58 compounds at a single concentration. Please click here to view a larger version of this figure.
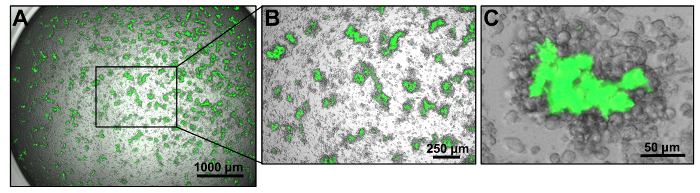
Figure 2: Generation of Mtb/macrophage aggregate structures in 96-well plate format. (A) Representative bright field and GFP merged images of Mtb-macrophage aggregates on Day 9 post infection. Images were captured with a 4X objective using an automated cell imaging program that documents the entire well by stitching a montage of 3 by 3 individually captured images. (B) An individual non-stitched image from the visual field shown in (A). (C) A representative merged bright field and GFP image of an Mtb/macrophage aggregate structure captured with a 10X objective. Please click here to view a larger version of this figure.
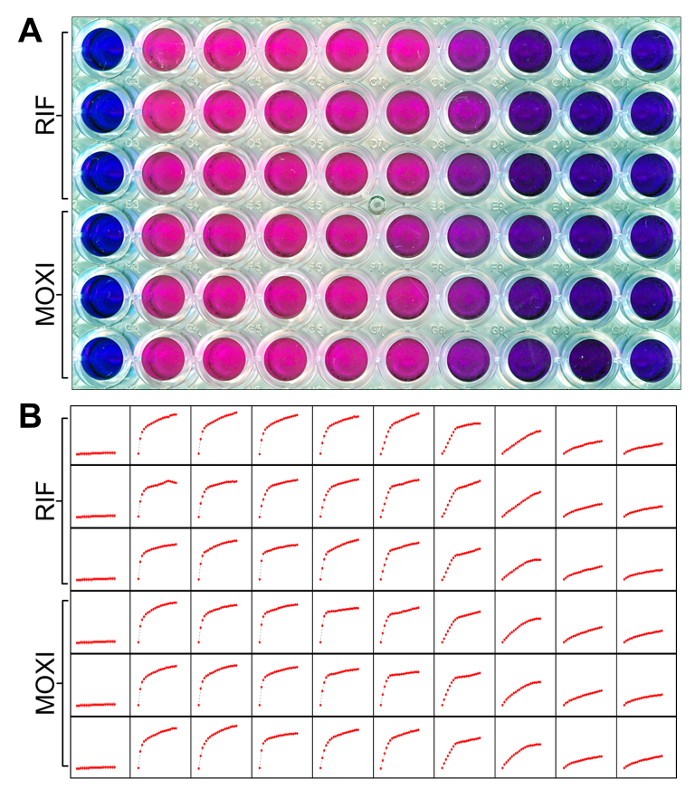
Figure 3: Using the resazurin microtiter assay to measure Mtb viability. (A) The presence of viable Mtb cells can be simply determined by the conversion of the blue resazurin dye to its pink, reduced form. Representative results are shown here using rifampicin (RIF) and moxifloxacin (MOXI) according to the template in Figure 1A. (B) To quantitatively measure the conversion of resazurin, fluorescence of individual wells (corresponding to Figure 3A) was monitored kinetically for 24 h as described in step 5. Representative mini-graphs show relative fluorescence units (y-axis) versus time (x-axis). Please click here to view a larger version of this figure.
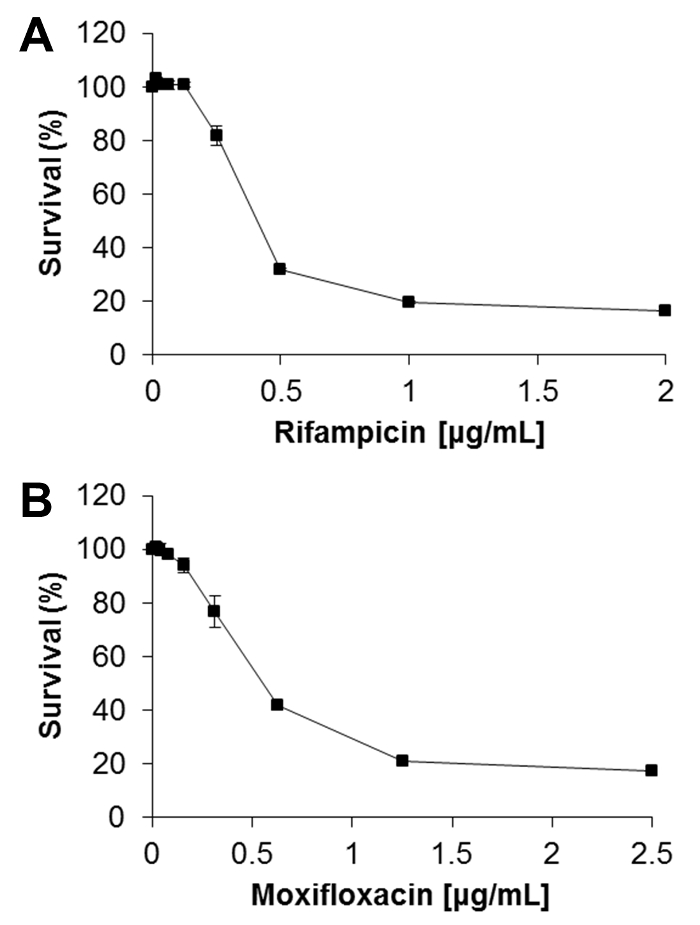
Figure 4: Determining drug susceptibility killing curves from REMA data. Relative fluorescence units at the time of maximal resazurin signal in (A) rifampicin and (B) moxifloxacin treated wells (from Figure 3B) were normalized to the no drug control (maximal Mtb growth) as 100% survival. Background control (Blank) signals were subtracted from every sample well. The percent survival was plotted for each individual concentration of drug treatment to generate a killing curve. Data in this figure represent the means ± S.D. of three independent experiments. Please click here to view a larger version of this figure.
Discussion
Here, we have described in detail an alternative Mtb infection model suitable for drug efficacy testing. This model takes into account two key factors that should be given more consideration during the early TB drug development process: the presence of physiologically relevant barriers to drug penetration and metabolic changes of Mtb during infection. While we have previously shown the benefits of our infection model and proposed the possibility of scaling up the infection for throughput compatibility16, here we demonstrate that this is indeed achievable by adapting the system directly into 96-well plate format. The ability to generate Mtb/macrophage aggregates directly in 96-well plates is advantageous as it removes the requirement to pipette and distribute the aggregates from a big culture flask into individual wells, which is inherently difficult as the sizes of the aggregates can reach sizes >500 µm. The large size of the infected macrophage aggregates would prevent the use of P200 multi-channel pipettes that are necessary for throughput assays as it would either clog the tips or extensive pipetting would break apart the aggregates. The modification of this infection system to 96-well plates reduces the amount of manipulation steps needed and generates uniform wells with similar amounts of Mtb/macrophage aggregates. As such, this assay system is particularly valuable not only in the early development process to predict the efficacy of lead candidate compounds in vivo, but in the throughput screening of drug libraries.
A key advantage of the described infection model is the utilization of a BSL-2 compatible Mtb strain. The ability to work under BSL-2 conditions is highly cost-effective, accelerates manipulation of the protocol, and most importantly enables laboratories that do not have access to a BSL-3 facility to perform these advanced drug screening assays. The utilized auxotrophic Mtb mc26206 strain is a derivative of H37Rv17,18 that retains all but 4 genes, panCD and leuCD, which do not affect drug resistance or tolerance16. Compared to other commonly used Mtb surrogates such as M. bovis BCG and M. smegmatis, Mtb mc26206 is most closely related to virulent Mtb H37Rv, the reference strain for pathogenesis studies. Importantly, it maintains the RD-1 locus, which is absent from BCG and plays a major role in Mtb virulence and granuloma formation19. Comparatively, Mtb mc26206 is also more relevant for TB-drug development assays than M. smegmatis, which is a fast-growing species that is genomically different from Mtb and has different antibiotic resistance profiles.
The assay described here is also valuable due to its high level of flexibility. The 96-well plates containing the Mtb/macrophage aggregates can be used not only for titration of drug treatments in triplicates as we have shown (Figure 3), but also for the screening of entire drug libraries (Figure 1B). At a single concentration, each 96-well plate can screen 58 compounds. This number would drastically increase to >300 compounds if the infection system is adapted to a 384-well plate, which is completely feasible based on that the fact that we obtain strong resazurin signals after only allowing the bacteria to replicate for 3 days. In fact, it would be relatively simple to adapt this system to 384-well plate format, as other than down-scaling the volumes proportionally to suit the smaller well format, the majority of the protocol would remain unchanged. To enable adequate signal for the resazurin assay, it may be required to extend the incubation in step 4.2.5 to 7 days to allow for enough bacterial replication.
While coupling this infection model to the resazurin assay as a read-out for Mtb viability has many benefits, namely its cost effectiveness and quick assay protocol while obtaining comparable results to other gold standard systems such as BACTEC 46020, it nevertheless does have some limitations. The resazurin assay cannot discriminate between bacteriostatic and bactericidal drugs since it only measures metabolic activity. This is an important constraint to keep in mind, as metabolic activity is inherently low in latent Mtb. However, the key component of this assay is the infection model, which can be compatible with read-outs for Mtb drug susceptibility other than the resazurin assay. For example, after drug treatment, wells could be directly plated out for colony unit formation (CFU) counting, the gold standard to test bactericidal activity of compounds (though this is not compatible with throughput screening). Alternatively, this system could be coupled to luciferase expressing Mtb, which has been shown to correlate closely to CFU counting21. To utilize a luciferase system to measure drug susceptibility, only two changes to the protocol would be required: i) replacement of Mtb-GFP with Mtb-luciferase and ii) the use of Bright-Glo luciferin reagent in place of resazurin in step 5. The use of luciferase expressing Mtb would shorten the development of the assay to 30 min while considerably increasing the assay sensitivity to levels that would be easily compatible with 384-well plate format.
In summary, we have successfully transitioned this infection model into a 96-well plate format that has relatively few manipulation steps, high reproducibility, and the ability to produce endless amounts of starting material, all key factors in throughput compatibility. This assay is also easily adaptable to many different formats and read-outs, making it a valuable asset to the early Mtb drug discovery process.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Frank Wolschendorf for access to the Cytation 3 automated imaging plate reader. This work was funded in part by NIH grant R01-AI104499 to OK. Parts of the work were performed in the UAB CFAR facilities and by the UAB CFAR Flow Cytometry Core/Joint UAB Flow Cytometry Core, which are funded by NIH/NIAID P30 AI027767 and by NIH 5P30 AR048311.
Materials
| 7H9 | BD Difco | 271310 | Follow manufacturer's recommendations |
| Middlebrook OADC | BD Biosciences | 212351 | |
| Tyloxapol | Sigma | T8761 | Prepare 20% stock solution in H2O; filter sterilize |
| D-Pantothenic acid hemicalcium salt | Sigma | P5710 | Prepare 24 mg/ml stock solution in H2O; filter sterilize |
| L-leucine | MP Biomedicals | 194694 | Prepare 50 mg/ml stock solution in H2O; filter sterilize |
| Hygromycin B | EMD Millipore | 400051 | Prepare 200 mg/ml stock solution in H2O |
| Nalgene Square PETG media bottle | Thermo Fisher | 2019-0030 | |
| RPMI 1640 media | Hyclone | SH30027.01 | |
| Fetal Bovine Serum | Atlanta Biologicals | S12450H | |
| L-glutamine | Corning | MT25005CI | |
| HEPES | Hyclone | SH30237.01 | |
| Cytation 3 plate reader | Biotek | Interchangable with any fluorescent plate reader and microscope | |
| Gen5 Software | Biotek | Recording and analysis of rezasurin coversion | |
| Rifampicin | Fisher Scientific | BP2679250 | Prepare 10 mg/ml stock solution in H2O |
| Moxifloxacin Hydrochloride | Acros Organics | 457960010 | Prepare 10 mg/ml stock solution in H2O |
| Resazurin Sodium Salt | Sigma | R7017 | Prepare 800 μg/mL stock solution in H2O; filter sterilize |
| Tween-80 | Fisher Scientific | T164500 | Prepare 20% stock solution in H2O; filter sterilize |
References
- Barry, C. E. Lessons from seven decades of antituberculosis drug discovery. Curr Top Med Chem. 11 (10), 1216-1225 (2011).
- Bass, J. B., et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 149 (5), 1359-1374 (1994).
- Koul, A., Arnoult, E., Lounis, N., Guillemont, J., Andries, K. The challenge of new drug discovery for tuberculosis. Nature. 469 (7331), 483-490 (2011).
- Palomino, J. C., Martin, A. TMC207 becomes bedaquiline, a new anti-TB drug. Future Microbiol. 8 (9), 1071-1080 (2013).
- Zuniga, E. S., Early, J., Parish, T. The future for early-stage tuberculosis drug discovery. Future Microbiol. 10 (2), 217-229 (2015).
- Evangelopoulos, D., Fonseca, d. a., D, J., Waddell, S. J. Understanding anti-tuberculosis drug efficacy: rethinking bacterial populations and how we model them. Int J Infect Dis. 32, 76-80 (2015).
- Ekins, S., et al. Looking back to the future: predicting in vivo efficacy of small molecules versus Mycobacterium tuberculosis. J Chem Inf Model. 54 (4), 1070-1082 (2014).
- Christophe, T., et al. High content screening identifies decaprenyl-phosphoribose 2′ epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog. 5 (10), e1000645 (2009).
- Hartkoorn, R. C., et al. Differential drug susceptibility of intracellular and extracellular tuberculosis, and the impact of P-glycoprotein. Tuberculosis (Edinb). 87 (3), 248-255 (2007).
- Queval, C. J., et al. A microscopic phenotypic assay for the quantification of intracellular mycobacteria adapted for high-throughput/high-content screening. J Vis Exp. (83), e51114 (2014).
- Sorrentino, F., et al. Development of an intracellular screen for new compounds able to inhibit Mycobacterium tuberculosis growth in human macrophages. Antimicrob Agents Chemother. 60 (1), (2015).
- Sarathy, J., Dartois, V., Dick, T., Gengenbacher, M. Reduced drug uptake in phenotypically resistant nutrient-starved nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother. 57 (4), 1648-1653 (2013).
- Dutta, N. K., Karakousis, P. C. Can the duration of tuberculosis treatment be shortened with higher dosages of rifampicin?. Front Microbiol. 6, 1117 (2015).
- van Ingen, J., et al. Why Do We Use 600 mg of Rifampicin in Tuberculosis Treatment?. Clin Infect Dis. 52 (9), e194-e199 (2011).
- Donald, P. R. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb). 90 (5), 279-292 (2010).
- Schaaf, K., et al. A Macrophage Infection Model to Predict Drug Efficacy Against Mycobacterium Tuberculosis. Assay Drug Dev Technol. 14 (6), 345-354 (2016).
- Sampson, S. L., et al. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun. 72 (5), 3031-3037 (2004).
- Jain, P., et al. Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. MBio. 5 (3), e01245-e01214 (2014).
- Davis, J. M., Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 136 (1), 37-49 (2009).
- Collins, L., Franzblau, S. G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 41 (5), 1004-1009 (1997).
- Snewin, V. A., et al. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect Immun. 67 (9), 4586-4593 (1999).

