Defining the Program of Maternal mRNA Translation during In vitro Maturation using a Single Oocyte Reporter Assay
Summary
This protocol describes a reporter assay to study the regulation of mRNA translation in single oocytes during in vitro maturation.
Abstract
Events associated with oocyte nuclear maturation have been well described. However, much less is known about the molecular pathways and processes that take place in the cytoplasm in preparation for fertilization and acquisition of totipotency. During oocyte maturation, changes in gene expression depend exclusively on the translation and degradation of maternal messenger RNAs (mRNAs) rather than on transcription. Execution of the translational program, therefore, plays a key role in establishing oocyte developmental competence to sustain embryo development. This paper is part of a focus on defining the program of maternal mRNA translation that takes place during meiotic maturation and at the oocyte-to-zygote transition. In this method paper, a strategy is presented to study the regulation of translation of target mRNAs during in vitro oocyte maturation. Here, a Ypet reporter is fused to the 3' untranslated region (UTR) of the gene of interest and then micro-injected into oocytes together with polyadenylated mRNA encoding for mCherry to control for injected volume. By using time-lapse microscopy to measure reporter accumulation, translation rates are calculated at different transitions during oocyte meiotic maturation. Here, the protocols for oocyte isolation and injection, time-lapse recording, and data analysis have been described, using the Ypet/interleukin-7 (IL-7)-3' UTR reporter as an example.
Introduction
A fully-grown mammalian oocyte undergoes rapid changes in preparation for fertilization and acquisition of totipotency. These changes are essential to sustain embryonic development after fertilization. Although the events associated with nuclear maturation are relatively well described, much less is known about the molecular processes and pathways in the oocyte cytoplasm. During the final stages of oocyte maturation, oocytes are transcriptionally silent, and gene expression is entirely dependent on mRNA translation and degradation1,2. The synthesis of proteins critical for developmental competence, therefore, relies on a program of timed translation of long-lived mRNAs that have been synthesized earlier during oocyte growth1,3. As part of a focus on defining this program of maternal mRNA translation executed during meiotic maturation and at the oocyte-to-zygote transition, this paper presents a strategy to study the activation and repression of the translation of target maternal mRNAs in single oocytes during in vitro meiotic maturation.
In this method, the YPet open reading frame is cloned upstream of the 3' UTR of the transcript of interest. Next, mRNAs encoding this reporter are micro-injected into oocytes together with polyadenylated mRNAs encoding mCherry to control for injected volume. Reporter accumulation is measured during in vitro oocyte meiotic maturation using time-lapse microscopy. The accumulation of yellow fluorescent protein (YFP) and mCherry is recorded in individual oocytes, and YFP signals are corrected by the plateaued level of the co-injected mCherry. After data acquisition, translation rates are calculated for different time intervals during in vitro oocyte meiotic maturation by calculating the slope of the curve obtained by curve-fitting.
This approach provides a tool to experimentally confirm changes in translation of selected endogenous mRNAs. In addition, this method facilitates the characterization of regulatory elements that control translation during oocyte meiotic maturation by manipulating cis-regulatory elements of the 3' UTR of target mRNAs4,5,6. Manipulation of the poly(A) tail length also allows insight into adenylase/deadenylase activity in oocytes5. Mutagenesis of cis-acting elements or RNA immunoprecipitation can be used to study interactions with cognate RNA binding proteins6,7. Additionally, this method can be used to identify essential components of the translation program that are critical for oocyte developmental competence by measuring target 3' UTR translation in models associated with decreased oocyte quality 8,9,10. This method paper presents a representative experiment where denuded oocytes of 21-day-old C57/BL6 mice have been micro-injected with a Ypet reporter fused to the 3' UTR of IL-7. The setup and protocol for oocyte injection, time-lapse recording, and data analysis have been described.
Protocol
The experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of California at San Francisco (protocol AN182026).
1. Preparation of media
- Add all components, as described in Table 1, to make the basic oocyte collection medium and oocyte maturation medium. For the basic collection medium, set the pH to 7.4. For both collection and maturation medium, add 3 mg/mL of bovine serum albumin (BSA) and 1 µM cilostamide on the day of use.
2. Preparation of mRNA encoding for Ypet-3' UTR and mCherry
- Obtain the 3′ UTR sequences of the mRNAs of interest.
NOTE: For this study, sequences were previously obtained from mouse oocyte cDNA. - Design primers to amplify the target 3' UTRs from oocyte cDNA and portions of the pcDNA 3.1 vector containing a Ypet coding sequence, V5-epitope tag, and a T7 promoter.
- Amplify using a high-fidelity DNA polymerase kit. Run the polymerase chain reaction (PCR) products on a gel to check if the fragments have the correct size, cut out the bands, and extract the DNA from the gel using a gel extraction kit according to the manufacturer's instructions.
- Fuse the PCR fragments to a vector using a PCR cloning kit.
NOTE: PCR fragments were incubated on ice for 4 h to facilitate a more efficient recombination process. This is contrary to the manufacturer's instructions, which recommend an incubation time of only 45 min. - Transfect PCR fragments into competent 5-α Escherichia coli bacteria.
- Add the mix and plasmid to the bacteria, and incubate on ice for 30 min.
- Heat shock the mix and plasmid by placing the mixture in a water bath at 42 °C for 45 s, and immediately cool on ice for 3 min.
- Add 500 µL of Super Optimal broth with Catabolite repression (SOC) medium and incubate for 1 h at 37 °C.
- Spin down at 7000 × g for 2 min, and remove most of the supernatant. Resuspend and plate on a Luria Broth (LB) agar plate with the appropriate selection antibiotic.
NOTE: Carbenicillin was used in this study. - Incubate overnight at 37 °C. Isolate the colonies by lightly pressing a pipette tip on one of the colonies and placing it in 3 mL of LB medium with 100 µg/mL of carbenicillin. Incubate for 12-24 h at 37 °C.
- Extract the DNA of the plasmids using the plasmid DNA isolation kit according to the manufacturer's instructions and confirm the sequences via DNA sequencing.
- For Ypet/3' UTR:
- Produce a linear PCR template for in vitro transcription. Use a forward primer upstream of the Ypet sequence and a reverse primer with 20 additional thymine residues. See Table 2 for the sequence of Ypet/IL-7 3' UTR and the sequences of the forward and reverse primers.
NOTE: These additional thymine residues will add oligo(A) to the linear mRNA after in vitro transcription. - In vitro transcribe the PCR product using a T7 transcription kit according to the manufacturer's instructions.
- Purify the resulting complementary RNA (cRNA) using a transcription clean-up kit according to the manufacturer's instructions.
- Elute the purified cRNA in RNAse-free water, measure concentration and evaluate integrity by agarose electrophoresis. Store at −80 °C.
- Produce a linear PCR template for in vitro transcription. Use a forward primer upstream of the Ypet sequence and a reverse primer with 20 additional thymine residues. See Table 2 for the sequence of Ypet/IL-7 3' UTR and the sequences of the forward and reverse primers.
- For mCherry:
- Produce a linear PCR template for in vitro transcription by using a high-fidelity restriction enzyme; use the restriction enzyme mfEI-HF if replicating this example. Digest in a digestion buffer overnight at 37 °C.
- Run a gel to purify the sample, and extract the linear DNA using a gel extraction kit according to the manufacturer's instructions.
- In vitro transcribe the PCR product using a T7 transcription kit according to the manufacturer's instructions.
- Polyadenylate the cRNA (150-200 nucleotides) using a poly(A) tailing kit according to the manufacturer's instructions.
- Purify the resulting cRNA using a transcription clean-up kit according to the manufacturer's instructions.
- Elute the purified cRNA in RNAse-free water, measure mRNA concentrations, and evaluate the message integrity by gel electrophoresis. Store at −80 °C.
3. Experimental procedure
NOTE: A schematic overview of oocyte micro-injection and subsequent time-lapse microscopy is given in Figure 1.
- Day 1
- Intraperitoneally inject 21-day-old mice with 5 IU of pregnant mare's serum gonadotropin to promote follicle growth to the antral stage11.
- Day 3
- Oocyte collection
- Sacrifice mice 44-48 h after priming to collect the ovaries, and place them in a plastic Petri dish with basic oocyte collection medium.
- Carefully open the antral follicles by making a small cut in the follicle wall with a 26 G needle. Isolate intact cumulus-enclosed oocytes (COCs) with several layers of cumulus cells using a mouth-operated glass pipette.
- Use a smaller pipette (slightly larger than the diameter of the oocyte), and mechanically denude the COCs by repeated pipetting.
NOTE: Alternatively, perform micro-injection on intact COCs. - Using a larger pipette, aspirate the denuded oocytes and place them in a Petri dish with maturation medium supplemented with 1 µM of the phosphodiesterase inhibitor, cilostamide, to prevent resumption of meiotic maturation12. Place the dish in the incubator for 2 h to let the oocytes recover from the stress induced by isolation of the oocytes from the follicles.
- Oocyte micro-injection
- Prepare the injection needles by placing a 10 cm long borosilicate glass capillary tube in a mechanical puller. For optimal injection, bend the needle tip in a 45° angle using a heated filament.
- Prepare polystyrene dishes with 20 µL droplets of basic oocyte collection medium, and cover the droplets with light mineral oil.
- Prepare a reporter mix by adding 12.5 µg/µL Ypet-3' UTR and 12.5 µg/µL mCherry. Prepare a larger volume and make aliquots for future experiments to ensure similar reporter concentrations. Store these aliquots at -80 °C. Upon thawing, first centrifuge the aliquot for 2 min at 20,000 x g, and transfer to a new microcentrifuge tube.
NOTE: This centrifugation will prevent the injection needle from getting clogged by potential aggregates in the reporter mix. - Load the injection needle by capillarity with approximately 0.5 µL of reporter mix.
- Place the holding pipette and loaded injection needle into the holders, and position in the droplet of oocyte collection medium. Aspirate some of the medium into the holding pipette.
- Open the injection needle by gently tapping it against the holding pipette.
- Place oocytes in a droplet of basic collection medium, and inject 5-10 pL of the reporter mix.
- Incubate the oocytes in the maturation medium with 1 µM cilostamide for 16 h to allow the mCherry signal to plateau.
- Prepare a Petri dish that will be used for time-lapse microscopy with at least two 20 µL droplets of maturation medium for each injected reporter: one droplet with 1 µM cilostamide for control prophase I-arrested oocytes and one droplet without cilostamide for maturing oocytes. Cover the droplets with light mineral oil and place in incubator.
- Time-lapse microscopy
- After pre-incubation, remove the injected oocytes from the incubator, and wash them four times in maturation medium without cilostamide. Keep some oocytes in maturation medium with 1 µM cilostamide as a prophase I-arrested oocyte control group.
- Transfer the injected oocytes to their respective droplets on the previously prepared time-lapse microscope dish. Cluster the oocytes by using a closed glass pipette (a closed pipette can be prepared by holding the tip in a flame for a few seconds).
NOTE: Clustering the oocytes helps to prevent their movement during the recording. - Place the dish under the microscope equipped with a light-emitting diode illumination system and a motorized stage equipped with an environmental chamber maintained at 37 °C and 5% CO2. To replicate this study, use the following parameters: filter set: dichroic mirror YFP/CFP/mCherry 69008BS; YFP channel (Excitation (Ex): S500/20 × 49057; Emission (EM): D535/30 m 47281), mCherry channel (Ex: 580/25 × 49829; Em: 632/60 m).
- Enter the appropriate settings for the time-lapse experiment (see Table of Materials for the software used in this study): Click on Apps | Multi Dimensional Acquisition. Select the first tab Main and select timelapse, multiple stage positions, and multiple wavelengths.
- Select the tab Saving to enter the location where the experiment should be saved.
- Select the tab Timelapse to enter the number of time points, duration, and time interval.
NOTE: The duration of the time-lapse experiment depends on the animal species studied, as timing of oocyte meiotic maturation differs among species. In this experiment with mouse oocytes, oocytes were recorded every 15 min for 16 h. - Select the tab Stage. Switch on brightfield and locate the position of the oocytes by opening a new window by selecting Acquire | Acquire | Show Live. Once the oocytes are located, switch back to the Multi Dimensional Acquisition window, and press + to set the location of the oocytes.
- Select the tab Wavelengths, and set 3 different wavelengths for brightfield (exposure 15 ms), YFP (exposure 150 ms for Ypet/IL7 3' UTR), and mCherry (exposure 75 ms for Ypet/IL7 3' UTR).
NOTE: Adjust the exposure for each Ypet/3' UTR reporter based on the level of reporter accumulation and the injected volume. Ensure that the YFP signal falls in the center of the range of detection at the start of the experiment to prevent underestimation or saturation in case of activation of translation. For mCherry, adjust the exposure for each batch of mCherry as the signal depends on the number of adenine nucleotides that were added during the polyadenylation procedure. Because of the variation in polyadenylation efficiency, use the same batch of mCherry in different experiments for a better comparison between experiments. - Start the time lapse experiment by clicking on Acquire. See Figure 2 and the Supplemental File for an example of a time-lapse recording in a single oocyte.
- Analysis of Ypet-3' UTR translation
- For this analysis (see Table of Materials for the software used in this study), perform two region measurements for each oocyte: the oocyte itself-click on ellipse region and surround the oocyte-and a small region close to the oocyte to be used for background subtraction-click on rectangular region.
NOTE: Ensure that the oocytes do not move out of the selected region during the time-lapse recording. Pay special attention to the placement of the region measurement around polar body extrusion, as this causes movement in the oocyte and may distort the recording. - Export the region measurement data to a spreadsheet by clicking first on Open Log and then on Log Data to export the data analysis software.
- For each individual oocyte and for all measured time-points, subtract the background region measurement from the oocyte region measurement. Do this for YFP and mCherry wavelengths separately.
- Record the timing of germinal vesicle breakdown (GVBD) and polar body extrusion (PBE) for later reference.
NOTE: Exclude maturing mouse oocytes when GVBD exceeds 2 h. - Plot YFP and mCherry expression over time for each oocyte to check for outliers.
NOTE: This should be a smooth curve, if it is not this may indicate that the oocyte moved during the recording. - For each individual oocyte, calculate average mCherry expression for the last ten time-points of the recording. For each time-point, divide the YFP expression by the averaged mCherry expression to correct for injected volume to obtain a YFP/mCherry ratio at every time-point for each individual oocyte.
- Calculate translation rates for a specific time-interval by fitting the slope of the curve by linear regression. Assess the differences in translation rates using statistical inference.
- For this analysis (see Table of Materials for the software used in this study), perform two region measurements for each oocyte: the oocyte itself-click on ellipse region and surround the oocyte-and a small region close to the oocyte to be used for background subtraction-click on rectangular region.
- Oocyte collection
Representative Results
Denuded prophase I-arrested oocytes of 21-day-old C57/BL6 mice were injected with a reporter mix containing mRNA encoding the Ypet reporter fused to the 3' UTR of IL-7 and mRNA encoding mCherry. YFP and mCherry expression was recorded in 39 oocytes, of which 30 were matured, and 9 were arrested in prophase I as a negative control. Three maturing oocytes were excluded for analysis because they either had a delayed GVBD (N=2) or moved in the dish during the recording (N=1). Figure 3 shows mCherry and YFP expression in prophase I and maturing oocytes. Figure 4 shows YFP expression of maturing oocytes corrected for plateaued mCherry expression (averaged mCherry expression in the last 10 time-points) to correct for the injected volume. Translation rates of the reporter were measured by curve-fitting (linear regression) the YFP/mCherry values in prophase I and in maturing oocytes during the first 0-2 h or 8-10 h after cilostamide release (Figure 5A). The accumulation of the reporter does not follow a linear pattern, as indicated by a significant difference in translation rates between 0-2 h and 8-10 h after cilostamide release (p<0.0001; Figure 5B). Therefore, these results indicate activation of IL-7 translation during oocyte meiotic maturation.
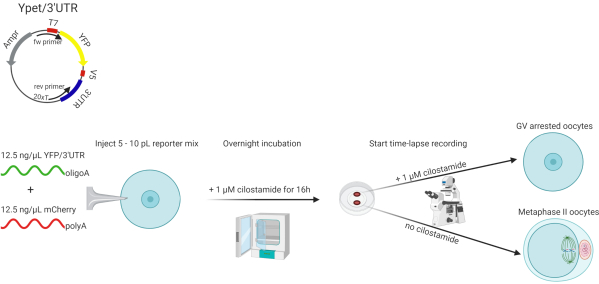
Figure 1: Schematic overview of the experimental procedure. Oligoadenylated Ypet/3' UTR and polyadenylated mRNA encoding mCherry are micro-injected into denuded oocytes of 21-day-old C57/BL6 mice. Oocytes are pre-incubated for 16 h in cilostamide containing maturation medium to allow the mCherry signal to reach a plateau. After pre-incubation, a time-lapse recording is started where oocytes are either kept in medium with cilostamide to create a prophase I-arrested control group or released in cilostamide-free medium to mature. Abbreviations: UTR = untranslated region; YFP = yellow fluorescent protein; fw primer = forward primer; rev primer = reverse primer; Ampr = ampicillin resistance; polyA = polyadenyl; oligoA = oligoadenyl; GV = germinal vesicle. Please click here to view a larger version of this figure.
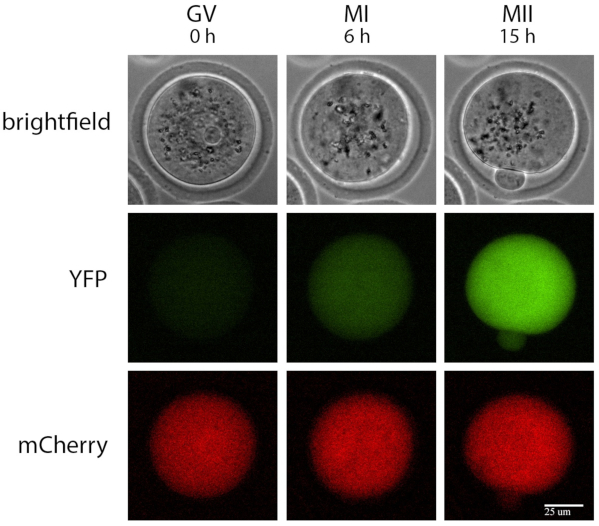
Figure 2: Example of a single oocyte time-lapse recording. Brightfield, YFP, and mCherry recordings of a single oocyte injected with mRNAs encoding Ypet/Interleukin-7 3' UTR and polyadenylated mCherry at prophase I, MI (6 h after cilostamide release), and MII (15 h after cilostamide release). Scale bar = 25 µm. Abbreviations: YFP = yellow fluorescent protein; GV = germinal vesicle; MI = metaphase I; MII = metaphase II. Please click here to view a larger version of this figure.
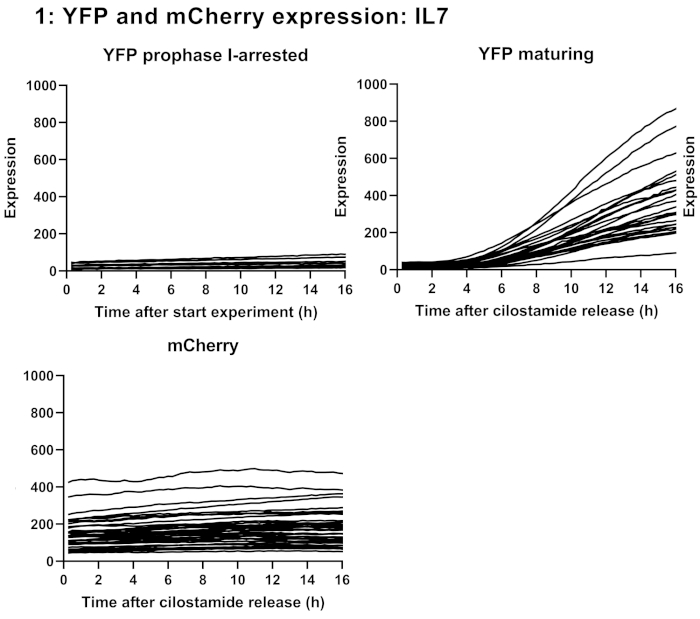
Figure 3: YFP and mCherry signals recorded by time-lapse microscopy. YFP and mCherry signals of oocytes injected with oligoadenylated Ypet/IL7 3' UTR and polyadenylated mRNA encoding mCherry. Oocytes were either kept in medium with cilostamide to generate a prophase I-arrested control group (N=9) or released in cilostamide-free medium to allow for maturation (N=30). Data are individual oocyte measurements. Abbreviations: IL-7 = interleukin-7; YFP = yellow fluorescent protein. Please click here to view a larger version of this figure.

Figure 4: YFP signal corrected for co-injected mCherry level. YFP signals of prophase I-arrested and maturing oocytes were corrected for injected volume by dividing the YFP signal by the average mCherry signal of the last 10 time-points. Individual YFP/mCherry ratios for (A) prophase I-arrested oocytes and (B) maturing oocytes and mean ± standard error of the mean YFP/mCherry ratios of (C) prophase I-arrested and (D) maturating oocytes. Abbreviations: YFP = yellow fluorescent protein; GVBD = germinal vesicle breakdown. Please click here to view a larger version of this figure.
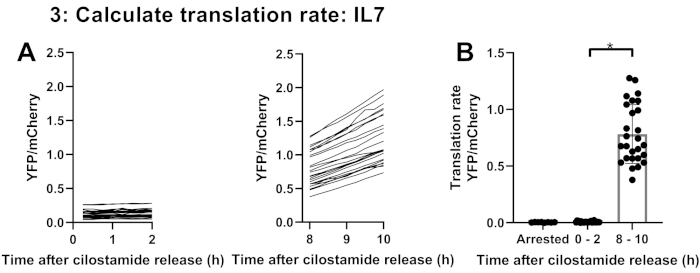
Figure 5: Calculated translation rates at 0-2 h and 8-10 h of maturation. (A) Yellow fluorescent protein (YFP) signals of single oocytes corrected for mCherry (YFP/mCherry) at 0-2 h or 8-10 h after cilostamide release and (B) translation rates (mean ± standard error of the mean) as calculated by curve-fitting (linear regression) the YFP/mCherry values at 0-2 h and 8-10 h after cilostamide release. Data were analyzed using the unpaired two-tailed t-test. *p < 0.0001. Please click here to view a larger version of this figure.

Figure 6: Example of repression of translation: Oosp2. Re-analyzed data of an experiment where oocytes were injected with Ypet-Oosp2 3′ UTR and polyadenylated mRNA encoding mCherry. YFP signals of prophase I-arrested (N=63) and maturing oocytes (N=72) were corrected for injected volume by dividing the YFP signal by the average mCherry signal of the last 10 time-points. YFP and mCherry expression data were obtained using a Xenon Arc lamp, unlike the IL-7 experiment where an LED light source was used. Data represent the mean ± standard error of the mean of individual oocyte measurements and were previously published in Luong et al.5. Abbreviations: YFP = yellow fluorescent protein; Oosp2 = oocyte secreted protein 2; UTR = untranslated region; IL-7 = interleukin-7; LED= light-emitting diode; GVBD = germinal vesicle breakdown. Please click here to view a larger version of this figure.
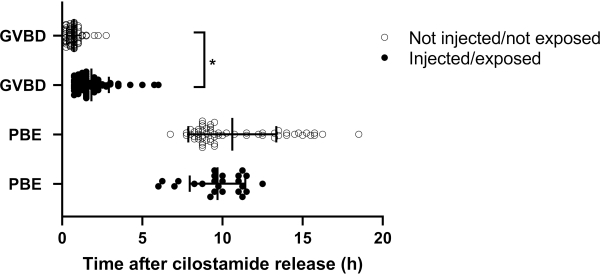
Figure 7: Effect of micro-injection and fluorescence exposure on timing of GVBD and PBE. Timing of GVBD and PBE of oocytes that were either micro-injected and exposed to fluorescence (injected), or not-injected and not exposed to fluorescence (not-injected). Data are individual oocyte measurements. Data were analyzed using the unpaired two-tailed t-test. *p<0.001. Abbreviations: GVBD = germinal vesicle breakdown; PBE= polar body extrusion. Please click here to view a larger version of this figure.
| Basic oocyte collection medium | ||
| Component | For 500 mL | |
| HEPES modified Minimum Essential Medium Eagle | 7.1 g | |
| Sodium bicarbonate | 252 mg | |
| Sodium pyruvate | 1.15 mL | |
| Penicillin/Streptomycin 100x | 5 mL | |
| Ultrapure distilled water (Invitrogen, 10977-015) | Up to 500 mL | |
| Maturation medium | ||
| Component | For 500 mL | |
| MEM alpha 1x | Up to 500 mL | |
| Sodium pyruvate | 1.15 mL | |
| Penicillin/Streptomycin 100x | 5 mL | |
Table 1: Preparation of media. List of components that need to be added to prepare basic oocyte collection medium and oocyte maturation medium.
| Sequence | |||||
| Ypet/Interleukin-7 3’ UTR | GAGAACCCACTGCTTACTGGCTTATCGAAATTAATACGACTCACTATAGGGAGACCCAAGCTG GCTAGTTAAGCTTGGTACCGAGCTCGGATCCACCGGTCGCCACCATGGTGAGCAAAGGCGA AGAGCTGTTCACCGGCGTGGTGCCCATCCTGGTGGAGCTGGACGGCGACGTGAACGGCC ACAAGTTCAGCGTGAGCGGCGAGGGCGAGGGCGACGCCACCTACGGCAAGCTGACCCTG AAGCTGCTGTGCACCACCGGCAAGCTGCCCGTGCCCTGGCCCACCCTGGTGACCACCCTG GGCTACGGCGTGCAGTGCTTCGCCCGGTACCCCGACCACATGAAGCAGCACGACTTCTTCA AGAGCGCCATGCCCGAGGGCTACGTGCAGGAGCGGACCATCTTCTTCAAGGACGACGGCAA CTACAAGACCCGGGCCGAGGTGAAGTTCGAGGGCGACACCCTGGTGAACCGGATCGAGCTGA AGGGCATCGACTTCAAGGAGGACGGCAACATCCTGGGCCACAAGCTGGAGTACAACTACAAC AGCCACAACGTGTACATCACCGCCGACAAGCAGAAGAACGGCATCAAGGCCAACTTCAAGAT CCGGCACAACATCGAGGACGGCGGCGTGCAGCTGGCCGACCACTACCAGCAGAACACCCC CATCGGCGACGGCCCCGTGCTGCTGCCCGACAACCACTACCTGAGCTACCAGAGCGCCCTG TTCAAGGACCCCAACGAGAAGCGGGACCACATGGTGCTGCTGGAGTTCCTGACCGCCGCC GGCATCACCGAGGGCATGAACGAGCTCTATAAGAGATCTTTCGAAGGTAAGCCTATCCCTAA CCCTCTCCTCGGTCTCGATTCTACGCGTACCGGTCATCATCACCATCACCATTGAACAGGAC ATGTAGTAACAACCTCCAAGAATCTACTGGTTCATATACTTGGAGAGGTTGAAACCCTTCCAG AAGTTCCTGGATGCCTCCTGCTCAAATAAGCCAAGCAGCTGAGAAATCTACAGTGAGGTATG AGATGATGGACACAGAAATGCAGCTGACTGCTGCCGTCAGCATATACATATAAAGATATATCAA CTATACAGATTTTTGTAATGCAATCATGTCAACTGCAATGCTTTTAAAACCGTTCCAAATGTTTC TAACACTACAAAGTCTACAAAAAGCAAGGCTATGAAGATTCAGAGTCACCACTGTTTTCTTAGC AAAATGATGGTATGGTTAAACATTCATTGGTGAACCACTGGGGGAGTGGAACTGTCCTGTTTTAG ACTGGAGATACTGGAGGGCTCACGGTGATGGATAATGCTCTTGAAAACAAGAGTCTATCTTAAAGC AGCAGCAAAAAGAAGCTTAAGGCACTTAAGGCATCAACAAATGTAGTTAAATATGAATGTATAACA CATAACTTCAGTAAAGAGCATAGCAGATATTTTTAAATAAAAGTATTTTTAAAGATAGAAATGCACTTAT TCCAAAGATACTGAACCTTAGTATTCAGTCGCTTTTGACACTTGTGTATAATAAAGCTTATATAACTGAA TTTTCAATTTGAAAAGTATATTTTTAAAAGAATAATATATGCTAGACTTTTAATTAATGTATATGTTTAATTT TGGCATTCTGTCTGTCTCTCTGTCTCTCTCTCTCTCTCTCTCTCTCTCTACCTATCTATCTATATATATA ATTTTCATATACTACCAATTGCGTACTTTGGATAGTGTCTCTTTTTAACCTAAATGACCTTTATTAACAC TGTCAGGTTCCCTTACTCTCGAGAGTGTTCATTGCTGCACTGTCATTTGATCCCAGTTTTATTGAACAC ATATCCTTTAACACACTCACGTCCAGATTTAGCAGGAGACTAGGACCCTATAACTTTGTTAAGAGAGAA AACACTAATTTCTTGTTTTATAGTAGGGTCTTATTCGTATCTAAGGCAGGCTAGGATTGCAGACATGAGC CAATATGCTTAATTAGAAACATTCTTTTTATGTTAAACTCATGTCTTTTACAAGATGCCTACATATATCCTAT GTATATGCCTGTTTAAATCCTTTTTTGTAAGGTCTGCTGTCTTCCTTCAGTTGTAATGGAAAGAAACACTA TGTTGTAGAGGCCAAATTTCTGAAAGTGATAAGGGTTTGCTTGTACTGAATTCTCATTCTCCTTGCTTT TTCCAGCCACGTGAGCATCTAGCTATCTATACGCTGGATGTATTTGACCGATGCCTGCTCCACTGGCAC ATTGCATGTGTGGTAGCCATGCCTTCTTGCTTCTCCTTTTCCCCAACCCCTATAATGCTCTACTCAGTGG TACAGATAGCTGGGATTATCACAATTTTGAGAGAAACACCAATTGTTTAAAGTTTGTTTCATAATCACCATTT GCCCAGAAAACAGTTCTCTCAACTTGTTTGCAACATGTAATAATTTAAGAAACTCAATTTTGTTAATGGACTT TCGATAACTTCCTTAGATATCCCACATCTCCTACGTGTCAGTCCTTTGTCCTGAGGAACTGGTAAAATGGGTA AGCCCTTAGCTAGCGAACTGAAGGCATTCGCATGTGTAAGATAATCTCTATACCTGCAAGGCTGTCTGGAT GGCTCCCTACCAATATTGAACAATATTCTGATTTTGGCAAAATAAAGGATAATATTTT |
||||
| Forward primer | GAGAACCCACTGCTTAC | ||||
| Reverse primer | TTTTTTTTTTTTTTTTTTTTAAAATATTATCCTTTATTTTG CCAAAATC |
||||
Table 2: Example of reporter and primer sequences Sequence of YFP/IL7 3'UTR reporter and sequences of forward and reverse primers that were used to produce a linear PCR template for in vitro transcription.
Supplemental File: Yellow fluorescent protein (YFP) and mCherry time-lapse recording. YFP and mCherry time-lapse recordings of a single oocyte injected with mRNAs encoding Ypet/Interleukin-7 3' UTR and polyadenylated mCherry. YFP channel (Ex: S500/20 × 49057; Em: D535/30 m 47281), mCherry channel (Ex: 580/25 × 49829; Em: 632/60 m). The oocytes were recorded every 15 min for 16 h (7 frames/second). Please click here to download this file.
Discussion
The presented method describes a strategy to study activation and repression of translation of target mRNA at different transitions during in vitro oocyte meiotic maturation. IL-7, a cytokine released by the oocyte that may be involved in oocyte-cumulus cell communication8,13, was chosen for the purpose of describing this method. IL-7 is known to be increasingly translated during oocyte maturation8 and allows for good visualization of translational activation using this method. If, however, translation occurs at a constant rate throughout the experiment, accumulation of a reporter will follow a linear pattern, and translation rates at the beginning and end of the experiment will be similar. This conclusion can be verified by goodness of fit (R) of a linear regression through the entire recording interval. A repression in reporter translation will present itself as the plateauing of the YFP signal.
An example of repression of 3' UTR translation, can be found in the study of Luong et al.5, where the authors report repression of Oocyte Secreted Protein 2 (Oosp2) during oocyte meiotic maturation. These data have been re-analyzed and are shown in Figure 6. The maturing oocytes show plateauing of the YFP signal, which indicates repression of translation, while the prophase I-arrested control group follows a linear pattern of reporter accumulation, which indicates similar translation rates at the beginning and end of the experiment. When studying repression of translation, it is especially important to include a prophase I-arrested control group to ensure that the plateauing of the YPF signal is not explained by a decrease in oocyte quality due to poor culture conditions, thus confirming the viability of the oocytes. Accumulation of the reporter can be validated by quantitative reverse-transcription PCR using primers for YFP or by western blotting by taking advantage of the V5-epitope tag included in the reporter in this protocol.
Plateauing of the YFP signal may not be solely due to actively regulated repression of translation, but may also be explained by the degradation of the reporter during oocyte meiotic progression. This may mirror destabilization of the endogenous mRNA, which is essential for the transition to embryonic genome expression14,15,16. This possibility can be verified by measuring target gene transcript levels in the prophase I stage vs. metaphase II stage to confirm stability of the mRNAs. When preparing oocyte cDNA, it is preferable to use random hexamer priming over oligo-dT priming. The latter method presents apparent differences in gene transcript levels that may reflect differences in poly(A) length of these transcripts rather than actual differences in transcript levels7.
There are several methods to study genome-wide endogenous mRNA translation in mouse oocytes. These methods include polysome profiling7,17,18 and RiboTag/RNA-Seq5,19,20. Ribosome profiling is another method to study genome-wide translation that has been used in yeast, which is another model organism that is used to study meiosis21,22. However, these genome-wide analyses to study mRNA translation in the mouse require the use of 150-200 oocytes per sample while the number of oocytes available for analysis is usually limited. The single-oocyte assay described herein to assess translation of 3' UTR of target mRNAs complements genome-wide analyses of translation, as it allows the characterization of elements of the 3' UTR that regulate the translation program during oocyte meiotic maturation4,5,6. In the past, luciferase-based assays were used to assess translation of the 3' UTR of target mRNAs10,20,23 as it is a very sensitive method that requires only a few oocytes per sample; however, the oocytes need to be lysed to detect the amount of luciferase in a sample.
Others have used a 3' UTR/green fluorescent protein (GFP) reporter to assess GFP accumulation in live mouse oocytes4. However, in these experiments, only two time-points were used: the beginning of incubation (prophase I) and the end of the incubation (metaphase II). This greatly diminishes the power of the measurements and increases the possibility of errors. Kinetic data provide accurate measurements based on rates (multiple points) and accurately define the time when translational activation or repression takes place. In addition, repression of translation cannot be assessed at these single time-points, which can lead to an inaccurate conclusion. Therefore, this method was adjusted by applying time-lapse microscopy to assess Ypet/3' UTR reporter accumulation throughout in vitro maturation of mouse oocytes5,6,9. By using this strategy, it is possible to study translation at different transitions during oocyte maturation.
There are several important aspects about this method to consider. First, the reporters used include an oligo(A) tail whose omission considerably reduces reporter accumulation. This may be due to both decreased translation as well reporter mRNA destabilization24,25. During the pre-incubation in prophase I, translation of an oligoadenylated probe adjusts to reflect the translation rate of the endogenous mRNA5. Second, it is important to realize that an increase in the number of recordings or duration of fluorescence exposure may potentially induce phototoxicity and thereby impair oocyte quality. Although the current experiment adopted 15-min intervals, the sampling rate can be decreased when a high fluorescence exposure must be used in case of low reporter expression.
To limit the amount of phototoxicity, a cold LED light source was used, which requires a shorter excitation26. To assess potential phototoxicity effects in the oocytes, the timing of GVBD and PBE was compared in oocytes that were either micro-injected and exposed to fluorescence or not injected and not exposed to fluorescence. The combination of micro-injection and fluorescence exposure was found to slightly delay GVBD (p<0.001), while the timing of PBE was similar (Figure 7).
This method has been used to study translational regulatory elements of the 3' UTR during in vitro oocyte meiotic maturation. Similarly, it may also be used to study functional 5' UTR elements essential for the regulation of translation or to study translation during in vitro oocyte fertilization. Although this method has been applied to human and mouse oocytes, it is also applicable to other animal species. Because this reporter assay is performed in single oocytes, it is especially suitable for use in mono-ovulatory species in which the number of oocytes available for analysis is limited. As opposed to using denuded oocytes, another application of this technique is the injection of the reporter into cumulus-enclosed oocytes, as the cumulus cells play a major role in the regulation of the translational program8,10,27. However, it should be taken into account that cumulus-enclosed oocytes are more likely to move away from the recording plane during the experiment as compared to denuded oocytes because of the movement of the cumulus cells during COC expansion. This may result in a larger proportion of oocytes that have to be excluded from analysis. In the future, cell-tracking software may be used that is able to track x, y, and z positions of the oocytes in the droplet, which may solve this issue.
In conclusion, the described single oocyte reporter assay represents a strategy to investigate the translational program executed at the oocyte-to-zygote transition. Increased knowledge of this translational program can provide important clues about the molecular regulation of oocyte developmental competence.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH R01 GM097165, GM116926 and Eunice Kennedy Shriver NICHD National Centers for Translational Research in Reproduction and Infertility P50 HD055764 to Marco Conti. Enrico M. Daldello was supported by a fellowship from the Lalor Foundation and Natasja G. J. Costermans was supported by a Rubicon fellowship from the Netherlands Organisation for Scientific Research (NWO).
Materials
| Preparation of media | |||
| Bovine Serum Albumin Powder Bioxtra | Sigma-Aldrich | SIAL-A3311 | |
| Cilostamide | EMD Millipore | 231085 | |
| MEM alpha | Gibco | 12561-056 | |
| Minimum Essential Medium Eagle | Sigma-Aldrich | M2645 | |
| Penicillin-Streptomycin 100x Solution, Sterile Filtered | Genesee Scientific Corporation (GenClone) | 25-512 | |
| Sodium Bicarbonate | JT-Baker | 3506-1 | |
| Sodium Pyruvate | Gibco | 11360-070 | |
| Ultrapure distilled water | Invitrogen | 10977-015 | |
| Preparation of mRNA encoding YFP/3' UTR and mCherry | |||
| Agarose | Apex Biomedical | 20-102QD | |
| Carbenicillin disodium salt | Sigma-Aldrich | C1389-1G | |
| Choo-Choo Cloning Kit | McLab | CCK-20 | |
| CutSmart Buffer (10x) | New England Biolabs | B7204 | |
| DNA loading dye (6x) | Thermo Scientific | R0611 | |
| dNTP Solution | New England Biolabs | N0447S | |
| DpnI | New England Biolabs | R0176 | |
| GeneRuler 1 kb DNA ladder | Thermo Fisher | SM1333 | |
| LB Agar Plates with 100 µg/mL Carbenicillin, Teknova | Teknova | L1010 | |
| LB Medium (Capsules) | MP Biomedicals | 3002-021 | |
| MEGAclear Transcription Clean-Up Kit | Life Technologies | AM1908 | |
| MfeI-HF restriction enzyme | New England Biolabs | R3589 | |
| mMESSAGE mMACHINE T7 Transcription Kit | Invitrogen | AM1344 | |
| Phusion High Fidelity DNA polymerase | New England Biolabs | M0530 | |
| Poly(A) Tailing kit | Invitrogen | AM1350 | |
| QIAprep Spin Miniprep Kit | Qiagen | 27106 | |
| QIAquick Gel Extraction Kit | Qiagen | 28704 | |
| S.O.C. medium | Thermo Fisher | 15544034 | |
| TAE buffer | Apex Biomedical | 20-193 | |
| Ultrapure Ethidium Bromide Solution | Life Technologies | 15585011 | |
| Oocyte collection | |||
| Aspirator tube assembly for calibrated micro-pipettes | Sigma-Aldrich | A5177-5EA | |
| Calibrated micro-pipettes | Drummond Scientific Company | 2-000-025 | |
| PMSG- 5000 | Mybiosource | MBS142665 | |
| PrecisionGlide Needle 26 G x 1/2 | BD | 305111 | |
| Syringe 1 ml | BD | 309659 | |
| Oocyte micro-injection | |||
| 35 mm Dish | No. 0 Coverslip | 20 mm Glass Diameter | Uncoated | MatTek | P35G-0-20-C | For time-lapse microscopy |
| Borosilicate glass with filament | Sutter Instrument | BF100-78-10 | |
| Oil for Embryo Culture | Irvine Scientific | 9305 | |
| Petri Dish | Falcon | 351006 | For micro-injection |
| Tissue Culture Dish | Falcon | 353001 | For oocyte incubation |
| VacuTip Holding Capillary | Eppendorf | 5195000036 | |
| Software | |||
| Biorender | BioRender | Preparation of Figure 1S | |
| MetaMorph, version 7.8.13.0 | Molecular Devices | For time-lapse microscopy, analysis of 3' UTR translation |
References
- Clarke, H. J. Post-transcriptional control of gene expression during mouse oogenesis. Results and Problems in Cell Differentiation. 55, 1-21 (2012).
- Gosden, R., Lee, B. Portrait of an oocyte: our obscure origin. Journal of Clinical Investigation. 120 (4), 973-983 (2010).
- Conti, M., Sousa Martins, J. P., Han, S. J., Franciosi, F., Menon, K. M. J., Goldstrohm, A. Translational control in the germ line. Posttranscriptional Mechanisms in Endocrine Regulation. , 129-156 (2015).
- Dai, X. -. X., et al. A combinational code for mRNA 3’UTR-mediated translational control in the mouse oocyte. Nucleic Acids Research. 47 (1), 328-340 (2019).
- Luong, X. G., Daldello, E. M., Rajkovic, G., Yang, C. R., Conti, M. Genome-wide analysis reveals a switch in the translational program upon oocyte meiotic resumption. Nucleic Acids Research. 48 (6), 3257-3276 (2020).
- Yang, C. R., et al. The RNA-binding protein DAZL functions as repressor and activator of mRNA translation during oocyte maturation. Nature Communications. 11, 1399 (2020).
- Chen, J., et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes & Development. 25 (7), 755-766 (2011).
- Cakmak, H., Franciosi, F., Musa Zamah, A., Cedars, M. I., Conti, M. Dynamic secretion during meiotic reentry integrates the function of the oocyte and cumulus cells. Proceedings of the National Academy of Sciences. 113 (9), 2424-2429 (2016).
- Daldello, E. M., Luong, X. G., Yang, C. R., Kuhn, J., Conti, M. Cyclin B2 is required for progression through meiosis in mouse oocytes. Development. 146 (8), (2019).
- Franciosi, F., Manandhar, S., Conti, M. FSH regulates mRNA translation in mouse oocytes and promotes developmental competence. Endocrinology. 157 (2), 872-882 (2016).
- Peters, H., Byskov, A. G., Himelstein-braw, R., Faber, M. Follicular growth: the basic event in the mouse and human ovary. Reproduction. 45 (3), 559-566 (1975).
- Shu, Y., et al. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Human Reproduction. 23 (3), 504-513 (2008).
- Cheng, Y., et al. Oocyte-expressed Interleukin 7 suppresses granulosa cell apoptosis and promotes oocyte maturation in rats. Biology of Reproduction. 84 (4), 707-714 (2011).
- Ma, J., Fukuda, Y., Schultz, R. M. Mobilization of dormant Cnot7 mRNA promotes deadenylation of maternal transcripts during mouse oocyte maturation. Biology of Reproduction. 93 (2), 1-12 (2015).
- Sha, Q. Q., et al. CNOT 6L couples the selective degradation of maternal transcripts to meiotic cell cycle progression in mouse oocyte. The EMBO journal. 37 (24), 99333 (2018).
- Yartseva, V., Giraldez, A. J. The maternal-to-zygotic transition during vertebrate development: A model for reprogramming. Current Topics in Developmental Biology. 113, 191-232 (2015).
- Mašek, T., Valášek, L., Pospíšek, M. Polysome analysis and RNA purification from sucrose gradients. Methods in Molecular Biology. 703, 293-309 (2011).
- Larsson, O., Tian, B., Sonenberg, N. Toward a genome-wide landscape of translational control. Cold Spring Harbor Perspectives in Biology. 5 (1), 012302 (2013).
- Martins, J. P. S., et al. DAZL and CPEB1 regulate mRNA translation synergistically during oocyte maturation. Journal of Cell Science. 129 (6), 1271-1282 (2016).
- Yang, Y., et al. Maternal mRNAs with distinct 3′ UTRs define the temporal pattern of Ccnb1 synthesis during mouse oocyte meiotic maturation. Genes & development. 31, 1302-1307 (2017).
- Cheng, Z., et al. Pervasive, coordinated protein-level changes driven by transcript isoform switching during meiosis. Cell. 172 (5), 910-923 (2018).
- Ingolia, N. T., Ghaemmaghami, S., Newman, J. R., Weissman, J. S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 324 (5924), 218-223 (2009).
- Piqué, M., López, J. M., Foissac, S., Guigó, R., Méndez, R. A combinatorial code for CPE-mediated translational control. Cell. 132 (3), 434-448 (2008).
- Morgan, M., et al. mRNA 3′ uridylation and poly(A) tail length sculpt the mammalian maternal transcriptome. Nature. 548 (7667), 347-351 (2017).
- Yang, F., Wang, W., Cetinbas, M., Sadreyev, R. I., Blower, M. D. Genome-wide analysis identifies cis-acting elements regulating mRNA polyadenylation and translation during vertebrate oocyte maturation. RNA. 26 (3), 324-344 (2020).
- Magidson, V., Khodjakov, A. Circumventing photodamage in live-cell microscopy. Methods in Cell Biology. 114, 545-560 (2013).
- Chen, J., et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nature Cell Biology. 15 (12), 1415-1423 (2013).

