A Mouse Ear Model for Allergic Contact Dermatitis Evaluation
Summary
Here, we describe the methods for inducing allergic contact dermatitis in mouse ears by 1-fluoro-2,4-dinitrobenzene (DNFB) and how to evaluate the severity of allergic contact dermatitis.
Abstract
Skin is the human body's first line of defense and one of the most exposed organs to environmental chemicals. Allergic contact dermatitis (ACD) is a common skin disease that manifests as a local rash, redness, and skin lesions. The occurrence and development of ACD are influenced by both genetic and environmental factors. Although many scholars have constructed a series of models of ACD in recent years, the experimental protocols of these models are all different, which makes it difficult for readers to establish them well. Therefore, a stable and efficient animal model is of great significance to further study the pathogenesis of atopic dermatitis. In this study, we detail a modeling method using 1-fluoro-2,4-dinitrobenzene (DNFB) to induce ACD-like symptoms in the ears of mice and describe several methods for assessing the severity of dermatitis during modeling. This experimental protocol has been successfully applied in some experiments and has a certain promotional role in the field of ACD research.
Introduction
Allergic contact dermatitis (ACD) is a common skin disease that is characterized by eczema-like symptoms at the contact site, edema and erythema in moderate cases, and papules, erosion, exudation, or even massive scars in severe cases1. It affects up to 20% of the population and can affect people of any age2. ACD often occurs in individuals who have been exposed to allergens repeatedly and can be caused by the individual's immune response to one or more allergens in their home or workplace3. Type IV delayed hypersensitivity is considered the main type of immune response in ACD4. In areas of the skin that have been repeatedly exposed to allergens, circulating memory T cells accumulate in large numbers and induce immune and inflammatory responses3,5,6. The purpose of this work is to propose a reliable laboratory technique for further investigation of immunological and inflammatory responses in the development of ACD.
The onset of ACD is usually due to contact hypersensitivity caused by repeated exposure to chemicals. Numerous researchers have developed various ACD animal models in house mice7,8, guinea pigs9,10, and other animals over the course of the last few decades, in order to simulate the onset of the disease. Most of the experimental methods consist of two stages: abdominal sensitization (induction) and providing stimuli on the back or the ear lobe (stimulation). Commonly used chemical substances mainly include 1-fluoro-2,4-dinitrobenzene (DNFB)/1-chloro-2,4-dinitrobenzene (DNCB)8,9,11, oxazolone12, urushiol13, etc. Among them, DNFB and DNCB are the most widely used, first reported in October 195810. The nickel sensitization model14 and the photoallergic contact dermatitis model15 are also frequently used.
We present an experimental method for building the ACD model. This method is summarized and optimized on the basis of previous studies and upon comparison with multiple experiments. Compared with other ACD models, this model has some advantages, such as small individual differences, short experimental periods, a small amount of chemical stimulation, etc. In addition, this study is applicable to mice, which are not only economical but also have more options for gene knockout or transgenic mice preparation16. We also describe the various methods used to monitor ACD progress in the experiment, such as measuring ear thickness, using Evans blue dye to measure inflammatory exudation, etc. This model can not only analyze mouse ears, blood, spleen, and other samples by laboratory means to explore the pathogenesis of ACD, but also is applicable for the preclinical evaluation of new therapeutic methods, which has a certain promotional significance.
Protocol
All the care and treatment of the mice were in accordance with the guidelines established by the Institutional Animal Care and Use Committee of Yangzhou University and were approved by the Institutional Animal Care and Use Committee under the project license SYXK(SU)2022-0044. BALB/c male and female mice aged 6-8 weeks were used in this study. Each group consisted of six mice (see Table of Materials). Cages were placed in a temperature-controlled chamber (22 ± 2 °C, 12 h light/dark cycle) with free access to food and water. An experimental flow diagram is shown in Figure 1.
1. Animal preparation
- Start the modeling after 1 week of acclimatization to the environment.
- Use an ultraviolet lamp and 75% alcohol disinfectant to clean and disinfect the environment and countertops prior to manipulating the mice.
NOTE: In order to avoid the influence of external factors, marking the mice for identification cannot be performed on the mouse ear; staining on the back or on the tail can be used as an alternative. - Use a small cotton swab to apply soapy water to the abdomen of the mice (approximately 1-2 cm2 in size). Shave the area in the direction of hair growth with a blade or shaver (see Table of Materials) at the beginning of modeling (day 0; Figure 2A).
NOTE: The use of a straight razor blade for hair removal requires a skilled operator. If not performed correctly, it might cause skin irritation. Consider using depilatory cream, clippers, or a safety razor for hair removal. - Weigh the mouse and compare the weight changes among each group.
2. Abdominal sensitization stimulation
- Ensure the full recovery of any minor injury to the abdomen skin induced by shaving. Apply abdominal sensitization 2 days after shaving (day 2).
- Prepare the 0.5% DNFB solution: dilute DNFB with an acetone:olive oil mixture at a 4:1 ratio (e.g., 400 µL of acetone mixed with 100 µL of olive oil; see Table of Materials). Use a pipette gun to blow and mix 20 times to thoroughly mix the DNFB solution. Before each administration of DNFB solution to the mouse, blow and mix it three to five times.
NOTE: Prepare the solution before use and wrap it in aluminum foil to protect it from direct sunlight. - Apply 25 µL of the 0.5% DNFB solution to the skin of the shaved area on the abdomen of the mice with a pipettor (Figure 2B).
- Dribble the DNFB solution over the middle of the abdominal shaving area and lightly spread with the smooth side of the pipettor tip to uniformly disperse it.
- At 30 s after DNFB stimulation, place the mice in empty cages without bedding to prevent them from rubbing off the DNFB solution. When the DNFB solution is completely dry (about 2 min), return the mice to their original cage.
- Wear gloves when handling the DNFB solution as it is strongly irritating to human skin.
3. Ear sensitization stimulation
- Prepare a 0.2% DNFB solution as above, the vehicle solution (a 4:1 mixture of acetone and olive oil), and pure water.
- Orient the mouse body and make the outside border of the auricle face downward during the entire operation to prevent the solution from entering the ear canal during DNFB stimulation.
- On days 4, 6, 8, and 10, use a pipettor to apply 20 µL of the 0.2% DNFB solution or vehicle solution slowly and uniformly to the inner surface of the left auricles of the mice. To avoid DNFB solution from entering the ear canal, use the smooth side of the pipettor tip to gently distribute the DNFB solution during administration. Leave the right ears untreated (Figure 2C).
- Wait until the DNFB solution is dry and place the mice back in the cage (about 30 s).
- Wear gloves when handling the DNFB solution.
4. Recording mouse weight and ACD symptoms
- Weigh the mouse every day, start on day 1, and compare with its corresponding weight on day 0; evaluate the effect of ACD on the body weight of mice as weight change (g) ± standard error of the mean (SEM).
- Take high resolution photos of the mouse ears to record ACD clinical symptoms every 2 days, starting on day 1.
5. Measurement of the auricle thickness
- Measure the auricle thickness every 2 days, starting on day 1. Measure and record both ears in detail.
- Use vernier calipers (see Table of Materials) to measure the auricle thickness at the same time each day for accurate results (Figure 3A). Stop the vernier calipers from continuing inward clamping when there is a slight blockage, to prevent tissue damage to the mouse ear. Keep the position fixed and record the data.
- Collect the thickness from three different locations on each auricle (Figure 3B). Record the average of the three data as a valid value. Evaluate the ear swelling in micrometers (µm) ± standard error of the mean (SEM).
6. Evaluation of the degree of inflammatory swelling
- Prepare 0.5% Evans blue dye (see Table of Materials) solution: dilute Evans blue dye with phosphate buffered saline (PBS) on day 11. Wear a lab coat and gloves at all times, as Evans blue dye is slightly toxic to humans.
- Immobilize the mice with a fixator: open the lid of the fixator (see Table of Materials), hold the mouse tail, make the head of the mouse face the fixator, and make the mouse instinctively climb into the fixator. Cover the lid, make the mouse tail come out of the hole on the lid, and adjust the length of the fixator to expose the whole mouse tail.
- Wipe the tail repeatedly with an alcohol cotton ball or soak it in warm water for 30 s, and gently pinch the root of the tail to fill and expand the veins on both sides. Perform the injection under the irradiation of a cold light source.
- Slowly inject Evans blue dye solution into the mouse tail vein using a 1 mm insulin needle. Wait for 15 min and then take pictures of the mouse ears.
NOTE: Put the mouse on the table and gently hold it to expose the ear region for image acquisition. Shortly after injection with the Evans blue dye solution and observing corresponding indications, use cervical dislocation to euthanize the mouse.
Representative Results
Under repeated DNFB stimulation, the mouse ears of the DNFB group displayed evident clinical symptoms comparable to ACD, with sensitive areas showing the typical symptoms of redness, dryness, and even erosion and exudation. However, ear administration of pure water (control group) or solvent control (vehicle group) did not produce similar symptoms (Figure 4).
Meanwhile, in the DNFB group, compared to the untreated right ear, the thickness of the left ear increased significantly after DNFB stimulation (Figure 5A), whereas there was no significant difference in the control and vehicle groups (Figure 5B). The left ears of the DNFB group mice obviously turned dark blue after the injection of Evans blue dye on the 11th day of modeling, which was visually different from the right ear. However, the left and right ears of mice in the control and vehicle groups were approximately the same color (Figure 5C).
Furthermore, the body weight changes of mice were analyzed. Mouse weight gain was slightly slowed by DNFB or simple vehicle stimulation (Figure 6A), but did not result in significant weight loss (Figure 6B). Simultaneously, the spleen was isolated immediately after the mice were sacrificed. The spleen index was calculated according to the mouse weight and spleen weight; the calculation formula was as follows:
Spleen index = spleen weight (g) / body weight (g) x 100
The result shows that repeated DNFB stimulation in the mouse ear resulted in spleen enlargement (Figure 6C) and an increase in the spleen index (Figure 6D), whereas the spleen index of mice in the vehicle group did not change significantly. It was proved that under the stimulation of DNFB, the immune response function of mice in the DNFB group was hyperactive.
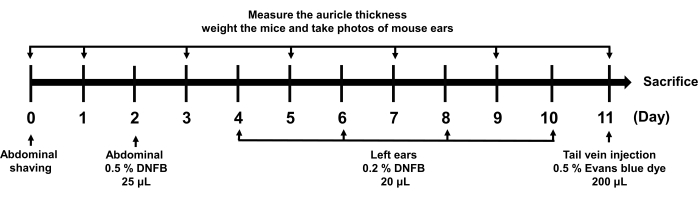
Figure 1: Schematic diagram of ACD molding time axis. The arrows indicate what was done at the corresponding time. The related operations involved include shaving, sensitization, auricle measurement, weighing, photo taking, and Evans blue dye application. Abbreviations: DNFB = 1-fluoro-2,4-dinitrobenzene. Please click here to view a larger version of this figure.
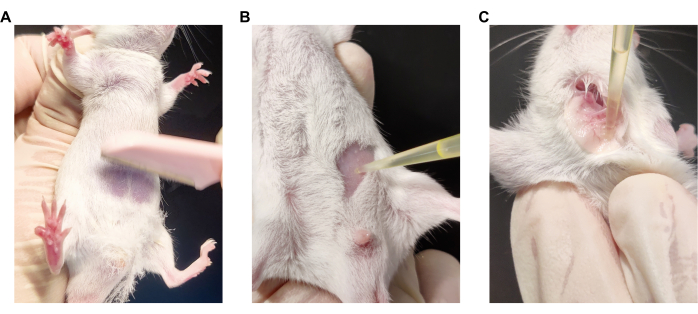
Figure 2: The operation method of the ACD model establishment. (A) Manipulation of abdominal shaving. (B) Manipulation of abdominal sensitizing stimulation. (C) Manipulation of ear sensitizing stimulation. Please click here to view a larger version of this figure.
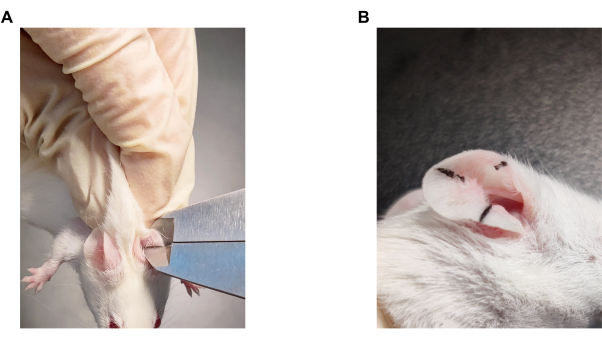
Figure 3: Evaluation method of ear swelling. (A) Manipulation of ear thickness measurements in mice. (B) The sites of ear thickness measurement in mice. Please click here to view a larger version of this figure.
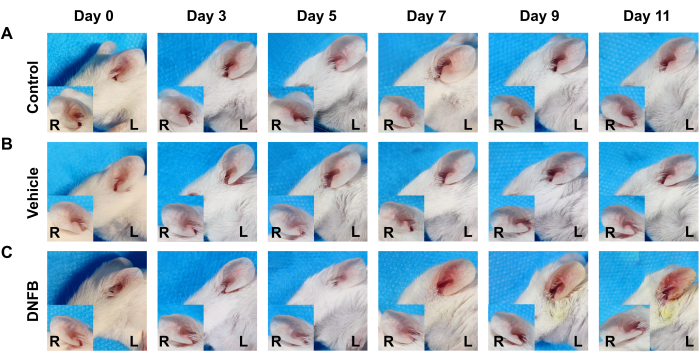
Figure 4: Representative picture of the effect of DNFB administration on the ears of mice over time. (A) Control group. (B) Vehicle group. (C) DNFB group. Please click here to view a larger version of this figure.
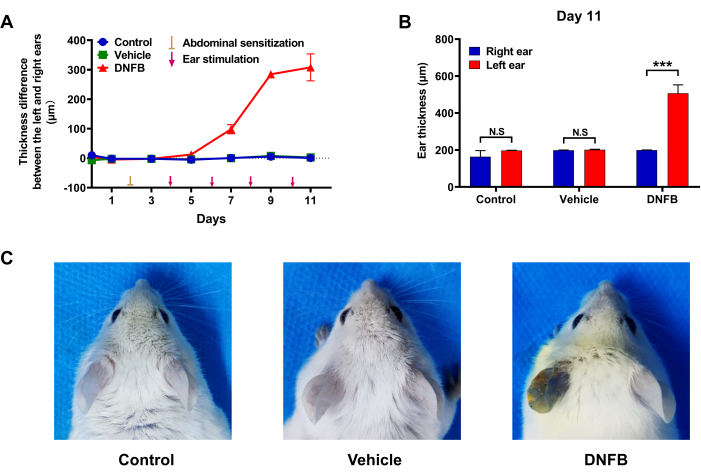
Figure 5: Effect of DNFB administration on ear swelling in mice. (A) Difference in ear thickness between the left and right ears of mice during modeling. (B) Comparison of left and right ear thickness of mice in each group at the end of modeling. (C) Effect of DNFB administration on ear vascular permeability in mice. (n = 6. ***p < 0.001, comparison between the right ears and left ears; N.S. = No significant). All data were expressed as the mean ± SEM. Different treatment analyses among groups were analyzed using an unpaired student's t-test or one-way analysis of variance with Dunnett's test. p values less than 0.05 were considered statistically significant. Please click here to view a larger version of this figure.
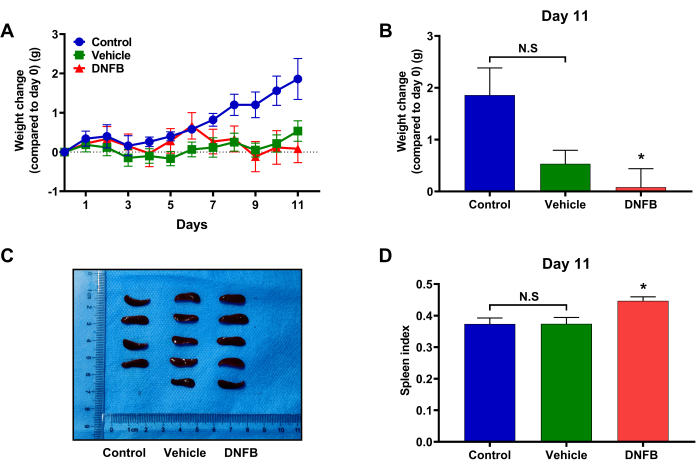
Figure 6: Effects of DNFB administration on body weight and spleen index in mice. (A) Body weight changes of mice in each group during modeling. (B) Comparison of body weight changes in mice in each group on day 11. (C) Comparison of spleen size in each group of mice. (D) Comparison of spleen index between groups of mice. (n = 6. *p < 0.05, compared with the control group; N.S. = No significant). All data were expressed as the mean ± SEM. Different treatment analyses among groups were analyzed using an unpaired student's t-test or one-way analysis of variance with Dunnett's test. p values less than 0.05 were considered statistically significant. Please click here to view a larger version of this figure.
Discussion
The protocol described here for inducing ACD-like symptoms in the ears of mice can be used to study the pathophysiology of ACD and as a screening tool for the development of new drugs.
There are two key steps to establish an ACD model: initial sensitization, and subsequent stimulation. The abdomen is usually the site of initial sensitization, but the subsequent stimulation site was chosen slightly differently. Previous studies have shown that most scholars choose to use chemical sensitizers such as DNFB/DNCB or oxazolone to establish ACD models on the back or neck of mice, and it is inevitable to use blades or trimmers to depilate the modeling area of mice17,18,19. However, this step can easily destroy the skin barrier and affect the subsequent experiments. Furthermore, the dripping drug is difficult to evenly distribute and is easily absorbed by the nearby hair, due to the large area on the nape of the neck and the influence of the surrounding hair.
In this experimental protocol, we found that performing the manipulation for subsequent stimulation in the inner surface of mouse auricles enabled us to alleviate some of above troubles, helping to establish a stable and highly reproducible ACD model. In accordance with our repeated experiments20, we also optimized and adjusted the interval of the sensitizing stimulus and the experimental period. In accordance with the given experimental method, a very obvious modeling effect can be obtained on the 10th day. In addition, as the modeling area is on the relatively independent inner side of the auricle, on which external factors have less interference, there is less difference in the severity of ACD in mice in the same experimental group in this experiment.
This experimental protocol also has some shortcomings. First, applying chemical sensitizers to the ear should be performed with caution to avoid chemicals entering the ear canal and harming the mice. Secondly, ACD models are often used as a means to study chronic itching in mice. In the ACD model established on the nape of mice, the bouts of scratching in mice could be intuitively observed, and the severity of the itching symptom in mice could be measured by this. Although scratching behavior was also observed in mice during our experiment, the mice also had spontaneous ear-cleaning habits, making it difficult to distinguish from pathological scratching behavior. This limited the use of this model in observing ACD-induced scratching behavior. Whether the protocol is applicable to this type of study is subject to further experimental verification.
To track the pathological course of ACD, a variety of monitoring methods were used, such as clinical ear symptoms, ear thickness measurement, and reflection of vascular permeability. These pathological indicators are more visible in the ear than in the neck and back skin. When measuring a mouse's ear thickness, measurement errors will occur due to mouse struggling behavior and the ear's uneven thickness. To reduce measuring mistakes, measurements should be performed in three different places on each ear. By injecting Evans dye to evaluate the vascular permeability of the modeled area, the severity of dermatitis can be seen, however, this also requires a high success rate of tail vein injection. If further comparative analysis is required, the absorbance of the supernatant of mouse ear tissue homogenate can be determined.
It is also worth mentioning that, in our previous research20, the ear tissue structure was well organized and less impacted by other disordered tissue structures (e.g., hair follicles), than in the neck and back skin tissue, which led to choosing this area for research.
In conclusion, the model of ACD described in this paper is a stable and efficient modeling method and is worthy of promotion in subsequent studies of allergic contact dermatitis.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) to N.-N. Y. (81904212); Jiangsu Traditional Chinese Medicine Science and Technology Project (YB201995); and the Special Funding Project for Postdoctoral Researchers in China (2020T130562).
Materials
| 1-Fluoro-2,4-dinitrobenzene (DNFB) | Merck | 200-734-3 | 1-Fluoro-2,4-dinitrobenzene, ≥99% |
| Acetone | Sinopharm Chemical Reagent Co. LTD | 10000418 | ≥99.5% |
| Aluminum foil | Cleanwrap | CF-2 | |
| Evans blue dye | Solarbio | 314-13-6 | Dye content approx. 80% |
| Mouse fixator | ZHUYANBANG | GEGD-SM1830 | |
| Olive oil | Solarbio | 8001-25-0 | 500 ml |
| Pipet tip | Biofount | FT-200 | 10 – 200 μl |
| Pipettor | Eppendorf AG | 3123000250 | 20 – 200 μl |
| Razor blade | Shanghai Gillette Co. LTD | 74-S | |
| Vernier calipers | Delixi Electric | DECHOTVCS1200 |
References
- Neale, H., Garza-Mayers, A. C., Tam, I., Yu, J. Pediatric allergic contact dermatitis. Part I: Clinical features and common contact allergens in children. Journal of the American Academy of Dermatology. 84 (2), 235-244 (2021).
- Koppes, S. A., et al. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermatitis. 77 (1), 1-16 (2017).
- Martin, S. F., et al. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy. 66 (9), 1152-1163 (2011).
- Kimber, I., Basketter, D. A., Gerberick, G. F., Dearman, R. J. Allergic contact dermatitis. International Immunopharmacology. 2 (2-3), 201-211 (2002).
- Vocanson, M., Hennino, A., Rozieres, A., Poyet, G., Nicolas, J. F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 64 (12), 1699-1714 (2009).
- Gamradt, P., et al. Inhibitory checkpoint receptors control CD8+ resident memory T cells to prevent skin allergy. The Journal of Allergy and Clinical Immunology. 143 (6), 2147.e9-2157.e9 (2019).
- Fraginals, R., Blasi, N. A., Lepoittevin, J. P., Benezra, C. A successful murine model for contact sensitization to a sesquiterpene-alpha-methylene-gamma-butyrolactone: sensitization to alantolactone in four strains of mice. The Journal of Investigative Dermatology. 97 (3), 473-477 (1991).
- Knop, J., Riechmann, R., Neumann, C., Macher, E. Modulation of suppressor mechanisms in allergic contact dermatitis: 5. Evidence that inhibition of suppressor T lymphocytes by Corynebacterium parvum is mediated by interferon. The Journal of Investigative Dermatology. 79 (6), 385-388 (1982).
- Polak, L., Scheper, R. J. Antigen-specific T-cell lines in DNCB-contact sensitivity in guinea pigs. The Journal of Investigative Dermatology. 80 (5), 398-402 (1983).
- Witten, V. H., March, C. H. Studies of the mechanism of allergic eczematous contact dermatitis. II. Use of C14 labelled 2:4-dinitrochlorobenzene in guinea pigs. The Journal of Investigative Dermatology. 31 (2), 97-102 (1958).
- Knop, J., Riechmann, R., Macher, E. Modulation of suppressor mechanism in allergic contact dermatitis. IV. Selective inhibition of suppressor T-lymphocytes by serum obtained from Corynebacterium parvum treated mice. The Journal of Investigative Dermatology. 77 (6), 469-473 (1981).
- Rubic-Schneider, T., et al. GPR91 deficiency exacerbates allergic contact dermatitis while reducing arthritic disease in mice. Allergy. 72 (3), 444-452 (2017).
- Stampf, J. L., Benezra, C., Byers, V., Castagnoli Jr, N. Induction of tolerance to poison ivy urushiol in the guinea pig by epicutaneous application of the structural analog 5-methyl-3-n-pentadecylcatechol. The Journal of Investigative Dermatology. 86 (5), 535-538 (1986).
- Dhingra, N., et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. The Journal of Allergy and Clinical Immunology. 134 (2), 362-372 (2014).
- Maguire Jr, H. C., Kaidbey, K. Experimental photoallergic contact dermatitis: a mouse model. The Journal of Investigative Dermatology. 79 (3), 147-152 (1982).
- Qiu, Z., et al. A dysregulated sebum-microbial metabolite-IL-33 axis initiates skin inflammation in atopic dermatitis. The Journal of Experimental Medicine. 219 (10), e2021397 (2022).
- Kim, H., et al. Anti-inflammatory activities of Dictamnus dasycarpus Turcz., root bark on allergic contact dermatitis induced by dinitrofluorobenzene in mice. Journal of Ethnopharmacology. 149 (2), 471-477 (2013).
- Zhou, P., et al. Effect of 6′-acetylpaeoniflorin on dinitrochlorobenzene-induced allergic contact dermatitis in BALB/c mice. Immunologic Research. 64 (4), 857-868 (2016).
- Donglang, G., et al. Comparative study on different skin pruritus mouse models. Frontiers in Medicine. 8, 630237 (2021).
- Yang, N., Shao, H., Deng, J., Liu, Y. Network pharmacology-based analysis to explore the therapeutic mechanism of Cortex Dictamni on atopic dermatitis. Journal of Ethnopharmacology. 304, 116023 (2023).

