发展多深的圆形横截面微内皮化的一个芯片的程序
Summary
Â微通道上的一个芯片平台开发回流焊的光致抗蚀剂光刻技术,软光刻技术和微流体的结合。模拟的三维(3D)的几何形状在体内微血管内皮化的微通道平台,控制连续灌注流量下运行,允许高品质和实时成像,并可以应用对微血管研究。
Abstract
努力一直专注于发 展在体外实验研究微血管,因为动物体内研究更费时,昂贵,观察和量化是非常具有挑战性的。然而,传统的在体外微血管检测体内微血管代表相对于三维(3D)的几何形状,并提供连续的流体流动的限制。使用回流焊的光致抗蚀剂光刻技术,软光刻技术,以及微流控芯片的组合,我们已经制定了多深的圆形横截面内皮化微的芯片,它模仿3D几何体内微血管和运行控制连续灌注下流程。使用了一个正的回流的光致抗蚀剂,制作母模的半圆形横截面的微通道网络。由对准和粘接的聚二甲基硅氧烷(PDMS)的微通道REPL从主模具icated,的圆柱形微通道网络已创建。微通道的直径可以得到很好的控制。此外,原代人脐静脉内皮细胞(HUVECs)表明,在芯片内接种细胞排列的微通道的内表面上为4天至2周之间的时间周期根据控制灌注持久。
Introduction
微血管,作为流通体系的一部分,调解血液和组织之间的相互作用,支持新陈代谢活动,定义组织微环境,在许多健康和病理条件下发挥了关键作用。再演在体外的功能微血管提供了一个平台,为研究复杂的血管现象。然而,传统的在体外微血管检测,如内皮细胞迁移测定,内皮细胞管腔形成的测定中,大鼠和小鼠的主动脉环测定,无法重新创建的三维(3D)的几何形状和连续的流量控制相对于体内微血管1-8。微血管的研究使用的动物模型中和在体内测定,如角膜血管生成法,鸡胚尿囊膜血管生成实验,Matrigel栓实验,是更费时,成本高,相对于观察和量化挑战,并引发伦理问题1,9-13。
显微制造和微流控芯片技术的进展已启用了各种各样的见解,到生物医学科学在削减高的实验与动物体内研究,如容易和严格控制的生物条件和动态流体环境中,不会有相关的成本和复杂性的同时一直未能与传统的大容量的技术。
在这里,我们提出了一种方法来构建内皮化微通道上的单芯片模拟3D几何体内微血管和控制的连续灌流采用回流焊的光致抗蚀剂光刻技术,软光刻技术和微流体的结合下运行。
Protocol
Representative Results
Discussion
1。主模具制造
血管形态计量学的设计和指导原则之一是被称为穆雷的第16条,其中规定,受整个网络分布的血管直径最小能量代价。报告还指出,在分叉的父容器的直径的立方等于总和的女儿血管直径的立方( 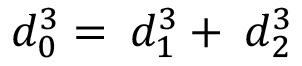 19)此外,泊肃叶定律已被用于估?…
19)此外,泊肃叶定律已被用于估?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
部分支持这项研究是由美国国家科学基金会(NSF 1227359),西弗吉尼亚的EPSCoR程序由美国国家科学基金会(EPS-1003907),西弗吉尼亚ADVANCE办公室主办,由美国国家科学基金会(1007978),和WVU PSCoR的分别。 WVU共享研究设施(无尘室设施)和微流控结合蜂窝片上实验室(芯片实验室)在西弗吉尼亚大学的研究工作是在微细。聚焦成像做在WVU显微镜成像设备。
Materials
| Reagent/Material | |||
| Reflow Photoresist | AZ Electronic Materials | AZP4620 | |
| Developer | AZ Electronic Materials | AZ 400K | |
| PDMS | Dow Corning Corporation | Sylgard 184 | |
| MCDB 131 Culture Medium | Invitrogen | 10372-019 | |
| NacBlue Nuclei Staining | Invitrogen | H1399 | |
| PKH Red Stain | Sigma | MINI26 and PKH26GL | |
| Fibronectin | Gibco | PHE0023 | |
| L-Glutamine | Sigma | G7513 | |
| Phosphate Buffered Saline | Invitrogen | 14040-133 | |
| HEPES Buffered Saline Solution | Lonza | CC-5024 | |
| Trypsin/EDTA | Invitrogen | 25300-062 | |
| Trypsin Neutralizing Solution | Lonza | CC-5002 | |
| PDMS Curing Agent | Dow Corning Corporation | Sylgard 184 | |
| Primary Human Umbilical Vein Endothelial Cells | Lonza | CC-2517 | |
| Fetal Bovine Serum | Lonza | 14-501F | |
| Diluent C | Sigma | CGLDIL | |
| Hoechst33342 | Invitrogen, Molecular Probes | R37605 | |
| Dextran | Sigma | 95771 | |
| 3.5% Paraformaldehyde | Electron Microscopy Science | 15710-S | |
| Equipment | |||
| Spinner | Laurell Technologies Corporation | WS-400BZ-6NPP/LITE | |
| Desiccator | BelArt Products | 999320237 | |
| Inverted Microscope | Nikon | Eclipse Ti | |
| Syringe Pump System | Harvard Apparatus | PHD Ultra | |
| Laminar Biosafety Hood | Thermo Scientific | 1300 Series A2 | |
| Planetary Centrifugal Mixer | Thinky | ARE-310 | |
| Isotemp Oven | Fisher Scientific | 13-246-516GAQ | |
| Optical Microscope | Zeiss | Invertoskop 40C | |
| Plasma Cleaner | Harrick Plasma | PDC-32G | |
| Hotplate | Barnstead/Thermolyne Cimarec | SP131635 | |
| Laser Scanning Confocal Microscope | Zeiss | LSM 510 |
References
- Adair, T. H. . Angiogenesis: Integrated systems physiology: from molecule to function to disease. , (2011).
- Goodwin, A. M. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc. Res. 74, 172-183 (2007).
- Smith, E. J., Staton, C. A. Tubule formation assays. Angiogenesis assays: A critical appraisal of current techniques. , 65-87 (2006).
- Nakatsu, M. N., Davis, J. J., Hughes, C. C. W. Optimized fibrin gel bead assay for the study of angiogenesis. J. Vis. Exp. (3), e186 (2007).
- Nicosia, R. F., Ottinetti, A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab. Invest. 63, 115-122 (1990).
- Aplin, A. C., Fogel, E., Zorzi, P., Nicosia, R. F. The aortic ring model of angiogenesis. Methods Enzymol. 443, 119-136 (2008).
- Nicosia, R. F. The aortic ring model of angiogenesis: A quarter century of search and discovery. J. Cell. Mol. Med. 13, 4113-4136 (2009).
- Griffith, L. G., Swart, M. A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7, 211-224 (2006).
- Folkman, J. History of angiogenesis. Angiogenesis: An integrative approach from science to medicine. , (2008).
- Auerbach, R., Lewis, R., Shinners, B., Kubai, L., Akhtar, N. Angiogenesis assays: A critical overview. Clin. Chem. 49, 32-40 (2003).
- Auerbach, R., Akhtar, N., Lewis, R. L., Shinners, B. L. Angiogenesis assays: Problems and pitfalls. Cancer Metastasis Rev. 19, 167-172 (2000).
- Staton, C. A., Reed, M. W., Brown, N. J. A critical analysis of current in vitro and in vivo angiogenesis assays. Int. J. Exp. Pathol. 90, 195-221 (2009).
- Staton, C. A., Stribbling, S. M., et al. Current methods for assaying angiogenesis in vitro and in vivo. Int. J. Exp. Pathol. 85, 233-248 (2004).
- Moraes, C., Mehta, G., Lesher-Perez, S. C., Takayama, S. Organs-on-a-Chip: A focus on compartmentalized microdevices. Ann. Biomed. Eng. 40 (6), 1211-1227 (2012).
- Huang, Z., Li, X., Martins-Green, M., Liu, Y. Microfabrication cylindrical microfluidic channel networks for microvascular research. Biomedical Microdevices. 14 (5), 873-883 (2012).
- Murray, C. D. The physiological principle of minimum work applied to the angle of branching of arteries. J. Gen. Physiol. 9 (6), 835-841 (1926).
- Zamir, M., Medeiros, J. A. Arterial branching in man and monkey. J. Gen. Physiol. 79, 353-360 (1982).
- Gafiychuk, V. V., Lubashevsky, I. A. On the principles of the vascular network branching. J. Theor. Biol. 212, 1-9 (2001).
- Sherman, T. F. On connecting large vessels to small. The meaning of Murray’s law. J. Gen. Physiol. 78 (4), 431-453 (1981).
- Kamiya, A., Bukhari, R., Togawa, T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull Math Biol. 46 (1), 127-137 (1984).
- LaBarbera, M. Principles of design of fluid transport systems in zoology. Science. 249, 992-1000 (1990).
- Emerson, D. R., Cieslicki, K., Gu, X., Barber, R. W. Biomimetic design of microfluidic manifolds based on a generalized Murray’s law. Lab Chip. 6, 447-454 (2006).
- Lu, H., Koo, L. Y., et al. Microfluidic shear devices for quantitative analysis of cell adhesion. Anal. Chem. 76, 5257-5264 (2004).
- Shevkoplyas, S. S., Gifford, S. C., Yoshida, T., Bitensky, M. W. Prototype of an in vitro model of the microcirculation. Microvasc. Res. 65, 132-136 (2003).
- Kaihara, S., Borenstein, J., et al. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 6, 105-117 (2000).
- Fisher, A. B., Chien, S., Barakat, A. I., Nerem, R. M. Endothelial cellular response to altered shear stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 281 (3), L529-L533 (2001).
- Nerem, R. M., Alexander, R. W., et al. The study of the influence of flow on vascular endothelial biology. Am. J. Med. Sci. 316 (3), 169-175 (1998).
- Daly, D., Stevens, R. F., Hutley, M. C., Davies, N. The manufacture of microlenses by melting photoresist. Meas. Sci. Technol. 1 (8), 759-766 (1990).
- Schilling, A., Merz, R., Ossmann, C., Herzig, H. P. Surface profiles of reflow microlenses under the influence of surface tension and gravity. Opt. Eng. 39 (8), 2171-2176 (2000).
- Young, B., Heath, J. W. . Wheater’s functional histology: A text and colour atlas. , (2000).
- O’Neill, F. T., Sheridan, J. T. Photoresist reflow method of microlens production. Part 1: Background and experiments. Optik Int. J. Light Electron Opt. 113, 391-404 (2002).
- de Gennes, P. G. Wetting: statics and dynamics. Rev. Mod. Phys. 57, 827-863 (1985).
- Elias, H. G. . An Introduction to Polymer Science. , (1997).
- Voinov, O. V. Dynamic edge angles of wetting upon spreading of a drop over a solid surface. J. Appl. Mech. Tech. Phys. 40, 86-92 (1999).
- Daly, D., Stevens, R. F., Hutley, M. C., Davies, N. The manufacture of microlenses by melting photoresist. Meas. Sci. Technol. 1, 759 (1990).
- Jay, T. R., Stern, M. B. Preshaping photoresist for refractive microlens fabrication. Opt. Eng. 33, 3552-3555 (1994).
- Nerem, R. M., Alexander, R. W., et al. The Study of the influence of flow on vascular endothelial biology. Am. J. Med. Sci. 316 (3), 169-175 (1998).
- Chien, S., Li, S., Shyy, Y. J. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 31, 162-169 (1998).
- Li, Y. S., Haga, J. H., Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 38, 1949-1971 (2005).
- Lee, E. J., Vunjak-Novakovic, G., Wang, Y., Niklason, L. E. A biocompatible endothelial cell delivery system for in vitro tissue engineering. Cell Transplant. 18, 731-743 (2009).
- Lee, E. J., Niklason, L. E. A novel flow bioreactor for in vitro microvascularization. Tissue Eng. Part C Methods. 16, 1191-1200 (2010).
- Chau, L., Doran, M., Cooper-White, J. A novel multishear microdevice for studying cell mechanics. Lab Chip. 9, 1897-1902 (2009).
- Meeson, A., Palmer, M., Calfon, M., Lang, R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 122, 3929-3938 (1996).
- Van Royen, N. J., Piek, J., Schaper, W., Bode, C., Buschmann, I. Arteriogenesis: mechanisms and modulation of collateral artery development. J. Nucl. Cardiol. 8, 687-693 (2001).
- Schaper, W. Therapeutic arteriogenesis has arrived. Circulation. 104 (17), 1994-1995 (2001).
- Tarbell, J. M. Shear stress and the endothelial transport barrier. Cardiovas. Res. 87 (2), 320-330 (2010).
- Potter, C. M., Lundberg, M. H., et al. Role of shear stress in endothelial cell morphology and expression of cyclooxygenase isoforms. Arterioscler. Thromb. Vasc Biol. 31, 384-391 (2011).
- Montesano, R. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J. Cell Biol. 97, 1648-1652 (1983).
- Darland, D. C., D’Amore, P. A. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 4 (1), 11-20 (2001).
- Lawley, T. J., Kubota, Y. Induction to morphologic differentiation of endothelial cells in culture. J. Invest. Dermatol. 93, 59S-61S (1989).
- Kanzawa, S., Endo, H., Shioya, N. Improved in vitro angiogenesis model by collagen density reduction and the use of type III collagen. Ann. Plast. Surg. 30, 244-251 (1993).
- Davis, G. E., Bayless, K. J., Mavila, A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat. Rec. 268, 252-275 (2002).
- Velazquez, O. C., Snyder, R., Liu, Z., Fairman, R. M., Herlyn, M. Fibroblast-dependent differentiation of human microvascular endothelial cells into capillary-like 3-dimensional networks. FASEB J. 16, 1316-1318 (2002).
- Donovan, D., Brown, N. J., Bishop, E. T. Comparison of three in vitro human “angiogenesis” assays with capillaries formed in vivo. Angiogenesis. 4, 113-121 (2001).
- Tang, D. G., Conti, C. J. Endothelial cell development, vasculogenesis, angiogenesis, and tumor neovascularization: an update. Semin. Thromb. Hemost. 30, 109-117 (2004).
- Takayama, S., McDonald, J. C., et al. Patterning cells and their environments using multiple laminar fluid flows in capillary networks. Proc. Natl. Acad. Sci. U.S.A. 96, 5545-5548 (1999).
- Cho, B. S., Schuster, T. G., et al. Passively driven integrated microfluidic system for separation of motile sperm. Anal. Chem. 75, 1671-1675 (2003).
- Parsa, H., Upadhyay, R., Sia, S. K. Uncovering the behaviors of individual cells within a multicellular microvascular community. Proc. Natl. Acad. Sci. U.S.A. 108 (12), 5133-5138 (2011).

