从猪肾和小鼠肝脏中提取和纯化FAHD1蛋白
Summary
该协议描述了如何从猪肾和小鼠肝脏中提取富马酰乙酰乙酸水解酶结构域蛋白1(FAHD1)。所列的方法可以适应于其他感兴趣的蛋白质并针对其他组织进行修饰。
Abstract
富马酰乙酰乙酸水解酶结构域含蛋白1(FAHD1)是真核生物中FAH超家族的第一个成员,在线粒体中充当草酰乙酸脱羧酶。本文介绍了一系列从猪肾和小鼠肝脏中提取和纯化FAHD1的方法。涵盖的方法包括使用快速蛋白质液相色谱 (FPLC) 进行离子交换色谱,使用 FPLC 进行制备和分析凝胶过滤,以及蛋白质组学方法。全蛋白提取后,探讨硫酸铵沉淀和离子交换色谱, 采用 离子交换和尺寸排阻色谱法提取FAHD1。这种具有代表性的方法可以适应其他感兴趣的蛋白质(在显着水平上表达)并针对其他组织进行修饰。从组织中纯化的蛋白质可以支持高质量抗体和/或有效和特异性药理抑制剂的开发。
Introduction
真核FAH结构域蛋白1(FAHD1)作为双功能草酰乙酸(OAA)脱羧酶(ODx)1和酰基丙酮酸水解酶(ApH)2。它定位于线粒体2中,属于酶1,2,3,4,5,6的广泛FAH超家族。虽然其ApH活性仅具有次要相关性,但FAHD1的ODx活性参与TCA循环通量1,7,8,9的调节。OAA不仅是三羧酸循环中柠檬酸盐合酶反应所必需的,而且还作为琥珀酸脱氢酶的竞争性抑制剂,作为电子传递系统的一部分和作为弹力代谢物。人脐静脉内皮细胞(HUVEC)中FAHD1基因表达的下调导致细胞增殖速率10的显着降低,线粒体膜电位的显着抑制,与同时切换到糖酵解有关。工作模型是指线粒体功能障碍相关衰老(MiDAS)11样表型8,其中线粒体OAA水平受到FAHD1活性1,8,9的严格调节。
重组蛋白更容易 通过 细菌12 的表达和纯化而不是从组织中获得。然而,在细菌中表达的蛋白质可能由于缺乏翻译后修饰而偏倚,或者可能只是有问题(即,由于质粒丢失,细菌应激反应,扭曲/未形成的二硫键,无或分泌不良,蛋白质聚集,蛋白水解裂解等)。对于某些应用,蛋白质需要从细胞裂解物或组织中获得,以便包括这种修饰和/或排除可能的伪影。从组织中纯化的蛋白质支持高质量抗体的开发,和/或针对选定酶的有效和特异性药理抑制剂,例如FAHD113。
本手稿介绍了从猪肾和小鼠肝脏中提取和纯化FAHD1的一系列方法。所描述的方法需要快速蛋白质液相色谱(FPLC),但使用常见的实验室设备。替代方法可以在其他地方找到14,15,16,17。在全蛋白提取后,所提出的方案涉及一个测试阶段,其中讨论了硫酸铵沉淀和离子交换色谱的子方案(图1)。在定义了这些亚方案之后,通过使用离子交换和FPLC的尺寸排阻色谱法, 通过 顺序策略提取感兴趣的蛋白质。基于这些指南,最终方案可以单独适应其他感兴趣的蛋白质。
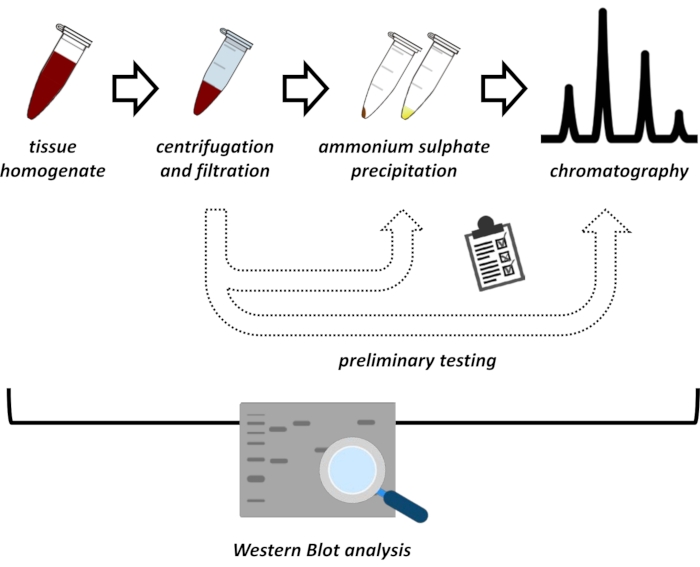
图 1:该协议的总体策略。从上到下:蛋白质是从组织中提取的。制备组织匀浆,离心和过滤。对于每对上清液和颗粒衍生样品,需要进行硫酸铵沉淀和离子交换色谱(FPLC)的测试以探针最佳条件。建立这些子方案后,可以通过硫酸铵沉淀,离子交换色谱和重复大小排阻色谱(FPLC)在不同pH和盐浓度下的顺序程序提取蛋白质。所有步骤都需要通过免疫印迹控制。请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
协议中的关键步骤
遵循处理蛋白质的常见准则至关重要,例如在冰上以及在中等pH和盐条件下工作。使用蛋白酶抑制剂对该方法有益,而强烈建议使用蛋白酶体抑制剂。冷冻和解冻样品可能总是导致蛋白质沉淀(至少部分),因此任何解冻的初始蛋白质裂解物等分试样(步骤2)都应连续处理,不得中断。一般建议解冻后的离心和过滤,以除去微沉淀。
从组织?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
作者非常感谢Ayse Öztürk和Eva Albertini的技术援助。用于生成肝组织的小鼠在Univ.-Doz的监督下维持。Pidder Jansen-Dürr博士(因斯布鲁克大学生物医学老龄化研究所,Rennweg 10,6020因斯布鲁克,奥地利)。
Materials
| 0.22 µm filter units | MERCK | SLGP033RS | Millex-HP, 0.22 µm, PES 33 mm, not steril |
| 0.45 µm filter units | MERCK | SLHP033NS | Millex-HP, 0.45 µm, PES 33 mm, not steril |
| 15 mL Falcon tubes | VWR | 734-0451 | centrifugal tubes |
| 50 mL Falcon tubes | VWR | 734-0448 | centrifugal tubes |
| 96-Well UV Microplate | Thermo-Fischer | 8404 | UV/VIS transparent flat-bottom 96 well plates |
| Acrylamide/Bis Solution (40%, 29:1 ratio) | BIO-RAD | #1610147 | 40% acrylamide/bis-acrylamide, 29:1 (3.3% crosslinker) solution for casting polyacrylamide gels |
| ÄKTA FPLC system | GE Healthcare Life Sciences / Cytiva | – | using the FPLC system by GE Healthcare; different custom versions exist; this work used the "ÄKTA pure" system |
| Amicon Ultra-15, PLGC Ultracel-PL Membran, 10 kDa | MERCK | UFC901024 | centrifigal filters for protein enrichment; 10 kDa molecular mass filter; 15 mL |
| Amicon Ultra-4, PLGC Ultracel-PL Membran, 10 kDa | MERCK | UFC801024 | centrifigal filters for protein enrichment; 10 kDa molecular mass filter; 4 mL |
| Ammonium sulfate powder | MERCK | A4418 | ammonium sulphate for molecular biology, ≥99.0% |
| Ammoniumpersulfat reagent grade, 98% | MERCK | 215589 | Catalyst for acrylamide gel polymerization. |
| Coomassie Brilliant blue R 250 | MERCK | 1125530025 | Coomassie Brilliant blue R 250 (C.I. 42660) for electrophoresis Trademark of Imperial Chemical Industries PLC. CAS 6104-59-2, pH 6.2 (10 g/l, H2O, 25 °C) |
| Dialysis tubing cellulose membrane | MERCK | D9277 | Cellulose membranes for the exchange of buffers via dialysis. |
| Eppendof tubes 1.5 mL | VWR | 525-1042 | microcentrifugal tubes; autoclaved |
| HiLoad 26/600 Superdex 75 pg | GE Healthcare Life Sciences / Cytiva | 28989334 | HiLoad Superdex 75 pg prepacked columns are for high-resolution size exclusion chromatography of recombinant proteins |
| Immun-Blot PVDF Membrane | BIO-RAD | #1620177 | PVDF membranes are protein blotting membranes optimized for fluorescent and multiplex fluorescent applications. |
| Mini Trans-Blot Electrophoretic Transfer Cell | BIO-RAD | #1703930 | Use the Mini Trans-Blot Cell for rapid blotting of Mini-PROTEAN precast and handcast gels. |
| Mini-PROTEAN Tetra Vertical Electrophoresis Cell for Mini Precast Gels | BIO-RAD | #1658004 | 4-gel vertical electrophoresis system, includes electrode assembly, companion running module, tank, lid with power cables, mini cell buffer dam. |
| Mono Q 10/100 GL | GE Healthcare Life Sciences / Cytiva | 17516701 | Mono Q columns are strong anion exchange chromatography columns for protein analysis or small scale, high resolution polishing of proteins. |
| Mono S 10/100 GL | GE Healthcare Life Sciences / Cytiva | 17516901 | Mono S columns are strong cation exchange chromatography columns for protein analysis or small scale high resolution polishing of proteins. |
| PageRuler Prestained Protein Ladder, 10 to 180 kDa | Thermo-Fischer | 26616 | A mixture of 10 blue-, orange-, and green-stained proteins (10 to 180 kDa) for use as size standards in protein electrophoresis (SDS-PAGE) and western blotting. |
| Pierce BCA Protein Assay Kit | Thermo-Fischer | 23225 | A two-component, high-precision, detergent-compatible protein assay for determination of protein concentration. |
| Sonifier 250; Ultrasonic Cell Disruptor w/ Converter | Branson | – | New models at https://www.emerson.com/documents/automation/brochure-sonifier-sfx250-sfx550-cell-disruptors-homogenizers-branson-en-us-168180.pdf |
| Swine Anti-Rabbit Immunoglobulins/HRP (affinity isolated) | Agilent Dako | P0399 | The antibody used for horseradish peroxidase conjugation reacts with rabbit immunoglobulins of all classes. |
| TEMED, 1,2-Bis(dimethylamino)ethane, TMEDA | MERCK | T9281 | TEMED (N,N,N′,N′-Tetramethylethylenediamine) is molecule which allows rapid polymerization of polyacrylamide gels. |
| Tube Roller | – | – | A general tube rotator roller; e.g. a new model at https://labstac.com/de/Mixer/Roller/c/71 |
| Tube Rotator | – | – | A general tube rotator wheel; e.g. a new model at https://labstac.com/de/Tube-Roller/p/MT123 |
| ULTRA-TURRAX; T 25 digital | IKA | 0003725000 | New models at https://www.ika.com/de/Produkte-Lab-Eq/Dispergierer-Dipergiergeraet-Homogenisierer-Homogenisator-csp-177/T-25-digital-ULTRA-TURRAX-cpdt-3725000/ |
References
- Pircher, H., et al. Identification of FAH domain-containing protein 1 (FAHD1) as oxaloacetate decarboxylase. Journal of Biological Chemistry. 290 (11), 6755-6762 (2015).
- Pircher, H., et al. Identification of human Fumarylacetoacetate Hydrolase Domain-containing Protein 1 (FAHD1) as a novel mitochondrial acylpyruvase. Journal of Biological Chemistry. 286 (42), 36500-36508 (2011).
- Kang, T. -. W., et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 479 (7374), 547-551 (2011).
- Hong, H., Seo, H., Park, W., Kim, K. K. -. J. Sequence, structure and function-based classification of the broadly conserved FAH superfamily reveals two distinct fumarylpyruvate hydrolase subfamilies. Environmental Microbiology. 22 (1), 270-285 (2020).
- Timm, D. E., Mueller, H. A., Bhanumoorthy, P., Harp, J. M., Bunick, G. J. Crystal structure and mechanism of a carbon-carbon bond hydrolase. Structure. 7 (9), 1023-1033 (1999).
- Bateman, R. L., et al. Mechanistic inferences from the crystal structure of Fumarylacetoacetate Hydrolase with a bound phosphorus-based inhibitor. Journal of Biological Chemistry. 276 (18), 15284-15291 (2001).
- Weiss, A. K. H., et al. Structural basis for the bi-functionality of human oxaloacetate decarboxylase FAHD1. Biochemical Journal. 475 (22), 3561-3576 (2018).
- Etemad, S., et al. Oxaloacetate decarboxylase FAHD1 – a new regulator of mitochondrial function and senescence. Mechanisms of Ageing and Development. 177, 22-29 (2019).
- Weiss, A. K. H., et al. Regulation of cellular senescence by eukaryotic members of the FAH superfamily – A role in calcium homeostasis. Mechanisms of Ageing and Development. 190, 111284 (2020).
- Petit, M., Koziel, R., Etemad, S., Pircher, H., Jansen-Dürr, P. Depletion of oxaloacetate decarboxylase FAHD1 inhibits mitochondrial electron transport and induces cellular senescence in human endothelial cells. Experimental Gerontology. 92, 7-12 (2017).
- Wiley, C. D., et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabolism. 23 (2), 303-314 (2016).
- Weiss, A. K. H., et al. Expression, purification, crystallization, and enzyme assays of Fumarylacetoacetate Hydrolase Domain-containing proteins. Journal of Visualized Experiments: JoVE. (148), e59729 (2019).
- Weiss, A. K. H., et al. Inhibitors of Fumarylacetoacetate Hydrolase Domain Containing Protein 1 (FAHD1). Molcules. 26 (16), 5009 (2021).
- Mizutani, H., Kunishima, N. Purification, crystallization and preliminary X-ray analysis of the fumarylacetoacetase family member TTHA0809 from Thermus thermophilus HB8. Acta Crystallographica Section F Structural Biology and Crystallization Communications. 63 (9), 792-794 (2007).
- Lee, C. H. A simple outline of methods for protein isolation and purification. Endocrinology and Metabolism. 32 (1), 18-22 (2017).
- Amer, H. E. A. Purification of proteins: Between meaning and different methods). Proteomics Technologies and Applications. , (2019).
- Niu, L., Yuan, H., Gong, F., Wu, X., Wang, W. Protein extraction methods shape much of the extracted proteomes. Frontiers in Plant Science. 9, 802 (2018).
- Gordon, J. A. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods in Enzymology. 201, 477-482 (1991).
- Gallagher, S. R. SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Current Protocols in Essential Laboratory Techniques. , (2012).
- . Effect of pH on Protein Size Exclusion Chromatography Available from: https://www.agilent.com/cs/library/applications/5990-8138EN.pdf (2011)
- Sørensen, B. K., et al. Silver staining of proteins on electroblotting membranes and intensification of silver staining of proteins separated by polyacrylamide gel electrophoresis. Analytical Biochemistry. 304 (1), 33-41 (2002).
- Fagerberg, L., et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Molecular & Cellular Proteomics. 13 (2), 397-406 (2014).
- . Cytiva Life Fundamentals of size exclusion chromatography Available from: https://www.cytivalifesciences.com/en/us/solutions/protein-research/knowledge-center/protein-purification-methods/size-exclusion-chromatography (2022)
- Rosano, G. L., Ceccarelli, E. A. Recombinant protein expression in Escherichia coli: advances and challenges. Frontiers in Microbiology. 5, 172 (2014).
- Rosano, G. L., Morales, E. S., Ceccarelli, E. A. New tools for recombinant protein production in Escherichia coli: A 5-year update. Protein Science: A Publication of the Protein Society. 28 (8), 1412-1422 (2019).

