Robust Ligature-Induced Model of Murine Periodontitis for the Evaluation of Oral Neutrophils
Summary
This article presents a protocol for establishing a ligature-induced model of murine periodontitis involving multiple maxillary molars, resulting in larger areas of the involved gingival tissue and bone for subsequent analysis as well as reduced animal usage. A technique to assess oral neutrophils in a manner analogous to human subjects is also described.
Abstract
The main advantages of studying the pathophysiology of periodontal disease utilizing murine models are the reduced cost of animals, array of genetically modified strains, the vast number of analyses that can be performed on harvested soft and hard tissues. However, many of these systems are subject to procedural criticisms. As an alternative, the ligature-induced model of periodontal disease, driven by the localized development and retention of a dysbiotic oral microbiome, can be employed, which is rapidly induced and relatively reliable. Unfortunately, the variants of ligature-induced murine periodontitis protocol are isolated to focal regions of the periodontium and subject to premature avulsion of the installed ligature. This minimizes the amount of tissue available for subsequent analyses and increases the number of animals required for study. This protocol describes the precise manipulations required to place extended molar ligatures with improved retention and usage of a novel rinse technique to recover oral neutrophils in mice with an alternative approach that mitigates the aforementioned technical challenges.
Introduction
Periodontal disease (PD) is an osteolytic condition associated with significant host morbidity and economic burden, which is manifested by gingival inflammation and loss of both soft tissue attachment and osseous support for the affected dentition1,2,3,4. This process is governed by interactions between the oral microbiota and innate immune system of the host. It is also associated with exacerbation of other systemic inflammatory diseases including diabetes, cardiovascular disease, and cancer5,6,7,8. Historically, it was hypothesized that PD pathogenesis is dependent on large quantities of specific bacteria such as Porphyromonas gingivalis9. However, recent evidence suggests that the microbial component of PD is mediated by the dental biofilm. The biofilm is an organized, complex community of numerous microorganisms that can exist in healthy symbiotic and destructive dysbiotic states10,11. The oral biofilm normally affords resistance to the host by preventing the establishment of foci of pathogenic bacteria and promotes ideal gingival tissue structure and function through regulation of the host immune response12,13. Perturbations of the equilibrious relationship between commensal organisms within the oral cavity and the host immune system may lead to alterations in tissue homeostasis, resulting in dysbacteriosis and development of the hallmark clinical and radiographic appearances of PD5,10,12,13,14.
Interestingly, the establishment of an oral dysbacteriosis, while required for the initiation of PD, is not sufficient to drive PD in all individuals, eluding toward the ability of the host immune response to subvert the transition of microbiota between symbiotic and dysbiotic states15. This places a particular spotlight on the means through which PD influences one of the leading characters of the innate immune system, namely the polymorphonuclear granulocyte (PMN), or neutrophil, from local and systemic perspectives16,17.
In humans, PMNs are recruited from the circulation at a rate of ~2 x 106 cells/h in healthy periodontal connective tissues, where they are the predominating leukocyte population. Here, they are subsequently expelled from the gingival sulcus into the oral cavity as a component of the gingival crevicular fluid. In the presence of PD, neutrophilia manifests within the circulation and oral cavity, where these effector cells possess a hyperinflammatory phenotype that leads to the aforementioned destruction of the periodontium17,18,19,20,21,22. Therefore, understanding the role of PMNs in PD and other systemic inflammatory conditions is of utmost importance.
Although it is widely accepted that chronic diseases are reciprocally linked to PD, the underlying mechanisms have yet to be elucidated, contributing to difficulties in the management of these morbid and potentially fatal systemic conditions. Multiple experimental animal models, each with unique advantages and disadvantages, have been utilized to study the pathophysiology of PD23,24. Focusing specifically on murine models, there are a variety of protocols through which the study of PD is facilitated; however, they possess several technical and physiologic shortcomings25,26,27,28,29,30,31.
First, the oral gavage mouse model requires numerous oral inoculations of human periodontal pathogens to generate gingival inflammation and bone loss. Additionally, it is generally preceded by a period of antibiotic treatment to subvert the murine commensal oral flora25. This model often requires specialized training to safely perform the oral gavage, uses only a small fraction of periodontal pathogens from the more complex human oral microbiome, and requires several months to establish alveolar bone loss.
In contrast, chemically induced murine models utilize the oral delivery of trinitrobenzene sulfonic acid (TNBS) or dextran sulfate sodium (DSS), agents commonly used in establishing murine models of colitis over a period of several months to induce periodontal bone loss26. Intraoral and extraoral abscess-based models are available, which involve the murine incisors and tissues of the dorsum as well as calvarium, respectively. In the former abscess model, several injections of bacteria are administered, creating multiple gingival abscesses and a dearth of alveolar bone loss, limiting their use in the study of PD. The latter abscess models are significantly more apt to studying bacterial virulence, inflammation, and bone resorption at sites outside of the oral cavity, which eliminates evaluation of the periodontium and oral microbiome27,28,29,30,31.
Using the ligature-induced model of periodontitis, a braided silk suture has commonly been installed circumferentially around the second molar. As an alternative, a single linear segment of suture material can be inserted between the first and second molars32,33. The goal of the ligature placement is to facilitate bacterial accumulation and generate dysbiosis within the gingival sulci, resulting in periodontal tissue inflammation and destruction of the tissues composing the periodontium. Most notably, this model is capable of producing significantly more alveolar bone loss compared to the more commonly used oral gavage model34. Further complicating the use of the oral gavage model is the natural resistance by several strains of mice (i.e., C57BL/6) to developing alveolar bone loss. This is also problematic, as this strain is the most frequently used in murine-based animal research35.
Existing procedures described by Marchesan et al. and Abe and Hajishengallis were devised to simplify the technical act of placing the ligature33,36. Unfortunately, the former protocol requires specialized 3D-printed equipment and possess the potential for premature ligature loss, thereby increasing animal use and the costs associated with additional time spent in the operating room. Furthermore, both protocols generate only small regions of the diseased periodontium available for a study.
The advantages that lie with this technique are grounded in the simultaneous study of oral dysbiosis and immunology that govern the periodontium, utilization of low-cost animals with diverse genetic backgrounds, and simple housing and husbandry practices. As such, goals should be to maximize volumes of diseased tissue and, in attempts to practice the principles of reduction in animal research, reduce animal consumption to a level as low as possible. This requires ensuring that all animals are capable of being included in experimental analyses37. However, it should be noted that no matter which animal model of periodontal disease is utilized, there is no single model that encompasses every element of human PD pathophysiology.
This new protocol employs the placement of a ligature around multiple maxillary molar teeth using instrumentation and materials that are found within most laboratories. It allows a sufficient amount of time to easily and confidently install a ligature that is unlikely to avulse prematurely. Finally, as PMNs coordinate destruction of the periodontium in PD, a novel methodology to recover oral neutrophils in a manner analogous to humans is also presented.
Protocol
All murine studies complied with the relevant ethical regulations and were approved by the University of Toronto Animal Care Committee and the Research Ethics Board (Protocol 20011930).
1. Ligature installation
NOTE: This is a non-sterile surgical procedure that can be carried out in a standard operating theater. The use of germ-free animals (not covered here) mandates handling within a biosafety cabinet, use of sterile instruments, and inoculation of the oral cavity with periodontal pathogens to cause the clinical manifestations of periodontitis.
- Administer intraperitoneal anesthesia to 8–12 week-old male C57BL/6 mice, which should be acclimated to their housing facility, using a 0.5" 26 G sterile hypodermic needle and 1 mL syringe according to approved institutional animal care and use committee (IACUC) guidelines.
NOTE: A combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthetics rapidly and reliably induces the appropriate level of anesthesia for the duration of the procedure. - Assess the anesthetic depth prior to and every 15 min during the procedure as evidenced by loss of the pedal reflex.
- Position the mouse on a heated surgical platform (Figure 1A). Stabilize the maxilla and mandible in the open position using elastic bands and prop the neck with a cotton roll to help maintain the maxilla in a more horizontal orientation (Figure 1B). Cover the body and tail of the animal to mitigate heat loss during the procedure.
- Position the surgical microscope at the desired magnification and lighting (if not mounted directly to the microscope) over the oral cavity for visualization of the dentition. While the desired magnification is operator-dependent, optimal visualization of the oral cavity is obtained at 16x.
- Install a sterile 5-0 braided silk suture around the first (M1) and second (M2) maxillary molars within the gingival sulcus using splinter forceps.
NOTE: Braided silk sutures of a smaller gauge can be used for this purpose to facilitate faster installation and reduce the possibility of iatrogenic soft tissue damage.- Position the distal tail of the suture on the palatal side of the dentition and insert the proximal segment between the contact of M2 and M3 (Figure 2A).
- Wrap the suture around the buccal surface of M2 and insert it between the contact of M1 and M2. Ensure that both ends of the suture are pulled tightly to drive the suture into the gingival sulcus and remove all slack (Figure 2B).
- Wrap the proximal suture segment around M1, below its height of contour, and insert it between the contact of M1 and M2. Pull the proximal tail of the suture tightly to drive the suture into the gingival sulcus and remove all slack (Figure 2C).
NOTE: If resistance is observed when inserting the suture between M1 and M2 or M2 and M3, the contact may be open slightly using a standard dental explorer. - Tie the ends of the suture with a surgeon's knot and trim the tails as short as possible. Place the knot in the gingival embrasure between M1 and M2 on the palatal side of the maxillary dentition (Figure 2D).
NOTE: Steps 1.4.1–1.4.4 can be repeated on the contralateral side, if required. - Sterilize the surgical instruments in a hot glass bead sterilizer between each animal subject.
CAUTION: The tips of the forceps are extremely sharp and can easily cause oral trauma and significant bleeding. Prepare small segments of gauze to remove blood from the oral cavity and apply pressure to actively bleeding wounds.
- After the ligature installation, remove the mouse from the surgical apparatus, place into a clean cage under a heat lamp and monitor until fully recovered.
- Individually house each mouse with the appropriate environmental enrichment and allow ad libitum access to filtered water and mashed standard chow in a temperature- and humidity-controlled environment (12-h light/12-h dark cycle) for 7–11 days.
NOTE: Mashed chow decreases the forces required for mastication thereby reducing pain associated with feeding, and aids in preventing premature loss of the installed ligature.
2. Sample collection
- Euthanize mice according to the approved IACUC guidelines.
NOTE: Individual euthanasia using a CO2 chamber followed by cervical dislocation is the preferred method for this protocol. This may be altered depending on experiments that require harvesting of additional tissues. - Using a pipette, immediately rinse the oral cavity with 100 µL of sterilized 4 °C 1x phosphate-buffered saline (PBS) without calcium and magnesium for 10 s.
- Repeat step 2.2 2x and place each rinse in a single 15 mL conical polypropylene sterile test tube.
- Transfer contents to a 50 mL conical polypropylene sterile test tube by running them through a 40 μm nylon mesh filter.
- Transfer these contents into a new 15 mL conical polypropylene sterile test tube.
NOTE: The final transfer of the sample to a smaller tube allows for the improved visualization of the cellular pellet in the upcoming steps.- If desired, remove the molar ligature(s) and place into 300 µL of sterilized 1x PBS (4 °C, without calcium and magnesium) in a separate 15 mL conical polypropylene sterile test tube. Agitate gently and remove the suture from the tube. This sample is treated identically to the oral rinse sample from this point forward.
- Add 33.3 µL of 16% paraformaldehyde (PFA) to facilitate the sample fixation.
- Vortex the samples immediately and incubate on ice for 15 min.
- Fill the tube to 15 mL with 1x PBS to dilute PFA, then centrifuge at 1000 x g and 4 °C for 5 min.
- Aspirate the supernatant and resuspend the pellet in 1 mL of fluorescence-activated cell sorting (FACS) buffer at 4 °C. Count cells on a hemocytometer or automated cell counter.
- Centrifuge the sample again at 1000 RCF and 4 °C for 5 min.
- Aspirate the supernatant and resuspend the pellet in an appropriate volume of FACS buffer for a final concentration of 0.5–1.0 x 106 cells/50 μL of FACS buffer.
3. Antibody staining for flow cytometric analysis
NOTE: Label and chill all required FACS tubes prior to use.
- Add 1 μL of rat serum and 2 μL of anti-mouse IgG antibody to 50 μL of the sample, vortex immediately, and block on ice for 20 min.
- Add the appropriate antibodies to each sample, vortex immediately, and incubate on ice for 30 min in the dark.
NOTE: Selection and volumes of antibodies depend on prior optimization pilot experiments tailored to this specific technique. - Wash samples with 1 mL of FACS buffer, vortexing briefly and centrifuging for 5 min at 1000 x g and 4 °C. Repeat this step 2x.
- Resuspend samples in 250 μL of FACS buffer, cover tubes with paraffin film, wrap in aluminum foil, and hold at 4 °C until analysis.
NOTE: It is ideal to analyze samples within hours of completing the protocol due to the loss of fluorescent signal during long periods of storage. However, samples may be analyzed 2–3 days later if absolutely required.
Representative Results
Representative flow cytometry data from oral rinse samples of a naive (Figure 3A) and inflamed (Figure 3B) murine oral cavity secondary to the ligature-induced periodontitis are provided. Recovery of PMNs from an installed ligature is also demonstrated (Figure 3C). Flow cytometer channel voltages were calibrated manually, and compensation was performed with single-stained compensation beads. PMNs were defined as Ly6G+veF4/80-ve using the outlined gating strategy38. A minimum of 500 gated PMN events were acquired from each oral rinse and ligature sample. PMN viability was determined to be approximately 37% by trypan blue staining. Representative images of alveolar bone levels as measured from the alveolar bone crest (ABC) to the cementoenamel junction (CEJ) for healthy (Figure 4A) and ligated mice (Figure 4B). The relative differences between these measurements (Figure 4C) are provided.
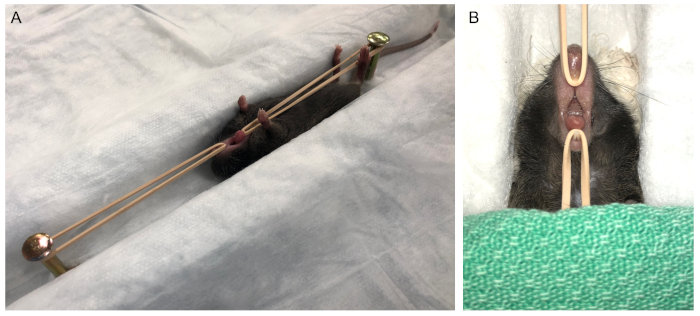
Figure 1: Animal positioning for molar ligation. (A) Access to the oral cavity is achieved by holding the mandible in a depressed position by placing mild traction on the maxillary and mandibular incisor dentition. (B) Gauze is placed under the skull to prevent movement of the head and to place the maxilla in a relatively horizontal position. The body and tail are covered to prevent heat loss prior to moving the surgical stage under the microscope. Please click here to view a larger version of this figure.
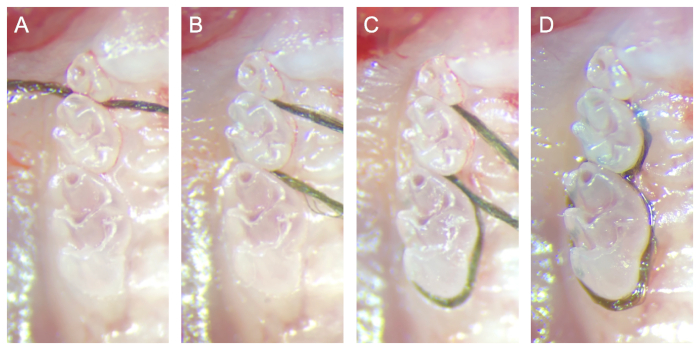
Figure 2: Sequential photograph of ligature installation. (A) The distal tail of the suture is positioned on the palatal side of the dentition, and the proximal segment is inserted between the contact of M2 and M3. (B) The suture is then wrapped around the buccal surface of M2 then inserted between the contact of M1 and M2. (C) The proximal suture segment is wrapped around M1 below its height of contour then inserted between the contact of M1 and M2. (D) The ends of the suture are tied with a surgeon's knot and trimmed as close as possible to the knot. The knot is placed in the gingival embrasure between M1 and M2 on the palatal side of the maxillary dentition. All photographs were acquired on dissected maxilla for recording procedural steps with an unobstructed view of the molar dentition. Please click here to view a larger version of this figure.
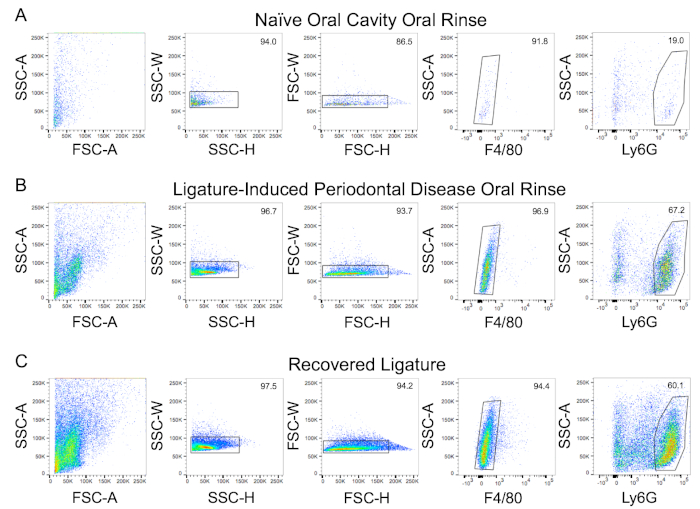
Figure 3: Representative FACS plots and gating strategy demonstrating oral neutrophil presence. Neutrophils were recovered from the following samples and assessed by flow cytometry: (A) oral rinse from a naïve mouse, (B) oral rinse, and (C) recovered ligatures from a mouse with ligature-induced periodontitis (bilaterally). Representative gating strategy scatterplots are shown. Singlets (SSC-H x SSC-W and FSC-H x FSC-W) and neutrophils (Ly6G+ve/F480-ve) were gated as shown. Numerical values reflect the percentage of cells within each gate. SSC-A = side scatter area; SSC-W = side scatter width; SSC-H = side scatter height; FSC-A = forward scatter area; FSC-W = forward scatter width; FSC-H = forward scatter height. Please click here to view a larger version of this figure.
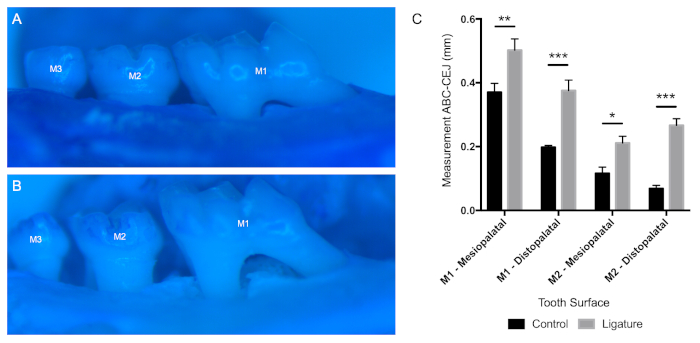
Figure 4: Alveolar bone loss differences between control and ligated animals. Representative photos demonstrating differences in alveolar bone loss between (A) control and (B) ligated mice. (C) Measurements from the cementoenamel junction (CEJ) to alveolar bone crest (ABC), along with the mesiopalatal and distopalatal line angles of M1 and M2, are shown (mean ± SEM, n = 3). P-values were determined by two-way ANOVA with a post-hoc Fisher's LSD test. (*p < 0.01; **p < 0.001; ***p < 0.0001). Please click here to view a larger version of this figure.
Discussion
The most critical element associated with use of the murine ligature-induced model of periodontitis is centered around the retention of the ligature until the time of sacrifice or intentional removal. The installed biofilm-retentive ligature is capable of inducing a significant loss of alveolar bone height in as few as 6 days, plateauing between the 11–16 day period39. The decision to sacrifice animal subjects before the maximal period of bone loss, rendering this a much shorter model of ligature-induced periodontitis, was selected to further reduce the incidence of premature ligature avulsion, defined as the loss of the ligature prior to the time of sacrifice, which renders the animal nondiagnostic.
The most commonly cited disadvantage of this model highlights the perceived technical difficulty of installing the ligature due to the miniscule dimensions of murine molar dentition, which range in circumference from 4.4 mm (M1) to 2.6 mm (M3)40. This issue can be mitigated through the involvement of surgical staff/trainees for ligature installation, which requires (at most) 10 min/animal and includes the delivery of anesthesia. Furthermore, placement of the ligature is a skill mastered through repetition, and it is no more intensive than many common lab-based techniques that require an elevated degree of manual dexterity. From a procedural standpoint, the use of intraperitoneal anesthesia provides extensive time to facilitate ligature installation. If required, it also allows replacement of any ligatures that are not installed in a satisfactory manner, as the localization of the suture to the gingival sulcus and knot between the contact of M1 and M2 is ideal for retention.
The proposed modifications of these protocols, which call for either 1) ligation of a single molar using a circumferential suture or 2) a single linear segment of suture placed between a single contact of the molar dentition, provides several advantages. From a technical perspective, the protocol utilizes equipment that is either readily available or can be constructed from pre-existing equipment in a similar manner to Abe and Hajishengallis. This obviates the need for special orders or manufacturing of equipment by 3D printing33. The installation of a continuous silk suture sling around multiple teeth secured with a surgeon's knot that cannot be disturbed by the tongue may also improve ligature retention.
During the course of this protocol, compared to the technique proposed by Marchesan et al. in which losses of up to 20% are anticipated, there was a complete absence of premature ligature avulsion36. While the incidence of ligature loss was not directly assessed by Abe and Hajishengallis, the technique has been applied in a number of studies41,42,43,44. From a technical perspective, iatrogenic soft tissue damage was avoided in ligated animals. Factors contributing to this phenomenon include a continuous observation of the oral cavity through a microscope, constant visualization of the tips of forceps, and use of clean forceps to prevent slipping off of the suture during placement.
Within the oral cavity, the elongated segments of suture material also dramatically increase the amount of diseased tissue available for analysis and can result in a marked decrease in animal use. This is because all subjects are able to be included in analysis, and pooling of tissue and rinse samples is not required. It should be noted that it is possible to ligate the contralateral maxillary molar dentition utilizing the previously reported techniques to increase the affected tissue area41. However, this modification is not possible in cases where a split-mouth design is required, lending support for the application of this new protocol. Finally, akin to the previous ligature-induced periodontitis protocols, use of this technique is not limited to 8–12 week-old male C57BL/6 mice. Mice of older age, either sex, and various genetic backgrounds may be acceptable depending on experimental design.
Unfortunately, this model still possesses some technical and methodologic faults. The use of specific pathogen-free mice as detailed above cannot mimic the progression of human periodontitis. This is due to several differences between the composition of their commensal oral biofilms, which limits the external validity of evaluating bacteria-bacteria and bacteria-host interactions45. This can theoretically be mitigated through the use of germ-free mice, which complicates the protocol significantly. Additional efforts and resources are required to ensure that animal subjects remain germ-free during the housing and surgical manipulation. Further measures must also be instituted to develop periodontitis, as these mice are resistant to ligature-induced PD in the absence of bacterial accumulation46,47.
Furthermore, mono-infection and co-infection ligature models fall short of recapitulating the human condition, as periodontal disease represents a more complex polymicrobial interaction between the biofilm and host immune system. In these circumstances, elucidating the roles of, and interactions between a limited number of human oral bacteria may be considered deficient and potentially disadvantageous, especially in the context of studying periodontium immunobiology.
Finally, recent evidence has implicated that the forces of mastication are capable of modifying gingival immunosurveillance through the accumulation of T helper 17 cells, an integral mediator of barrier immunity48. As such, the use of a soft diet, especially in older animals, may act a potential confounder. This may reduce the potential for the normal physiologic bone loss in regions where ligatures have been applied. In light of this evidence, careful consideration should be given to the use of aged animals and appropriate age-matched controls where these findings have been noted to be most prominent.
The use of this relatively simple model can be extended to evaluating alveolar bone loss by micro-computed tomography, histologic analyses of both the alveolar bone and surrounding attached gingiva, and conducting oral microbiota characterization, all of which have been accomplished with the existing ligature-induced PD models33,49. Although rarely discussed or utilized, it is also feasible to assess the effects of treatment, (as well as the repair and regeneration of the periodontium) in the setting of ligature-induced murine periodontitis by removing the ligature under general anesthesia with fine-point splinter forceps39. Finally, use of this model may be extended to studying the systemic effects of periodontal disease due to the increased inflammatory load caused by the ligation of more than one tooth, as well as the left and right maxillary molar dentition, if desired.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
J. W. C. is supported by the Canadian Institutes of Health Research (CIHR). The authors would like to thank Dr. Chunxiang Sun for her assistance in performing the trypan blue staining.
Materials
| Anti-mouse F4/80 Antibody | BioLegend | 123131 | BV421, Clone BM8 |
| Anti-mouse Ly6G Antibody | BD | 560602 | PerCP-Cy5.5, Clone 1A8 |
| C57BL/6 Male Mice | Charles River | 8 to 12 weeks old | |
| Conical Centrifuge Tube | FroggaBio | TB15-500 | 15 mL |
| Conical Centrifuge Tube | FroggaBio | TB50-500 | 50 mL |
| FACS Buffer | Multiple | 1% BSA (BioShop), 2mM EDTA (Merck), 1x HBSS-/- (Gibco) | |
| FACSDiva | BD | v8.0.1 | |
| Fibre-Lite | Dolan-Jenner | Model 180 | |
| FlowJo | Tree Star | v10.0.8r1 | |
| Heat Therapy Pump | Hallowell | HTP-1500 | |
| Hot Glass Bead Sterilizer | Electron Microscopy Sciences | 66118-10 | Germinator 500 |
| Iris Scissors | Almedic | 7602-A8-684 | Straight |
| Ketamine | Vetoquinol | 100mg/mL | |
| LSRFortessa | BD | X-20 | |
| Mouse Serum | Sigma | M5905-5ML | |
| Nylon Mesh Filter | Fisher Scientific | 22-363-547 | 40 µm |
| Paraformaldehyde | Fisher Scientific | 28908 | 16% (w/v), Methanol Free |
| Phosphate-buffered Saline | Sigma | D1408-500ML | Without CaCl2 and MgCl2, 10x |
| Plastic Disposable Syringes | BD | 309659 | 1 mL |
| Rat Serum | Sigma | R9759-5ML | |
| Silk Suture | Covidien | SS652 | C13 USP 5-0 |
| Splinter Forceps | Almedic | 7726-A10-700 | #1 |
| Splinter Forceps | Almedic | 7727-A10-704 | #5 |
| Stereo Dissecting Microscope | Carl Zeiss | 28865 | Photo-Zusatz |
| Sterile Hypodemic Needle | BD | 305111 | 26G X 1/2" |
| Syringe | BD | 309659 | 1 mL |
| Xylazine | Rompun | 20mg/mL |
Referenzen
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends in Immunology. 35 (1), 3-11 (2014).
- Pihlstrom, B. L., Michalowicz, B. S., Johnson, N. W. Periodontal diseases. Lancet. 366 (9499), 1809-1820 (2005).
- Richards, D. Oral Diseases affect some 3.9 Billion people. Evidence-Based Dentistry. 14 (2), 35 (2013).
- Listl, S., Galloway, J., Mossey, P. A., Marcenes, W. Global Economic Impact of Dental Diseases. Journal of Dental Research. 94 (10), 1355-1361 (2015).
- Hajishengallis, G. Periodontitis: from microbial immune subversion to systemic inflammation. Nature Reviews Immunology. 15 (1), 30-44 (2015).
- Preshaw, P. M., et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 55 (1), 21-31 (2012).
- Kampits, C., et al. Periodontal disease and inflammatory blood cytokines in patients with stable coronary artery disease. Journal of Applied Oral Sciences. 24 (4), 352-358 (2016).
- Fitzpatrick, S. G., Katz, J. The association between periodontal disease and cancer: A review of the literature. Journal of Dentistry. 38 (2), 83-95 (2010).
- Socransky, S. S., Haffajee, A. D. Periodontal microbial ecology. Periodontology 2000. 38 (1), 135-187 (2005).
- Marsh, P. D. Microbial Ecology of Dental Plaque and its Significance in Health and Disease. Advances in Dental Research. 8 (2), 263-271 (1994).
- Berezow, A. B., Darveau, R. P. Microbial shift and periodontitis. Periodontology 2000. 55 (1), 36-47 (2011).
- Roberts, F. A., Darveau, R. P. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: symbiosis and dysbiosis. Periodontology 2000. 69 (1), 18-27 (2015).
- Macpherson, A. J., Harris, N. L. Interactions between commensal intestinal bacteria and the immune system. Nature Reviews Immunology. 4 (6), 478-485 (2004).
- Hajishengallis, G., et al. Low-Abundance Biofilm Species Orchestrates Inflammatory Periodontal Disease through the Commensal Microbiota and Complement. Cell Host Microbe. 10 (5), 497-506 (2011).
- Löe, H., Anerud, A., Boysen, H., Morrison, E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. Journal of Clinical Periodontology. 13 (5), 431-445 (1986).
- Lakschevitz, F. S., et al. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Experimental Cell Research. 342 (2), 200-209 (2016).
- Fine, N., et al. Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. Journal of Dental Research. 95 (8), 931-938 (2016).
- Landzberg, M., Doering, H., Aboodi, G. M., Tenenbaum, H. C., Glogauer, M. Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease. Journal of Periodontal Research. 50 (3), 330-336 (2015).
- Bender, J. S., Thang, H., Glogauer, M. Novel rinse assay for the quantification of oral neutrophils and the monitoring of chronic periodontal disease. Journal of Periodontal Research. 41 (3), 214-220 (2006).
- Johnstone, A. M., Koh, A., Goldberg, M. B., Glogauer, M. A Hyperactive Neutrophil Phenotype in Patients With Refractory Periodontitis. Journal of Periodontology. 78 (9), 1788-1794 (2007).
- Figueredo, C. M. S., Fischer, R. G., Gustafsson, A. Aberrant Neutrophil Reactions in Periodontitis. Journal of Periodontology. 76 (6), 951-955 (2005).
- Christan, C., Dietrich, T., Hägewald, S., Kage, A., Bernimoulin, J. -. P. White blood cell count in generalized aggressive periodontitis after non-surgical therapy. Journal of Clinical Periodontology. 29 (3), 201-206 (2002).
- Oz, H. S., Puleo, D. A. Animal models for periodontal disease. Journal of Biomedicine and Biotechnology. , 1-8 (2011).
- Struillou, X., Boutigny, H., Soueidan, A., Layrolle, P. Experimental animal models in periodontology: a review. Open Dentistry Journal. 4 (1), 37-47 (2010).
- Baker, P. J., Evans, R. T., Roopenian, D. C. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Archives of Oral Biology. 39 (12), 1035-1040 (1994).
- Oz, H. S., Ebersole, J. L. A novel murine model for chronic inflammatory alveolar bone loss. Journal of Periodontal Research. 45 (1), 94-99 (2010).
- Zubery, Y., et al. Bone resorption caused by three periodontal pathogens in vivo in mice is mediated in part by prostaglandin. Infections and Immunity. 66 (9), 4158-4162 (1998).
- Feuille, F., Ebersole, J. L., Kesavalu, L., Stepfen, M. J., Holt, S. C. Mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a murine lesion model: potential synergistic effects on virulence. Infections and Immunity. 64 (6), 2094-2100 (1996).
- Yoshimura, M., et al. Proteome analysis of Porphyromonas gingivalis cells placed in a subcutaneous chamber of mice. Oral Microbiology and Immunology. 23 (5), 413-418 (2008).
- Kesavalu, L., Ebersole, J. L., Machen, R. L., Holt, S. C. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infections and Immunity. 60 (4), 1455-1464 (1992).
- Liu, P., Haake, S. K., Gallo, R. L., Huang, C. A novel vaccine targeting Fusobacterium nucleatum against abscesses and halitosis. Vaccine. 27 (10), 1589-1595 (2009).
- Jiao, Y., et al. Induction of Bone Loss by Pathobiont-Mediated Nod1 Signaling in the Oral Cavity. Cell Host Microbe. 13 (5), 595-601 (2013).
- Abe, T., Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. Journal of Immunological Methods. 394 (1-2), 49-54 (2013).
- de Molon, R. S., et al. Long-term evaluation of oral gavage with periodontopathogens or ligature induction of experimental periodontal disease in mice. Clinical Oral Investigations. 20 (6), 1203-1216 (2016).
- Baker, P. J., Dixon, M., Roopenian, D. C. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infections and Immunity. 68 (10), 5864-5868 (2000).
- Marchesan, J., et al. An experimental murine model to study periodontitis. Nature Protocols. 13 (10), 2247-2267 (2018).
- Flecknell, P. Replacement, reduction and refinement. ALTEX: Alternatives to Animal Experiments. 19 (2), 73-78 (2002).
- Fine, N., et al. Primed PMNs in healthy mouse and human circulation are first responders during acute inflammation. Blood Advances. 3 (10), 1622-1637 (2019).
- Viniegra, A., et al. Resolving Macrophages Counter Osteolysis by Anabolic Actions on Bone Cells. Journal of Dental Research. 97 (10), 1160-1169 (2018).
- Häärä, O., et al. Ectodysplasin regulates activator-inhibitor balance in murine tooth development through Fgf20 signaling. Development. 139 (17), 3189-3199 (2012).
- Tsukasaki, M., et al. Host defense against oral microbiota by bone-damaging T cells. Nature Communications. 9 (1), 1-11 (2018).
- Hiyari, S., et al. Ligature-induced peri-implantitis and periodontitis in mice. Journal of Clinical Periodontology. 45 (1), 89-99 (2018).
- Eskan, M. A., et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nature Immunology. 13 (5), 465-473 (2012).
- Dutzan, N., et al. A dysbiotic microbiome triggers T H 17 cells to mediate oral mucosal immunopathology in mice and humans. Science Translational Medicine. 10 (463), 1-12 (2018).
- Chun, J., Kim, K. Y., Lee, J., Choi, Y. The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiology. 10 (1), 1-8 (2010).
- Rovin, S., Costich, E. R., Gordon, H. A. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. Journal of Periodontal Research. 1 (3), 193-204 (1966).
- Martín, R., Bermúdez-Humarán, L. G., Langella, P. Gnotobiotic Rodents: An In Vivo Model for the Study of Microbe-Microbe Interactions. Frontiers in Microbiology. 7, 1-7 (2016).
- Dutzan, N., et al. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity. 46 (1), 133-147 (2017).
- Sima, C., et al. Nuclear Factor Erythroid 2-Related Factor 2 Down-Regulation in Oral Neutrophils Is Associated with Periodontal Oxidative Damage and Severe Chronic Periodontitis. The American Journal of Pathology. 186 (6), 1417-1426 (2016).

