模拟早期椎间盘疾病的发炎性、退行性器官培养模型。
Summary
该协议提出了一种新的实验模型的发炎,退化牛器官培养,以模拟早期椎间盘退化。
Abstract
症状性椎间盘 (IVD) 退化 (IDD) 是一个主要的社会经济负担,其特征是炎症和组织退化。由于缺乏因果疗法,迫切需要创新的实验器官培养模型,以研究疾病进展中涉及的机制,寻找治疗目标,减少对动物模型的需求。我们在这里展示一个新的,三维的器官培养模型协议,模仿发炎和催化微环境,这是在DD期间存在。
最初,牛的静脉注射器在组织培养介质中被解剖、清洁和培养。动态生理或病理负荷应用于定制生物反应器,每天2小时。IVDs被分配给一个对照组(高葡萄糖介质,生理负荷,磷酸盐缓冲盐水注射)和病理组(低葡萄糖介质,病理负荷,肿瘤坏死因子-阿尔法注射)四天。对IVD采集的细胞核细胞进行基因表达分析,对条件器官培养介质进行酶相关免疫分析。
我们的数据表明,与对照组相比,在病理组加载后,炎症标记的表达更高,圆盘高度降低。此协议是可靠的模拟IVD炎症和退化,可以进一步扩大其应用范围。
Introduction
背痛 (LBP) 可以影响所有年龄段的个人,是全世界残疾的主要原因1,2,3.与LBP相关的总成本每年超过1000亿美元4,5。症状性椎间盘 (IVD) 退化 (IDD), 一种以炎症和组织退化为特征的疾病, 是 LBP6,7的主要原因 .具体来说,IDD 的特点是 IVD 的细胞外基质 (ECM) 逐渐分解,由导致加速病理学、神经紊乱并最终残疾的多种因素诱导和触发。此外,IDD与释放发炎细胞因子、改变脊柱生物力学、血管生成和神经内生有关,这增加了疼痛感,共引起慢性LBP(主动不和谐)6,8。迄今为止,治疗方案包括切除术和随后融合相邻的椎骨,植入IVD假肢,或非手术方法,如非类固醇抗炎药物,阿片类药物,和肌肉松弛剂的IDD9患者。目前的标准治疗方案,外科和非手术,只是部分有效,未能解决潜在的生物学问题9,10。早期退行性椎间盘疾病的特点是初始炎症组织反应,特别是肿瘤坏死因子-阿尔法(TNF-阿尔法)表达11的增加。这些早期的椎间盘变化主要发生在细胞水平,而不会破坏光盘架构,以前可以模仿营养缺乏在亲炎症条件下12。因此,精确模拟体内情况,以调查这些退化机制,并找到合适的治疗目标至关重要。此外,在这些分子特性的模拟中,光盘的机械加载环境在IVD的病理和生理变化中起着关键作用。因此,结合这些方法将使我们向前迈出一步,以模拟体内静脉注射器的复杂微环境。目前没有研究考虑动态加载的方面,以及亲炎症和营养设置,以最好的我们所知。
虽然大型动物模型允许调查潜在的相关体内相互作用,他们是昂贵和工作密集型。此外,由于动物模型在研究中的使用长期以来一直是一个有争议的问题,因此减少回答重要研究问题所需的动物数量是人们非常感兴趣的。最后,目前还没有理想的动物模型模仿IDD在IVD研究13,14。因此,有必要建立一个具有成本效益和可靠的替代方法,例如一种器官培养模型,以模拟 IDD 以及相关的炎症和退行性过程。最近,本议定书的应用,建立一个发炎和退行性器官培养模型,以模拟早期椎间盘疾病,使我们能够研究抗炎药物在DD器官培养15的影响。
在这里,我们描述了如何获得牛椎间盘,并通过直接 α在低营养介质条件下在生物反应器中产生催化和增生微环境诱导早期 IDD 状态。 图1 说明了实验模型,并显示了用于模拟退行性和生理负荷条件的生物反应器。
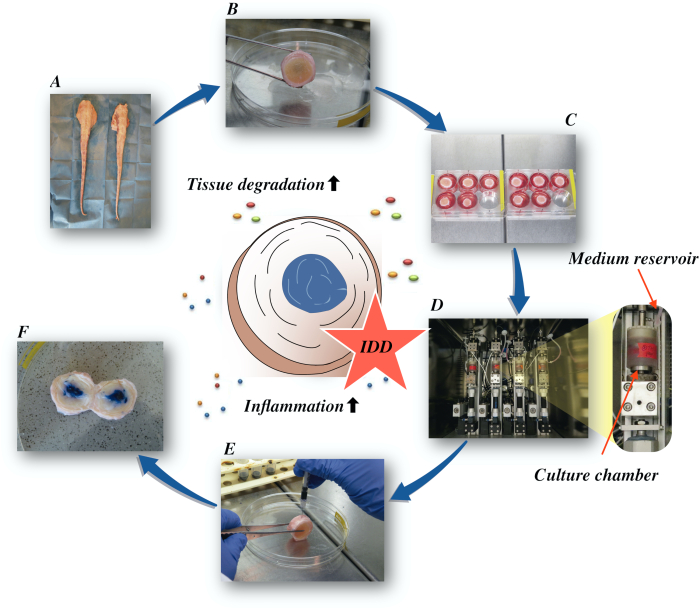
图1:实验设置的插图。A:牛尾巴: B:解剖牛间椎间盘: C:将光盘转移到具有文化媒介的井板: D:在生物反应器中加载模拟: E:静脉注射技术: F: 注射PBS/三潘蓝色染料后IVD显示分布。IDD:椎间盘退化。 请单击此处查看此图的较大版本。
Protocol
Representative Results
Discussion
我们在这里提供了一个详细的协议,以模拟退行性和炎症性IVDD。此协议可用于对导致对光盘的破坏性影响的炎症通路进行详细检查。此外,该协议可以帮助确定在疾病进展中涉及的有希望的治疗目标。
我们最近发现,人类重组TNF-α可诱发牛和人类NP细胞21的炎症,这是根据该领域的其他研究证实,TNF-α可用于炎症模拟IVD细胞22,23。…
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
这项工作得到了 AO 基金会和 AO 斯平国际的支持。巴巴克·萨拉维得到了德国脊柱基金会和德国骨关节炎基金会的奖学金支持。格诺特·朗得到了德国弗赖堡大学医学院高级临床科学家贝尔塔-奥滕斯坦方案的支持。
Materials
| 1-Bromo-3-chloropropane(BCP) | Sigma-Aldrich, St. Louis, USA | B9673 | |
| Ascorbate-2-phosphate | Sigma-Aldrich, St. Louis, USA | A8960 | |
| Band saw | Exakt Apparatebau, Norderstedt, Germany | model 30/833 | |
| Betadine | Munndipharma, Frankfurt, Germany | ||
| Bovine IL-8 Do.it-Yourself ELISA | Kingfisher Biotech, St. Paul, USA | DIY1028B-003 | |
| Corning ITS Premix | Corning Inc., New York, USA | 354350 | |
| DMEM high glucose | Gibco by life technologies, Carlsbad, USA | 10741574 | |
| DMEM low glucose | Gibco by life technologies, Carlsbad, USA | 11564446 | |
| Ethanol for molecular biology | Sigma-Aldrich, St. Louis, USA | 09-0851 | |
| Fetal Bovine Serum (FBS) | Gibco by life technologies, Carlsbad, USA | A4766801 | |
| Non-essential amino acid solution | Gibco by life technologies, Carlsbad, USA | 11140050 | |
| Penicillin/Streptomycin(P/S) | gibco by life technologies, Carlsbad, USA | 11548876 | |
| Phosphate Buffer Solution, tablet | Sigma-Aldrich, St. Louis, USA | P4417 | |
| Pronase | Sigma-Aldrich, St. Louis, USA | 10165921001 | |
| Primocin | InvivoGen, Sandiego, USA | ant-pm-05 | |
| Pulsavac Jet Lavage System | Zimmer, IN,USA | ||
| TissueLyser II | Quiagen, Venlo, Netherlands | 85300 | |
| Streptavidinn-HRP | Kingfisher Biotech, St. Paul, USA | AR0068-001 | |
| Superscript VILO | Invitrogen by life Technologies, Carlsbad, USA | 10704274 | |
| cDNA Synthesis Kit | Applied Biosystems by life technologies | 10400745 | |
| TaqMan Universal Master Mix | Applied Biosystems by life technologies | ||
| TNF-alpha, recombinant human protein | R&D systems, Minnesota, USA | 210-TA-005 | |
| TRI Reagent | Molecular Research Center, Cincinnati, USA | TR 118 | |
| Tris-EDTA buffer solution | sigma-Aldrich, St. Louis, USA | 93283 | |
| Gene bIL-6 | Applied Biosystems by life technologies | Custom made probes | Primer fw (5′–3′) TTC CAA AAA TGG AGG AAA AGG A Primer rev (5′–3′) TCC AGA AGA CCA GCA GTG GTT Probe (5′FAM/3′TAMRA) CTT CCA ATC TGG GTT CAA TCA GGC GATT |
| Gene bIL8 | Applied Biosystems by life technologies | Bt03211906_m1 | |
| Gene bTNF-alpha | Applied Biosystems by life technologies | Custom made probes | Primer fw (5′–3′) CCT CTT CTC AAG CCT CAA GTA ACA A Primer rev (5′–3′) GAG CTG CCC CGG AGA GTT Probe (5′FAM/3′TAMRA) ATG TCG GCT ACA ACG TGG GCT ACC G |
| GENE bIL1beta | Applied Biosystems by life technologies | Custom made probes | Primer fw (5′–3′) TTA CTA CAG TGA CGA GAA TGA GCT GTT Primer rev (5′–3′) GGT CCA GGT GTT GGA TGC A Probe (5′FAM/3′TAMRA) CTC TTC ATC TGT TTA GGG TCA TCA GCC TCA A |
| RPLP0 | Applied Biosystems by life technologies | Bt03218086_m1 |
Referencias
- Vos, T., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 390 (10100), 1211-1259 (2017).
- Hoy, D., et al. Measuring the global burden of low back pain. Best Practice & Research Clinical Rheumatology. 24 (2), 155-165 (2010).
- Thiese, M. S., et al. Prevalence of low back pain by anatomic location and intensity in an occupational population. BMC Musculoskeletal Disorders. 15 (1), 283 (2014).
- Katz, J. N. Lumbar Disc Disorders and Low-Back Pain: Socioeconomic Factors and Consequences. The Journal of Bone and Joint Surgery (American). 88, 21 (2006).
- Vlaeyen, J. W. S., et al. Low back pain. Nature Reviews Disease Primers. 4 (1), 52 (2018).
- Khan, A. N., et al. Inflammatory biomarkers of low back pain and disc degeneration: a review: Biomarkers of disc degeneration and back pain. Annals of the New York Academy of Sciences. 1410 (1), 68-84 (2017).
- Kim, H. S., Wu, P. H., Jang, I. T. Lumbar Degenerative Disease Part 1: Anatomy and Pathophysiology of Intervertebral Discogenic Pain and Radiofrequency Ablation of Basivertebral and Sinuvertebral Nerve Treatment for Chronic Discogenic Back Pain: A Prospective Case Series and Review of Literature. International Journal of Molecular Sciences. 21 (4), 1483 (2020).
- Adams, M. A., Roughley, P. J. What is Intervertebral Disc Degeneration, and What Causes It. Spine. 31 (18), 2151-2161 (2006).
- Wu, P. H., Kim, H. S., Jang, I. T. Intervertebral Disc Diseases Part 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease. International Journal of Molecular Sciences. 21 (6), 2135 (2020).
- Lurie, J. D., et al. Surgical Versus Nonoperative Treatment for Lumbar Disc Herniation: Eight-Year Results for the Spine Patient Outcomes Research Trial. Spine. 39 (1), 3-16 (2014).
- Risbud, M. V., Shapiro, I. M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nature Reviews Rheumatology. 10 (1), 44-56 (2014).
- Ponnappan, R. K., et al. An organ culture system to model early degenerative changes of the intervertebral disc. Arthritis Research & Therapy. 13 (5), 171 (2011).
- O’Connell, G. D., Vresilovic, E. J., Elliott, D. M. Comparison of Animals Used in Disc Research to Human Lumbar Disc Geometry. Spine. 32 (3), 328-333 (2007).
- Stannard, J. T., et al. Development of a whole organ culture model for intervertebral disc disease. Journal of Orthopaedic Translation. 5, 1-8 (2016).
- Li, Z., et al. Preclinical ex-vivo Testing of Anti-inflammatory Drugs in a Bovine Intervertebral Degenerative Disc Model. Frontiers in Bioengineering and Biotechnology. 8, 583 (2020).
- Li, Z., et al. Development of an ex vivo cavity model to study repair strategies in loaded intervertebral discs. European Spine Journal. 25 (9), 2898-2908 (2016).
- Kazezian, Z., Li, Z., Alini, M., Grad, S., Pandit, A. Injectable hyaluronic acid down-regulates interferon signaling molecules, IGFBP3 and IFIT3 in the bovine intervertebral disc. Acta Biomaterialia. 52, 118-129 (2017).
- Caprez, S., Menzel, U., Li, Z., Grad, S., Alini, M., Peroglio, M. Isolation of high-quality RNA from intervertebral disc tissue via pronase predigestion and tissue pulverization. JOR Spine. 1 (2), 1017 (2018).
- Lopa, S., Ceriani, C., Cecchinato, R., Zagra, L., Moretti, M., Colombini, A. Stability of housekeeping genes in human intervertebral disc, endplate and articular cartilage cells in multiple conditions for reliable transcriptional analysis. European Cells & Materials. 31, 395-406 (2016).
- Lang, G., et al. An intervertebral disc whole organ culture system to investigate proinflammatory and degenerative disc disease condition. Journal of Tissue Engineering and Regenerative Medicine. 12 (4), 2051-2061 (2018).
- Du, J., et al. Proinflammatory intervertebral disc cell and organ culture models induced by tumor necrosis factor alpha. JOR Spine. 3, 1104 (2020).
- Purmessur, D., Walter, B. A., Roughley, P. J., Laudier, D. M., Hecht, A. C., Iatridis, J. A role for TNFα in intervertebral disc degeneration: A non-recoverable catabolic shift. Biochemical and Biophysical Research Communications. 433 (1), 151-156 (2013).
- Walter, B. A., Likhitpanichkul, M., Illien-Junger, S., Roughley, P. J., Hecht, A. C., Iatridis, J. C. TNFα Transport Induced by Dynamic Loading Alters Biomechanics of Intact Intervertebral Discs. PLOS One. 10 (3), 0118358 (2015).
- Gullbrand, S. E., et al. A large animal model that recapitulates the spectrum of human intervertebral disc degeneration. Osteoarthritis and Cartilage. 25 (1), 146-156 (2017).
- Willems, N., et al. Safety of intradiscal injection and biocompatibility of polyester amide microspheres in a canine model predisposed to intervertebral disc degeneration: intradiscal application of pea microspheres. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 105 (4), 707-714 (2017).
- Michalek, A. J., Buckley, M. R., Bonassar, L. J., Cohen, I., Iatridis, J. C. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. The Spine Journal. 10 (12), 1098-1105 (2010).
- Illien-Jünger, S., et al. The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine. 35 (19), 1744-1752 (2010).
- Gantenbein, B., et al. Organ culture bioreactors–platforms to study human intervertebral disc degeneration and regenerative therapy. Current Stem Cell Research & Therapy. 10 (4), 339-352 (2015).
- Boubriak, O. A., Watson, N., Sivan, S. S., Stubbens, N., Urban, J. P. G. Factors regulating viable cell density in the intervertebral disc: blood supply in relation to disc height. Journal of Anatomy. 222 (3), 341-348 (2013).
- Maroudas, A., Stockwell, R. A., Nachemson, A., Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. Journal of Anatomy. 120, 113-130 (1975).
- Beckstein, J. C., Sen, S., Schaer, T. P., Vresilovic, E. J., Elliott, D. M. Comparison of Animal Discs Used in Disc Research to Human Lumbar Disc: Axial Compression Mechanics and Glycosaminoglycan Content. Spine. 33 (6), 166-173 (2008).
- Walter, B. A., Illien-Jünger, S., Nasser, P. R., Hecht, A. C., Iatridis, J. C. Development and validation of a bioreactor system for dynamic loading and mechanical characterization of whole human intervertebral discs in organ culture. Journal of Biomechanics. 47 (9), 2095-2101 (2014).
- Rajan, N. E., et al. Toll-Like Receptor 4 (TLR4) Expression and Stimulation in a Model of Intervertebral Disc Inflammation and Degeneration. Spine. 38 (16), 1343-1351 (2013).
- vanden Akker, G. G., Rorije, A. J., Davidson, E. N. B., vander Kraan, P. M. Phenotypic marker genes distinguish inner and outer annulus fibrosus from nucleus pulposus tissue in the bovine intervertebral disc. Osteoarthritis and Cartilage. 25, 402 (2017).
- Du, J., et al. Functional cell phenotype induction with TGF-β1 and collagen-polyurethane scaffold for annulus fibrosus rupture repair. European Cells & Materials. 39, 1-17 (2020).
- Risbud, M. V., et al. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 33 (3), 283-293 (2015).

