分离近端液研究胰腺腺癌肿瘤微环境
Summary
胰液是人类胰腺癌生物标志物的宝贵来源。我们在这里描述一种术中收集程序的方法。为了克服在小鼠模型中采用该程序的挑战,我们建议使用替代样品,肿瘤组织液,并在此处描述其分离的两种方案。
Abstract
胰腺腺癌(PDAC)是癌症相关死亡的第四大原因,很快就会成为第二大原因。迫切需要与特定胰腺病变相关的变量,以帮助术前鉴别诊断和患者分析。胰液是一种相对未开发的体液,由于其靠近肿瘤部位,它反映了周围组织的变化。在这里,我们详细描述了术中收集程序。不幸的是,将胰液收集转化为PDAC的小鼠模型以进行机理研究在技术上非常具有挑战性。肿瘤间质液(TIF)是血液和血浆外的细胞外液,用于沐浴肿瘤和基质细胞。与胰液类似,由于其收集和浓缩在血浆中稀释的分子的特性,TIF可以用作微环境改变的指标和疾病相关生物标志物的宝贵来源。由于TIF不易获得,因此提出了各种技术来隔离TIF。我们在这里描述了两种简单且技术要求不高的分离方法:组织离心和组织洗脱。
Introduction
胰腺导管腺癌(PDAC)是最具侵袭性的肿瘤之一,很快就会成为第二大死因1,2,3。它以其免疫抑制微环境和对免疫治疗方案无反应而闻名4。目前,手术切除仍然是PDAC的唯一治愈选择,但早期复发和术后并发症的频率很高。直到晚期才有特定症状,无法进行早期诊断,从而导致疾病的最后期限。此外,PDAC与其他良性胰腺病变之间的症状重叠会阻碍使用当前的诊断策略实现及时可靠的诊断。识别与特定胰腺病变相关的变量可以促进手术决策过程并改善患者分析。
使用易于获取的体液(例如血液5,6,7,尿液8,唾液9和胰液10,11,12)在生物标志物发现方面取得了有希望的结果。许多研究利用全面的“组学”方法,如基因组学、蛋白质组学和代谢组学技术,来识别可以区分PDAC和其他良性胰腺疾病的候选分子或特征。我们最近证明,胰液是一种相对未开发的体液,可用于识别具有不同临床特征的患者的代谢特征12。胰液是一种富含蛋白质的液体,它积累胰腺导管细胞的分泌组并流向主胰管,然后流向主胆总管。由于它靠近胰腺,它可能受到肿瘤肿块引起的微环境扰动的强烈影响(图1),因此比血液或尿液或基于组织的分析更具信息性。一些研究已经探索了胰液使用各种方法鉴定疾病新型生物标志物的潜力,包括细胞学分析13,质谱分析14,15,评估遗传和表观遗传标志物,如K-ras和p53突变16,17,DNA甲基化改变18和miRNA19.从技术上讲,胰液可以通过术中或微创手术收集,例如内窥镜超声、逆行胰胆管造影或通过内镜收集十二指肠液分泌物20。目前尚不清楚所使用的收集技术在多大程度上影响胰液组成。我们在这里描述了术中收集程序,并表明胰液可以代表PDAC生物标志物的宝贵来源。
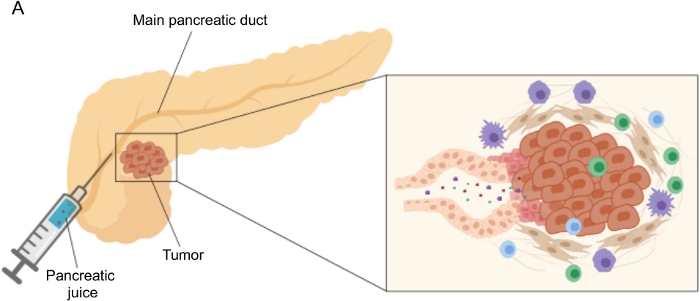
图1:胰液收集的示意图。 (A)描述胰液分泌到胰管中的示意图,并在手术过程中收集胰液。插图显示了肿瘤微环境的特写:胰液收集胰管中肿瘤和基质细胞释放的分子。 请点击此处查看此图的大图。
在PDAC的遗传和原位小鼠模型中收集胰液将受到赞赏,以便在临床前机制研究中利用这种生物流体;然而,这种手术在技术上可能非常具有挑战性,对于皮下肿瘤等更简单的模型是不可行的。出于这个原因,我们将肿瘤间质液(TIF)确定为胰液的替代来源,因为它具有作为周围扰动指标的相似特征。间质液(IF)是在血液和淋巴管外发现的细胞外液体,可沐浴组织细胞21。IF组成受器官血液循环和局部分泌的影响;事实上,周围细胞在IF21中积极产生和分泌蛋白质。间质反映了周围组织的微环境变化,因此可以代表在几种病理环境中发现生物标志物的宝贵来源,例如肿瘤。TIF中高浓度的局部分泌蛋白可用于鉴定要作为血浆22,23,24中的预后或诊断生物标志物进行测试的候选分子。一些研究已经证明TIF是高通量蛋白质组学方法的合适样品,例如质谱技术23,24,25,多重ELISA方法26和microRNA分析27。
已经提出了几种用于分离肿瘤中IF的方法,其大致可分为体内(毛细管超滤28,29,30,31和微透析32,33,34,35)和离体方法(组织离心22,36,37,38和组织洗脱39,40,41,42)。这些技术已经进行了广泛的详细审查43,44。适当方法的选择应考虑下游分析和应用以及回收量等问题。我们最近使用这种方法作为原理证明,以证明来自两种小鼠胰腺腺癌细胞系的肿瘤的不同代谢活性12。基于文献24,38,我们选择使用低速离心方法,以避免细胞破裂和细胞内内容物稀释。TIF中葡萄糖和乳酸的含量都反映了两种不同细胞系的不同糖酵解特性。在这里,我们详细描述了两种最常用的TIF分离方法的方案:组织离心和组织洗脱(图2)。
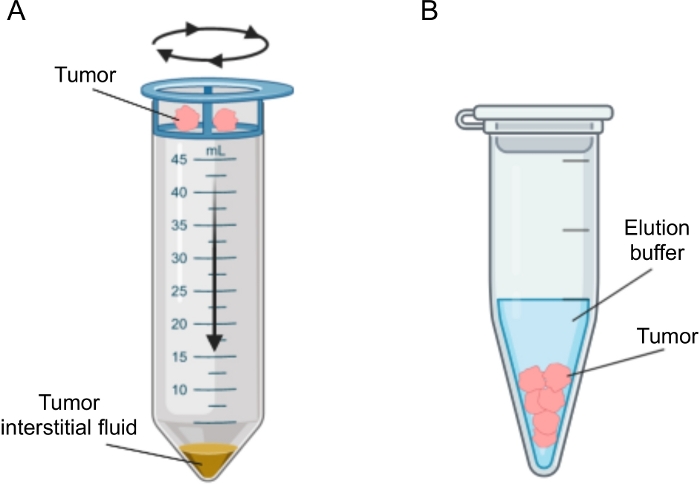
图2:肿瘤间质液分离方法的示意图。 方案中详细描述的技术的示意图,即组织离心(A)和组织洗脱(B)。 请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
在这项研究中,我们描述了术中收集胰液的技术,这是一种在很大程度上未开发的液体活检。我们最近表明,胰液可以用作疾病12代谢标志物的来源。对其他液体活检(如血液5、6、7、尿液8 和唾液9)的代谢组学分析在区分 PDAC 与健康受试者或胰腺炎方面显示出有希望的结?…
Divulgations
The authors have nothing to disclose.
Acknowledgements
我们感谢罗伯塔·米廖雷的技术援助。导致这些结果的研究已获得意大利人协会(AIRC)在IG2016-ID.18443项目下的资助 – P.I. Marchesi Federica。资助者在研究设计、数据收集和分析、发表决定或手稿准备方面没有任何作用。
Materials
| 1 mL syringe | BD Biosciences | 309659 | |
| 1.5 mL Eppendorf tube | Greiner BioOne | GR616201 | |
| 20 µm nylon cell strainer | pluriSelect | 43-50020-03 | |
| 25G needle | BD Biosciences | 305122 | |
| 3 mL K2EDTA vacutainer | BD Biosciences | 366473 | |
| 3 mL syringe | BD Biosciences | 309656 | |
| 50 mL Falcon tube | Corning | 352098 | |
| Clamps | Medicon | 06.20.12 | |
| Disposable scalpel | Medicom | 9000-10 | |
| Fetal bovine serum | Microtech | MG10432 | |
| Flat-tipped forceps | Medicon | 06.00.10 | |
| Penicillin-Streptomycin | Lonza | ECB3001D | |
| Phosphate-Buffered Saline (PBS) | Sigma-Aldrich | D8537 | |
| Protease inhibitor cocktail | Roche | 34044100 | |
| RPMI medium | Euroclone | ECB9006L | |
| Scissors | Medicon | 02.04.09 | |
| Trypsin/EDTA 1x | Lonza | BE17-161F | |
| Ultraglutamine 100x | Lonza | BE17-605E/U1 |
References
- Costello, E., Greenhalf, W., Neoptolemos, J. P. New biomarkers and targets in pancreatic cancer and their application to treatment. Nature Reviews Gastroenterology & Hepatology. 9 (8), 435-444 (2012).
- Siegel, R. L., Miller, K. D., Jemal, A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians. 70 (1), 7-30 (2020).
- Neoptolemos, J. P., et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nature Reviews Gastroenterology & Hepatology. 15 (6), 333-348 (2018).
- Sahin, I. H., Askan, G., Hu, Z. I., O’Reilly, E. M. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity. Annals of Oncology. 28 (12), 2950-2961 (2017).
- Mayerle, J., et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut. 67 (1), 128-137 (2018).
- Bathe, O. F., et al. Feasibility of identifying pancreatic cancer based on serum metabolomics. Cancer Epidemiology, Biomarkers & Prevention. 20 (1), 140-147 (2011).
- Mayers, J. R., et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nature Medicine. 20 (10), 1193-1198 (2014).
- Napoli, C., et al. Urine metabolic signature of pancreatic ductal adenocarcinoma by (1)h nuclear magnetic resonance: identification, mapping, and evolution. Journal of Proteome Research. 11 (1), 1274-1283 (2012).
- Sugimoto, M., Wong, D. T., Hirayama, A., Soga, T., Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 6 (1), 78-95 (2010).
- Chen, R., et al. Comparison of pancreas juice proteins from cancer versus pancreatitis using quantitative proteomic analysis. Pancreas. 34 (1), 70-79 (2007).
- Mori, Y., et al. A minimally invasive and simple screening test for detection of pancreatic ductal adenocarcinoma using biomarkers in duodenal juice. Pancreas. 42 (2), 187-192 (2013).
- Cortese, N., et al. Metabolome of Pancreatic Juice Delineates Distinct Clinical Profiles of Pancreatic Cancer and Reveals a Link between Glucose Metabolism and PD-1+ Cells. Cancer Immunology Research. , (2020).
- Tanaka, M., et al. Cytologic Analysis of Pancreatic Juice Increases Specificity of Detection of Malignant IPMN-A Systematic Review. Clinical Gastroenterology and Hepatology. 17 (11), 2199-2211 (2019).
- Chen, K. T., et al. Potential prognostic biomarkers of pancreatic cancer. Pancreas. 43 (1), 22-27 (2014).
- Tian, M., et al. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 8, 241 (2008).
- Shi, C., et al. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biology & Therapy. 7 (3), 353-360 (2008).
- Rogers, C. D., et al. Differentiating pancreatic lesions by microarray and QPCR analysis of pancreatic juice RNAs. Cancer Biology & Therapy. 5 (10), 1383-1389 (2006).
- Matsubayashi, H., et al. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Recherche en cancérologie. 66 (2), 1208-1217 (2006).
- Cote, G. A., et al. A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile. The American Journal of Gastroenterology. 109 (12), 1942-1952 (2014).
- Yu, J., et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. , (2016).
- Wiig, H., Swartz, M. A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiological Reviews. 92 (3), 1005-1060 (2012).
- Haslene-Hox, H., et al. A new method for isolation of interstitial fluid from human solid tumors applied to proteomic analysis of ovarian carcinoma tissue. PLoS One. 6 (4), 19217 (2011).
- Zhang, J., et al. In-depth proteomic analysis of tissue interstitial fluid for hepatocellular carcinoma serum biomarker discovery. British Journal of Cancer. 117 (11), 1676-1684 (2017).
- Sullivan, M. R., et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife. 8, (2019).
- Matas-Nadal, C., et al. Evaluation of Tumor Interstitial Fluid-Extraction Methods for Proteome Analysis: Comparison of Biopsy Elution versus Centrifugation. Journal of Proteome Research. 19 (7), 2598-2605 (2020).
- Espinoza, J. A., et al. Cytokine profiling of tumor interstitial fluid of the breast and its relationship with lymphocyte infiltration and clinicopathological characteristics. Oncoimmunology. 5 (12), 1248015 (2016).
- Halvorsen, A. R., et al. Profiling of microRNAs in tumor interstitial fluid of breast tumors – a novel resource to identify biomarkers for prognostic classification and detection of cancer. Molecular Oncology. 11 (2), 220-234 (2017).
- Yang, S., Huang, C. M. Recent advances in protein profiling of tissues and tissue fluids. Expert Review of Proteomics. 4, 515-529 (2007).
- Huang, C. M., et al. Mass spectrometric proteomics profiles of in vivo tumor secretomes: capillary ultrafiltration sampling of regressive tumor masses. Proteomics. 6 (22), 6107-6116 (2006).
- Leegsma-Vogt, G., Janle, E., Ash, S. R., Venema, K., Korf, J. Utilization of in vivo ultrafiltration in biomedical research and clinical applications. Life Sciences. 73 (16), 2005-2018 (2003).
- Schneiderheinze, J. M., Hogan, B. L. Selective in vivo and in vitro sampling of proteins using miniature ultrafiltration sampling probes. Analytical Chemistry. 68 (21), 3758-3762 (1996).
- Hardt, M., Lam, D. K., Dolan, J. C., Schmidt, B. L. Surveying proteolytic processes in human cancer microenvironments by microdialysis and activity-based mass spectrometry. Proteomics Clinical Applications. 5 (11-12), 636-643 (2011).
- Xu, B. J., et al. Microdialysis combined with proteomics for protein identification in breast tumor microenvironment in vivo. Cancer Microenvironment. 4 (1), 61-71 (2010).
- Bendrik, C., Dabrosin, C. Estradiol increases IL-8 secretion of normal human breast tissue and breast cancer in vivo. The Journal of Immunology. 182 (1), 371-378 (2009).
- Ao, X., Stenken, J. A. Microdialysis sampling of cytokines. Methods. 38 (4), 331-341 (2006).
- Ho, P. C., et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 162 (6), 1217-1228 (2015).
- Choi, J., et al. Intraperitoneal immunotherapy for metastatic ovarian carcinoma: Resistance of intratumoral collagen to antibody penetration. Clinical Cancer Research. 12 (6), 1906-1912 (2006).
- Wiig, H., Aukland, K., Tenstad, O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. The American Journal of Physiology-Heart and Circulatory Physiology. 284 (1), 416-424 (2003).
- Li, S., Wang, R., Zhang, M., Wang, L., Cheng, S. Proteomic analysis of non-small cell lung cancer tissue interstitial fluids. World Journal of Surgical Oncology. 11, 173 (2013).
- Fijneman, R. J., et al. Proximal fluid proteome profiling of mouse colon tumors reveals biomarkers for early diagnosis of human colorectal cancer. Clinical Cancer Research. 18 (9), 2613-2624 (2012).
- Teng, P. N., Hood, B. L., Sun, M., Dhir, R., Conrads, T. P. Differential proteomic analysis of renal cell carcinoma tissue interstitial fluid. Journal of Proteome Research. 10 (3), 1333-1342 (2011).
- Turtoi, A., et al. Novel comprehensive approach for accessible biomarker identification and absolute quantification from precious human tissues. Journal of Proteome Research. 10 (7), 3160-3182 (2011).
- Wagner, M., Wiig, H. Tumor Interstitial Fluid Formation, Characterization, and Clinical Implications. Frontiers in Oncology. 5, 115 (2015).
- Haslene-Hox, H., Tenstad, O., Wiig, H. Interstitial fluid-a reflection of the tumor cell microenvironment and secretome. Biochimica Biophysica Acta. 1834 (11), 2336-2346 (2013).
- Hsieh, S. Y., et al. Secreted ERBB3 isoforms are serum markers for early hepatoma in patients with chronic hepatitis and cirrhosis. Journal of Proteome Research. 10, 4715-4724 (2011).
- Sun, W., et al. Characterization of the liver tissue interstitial fluid (TIF) proteome indicates potential for application in liver disease biomarker discovery. Journal of Proteome Research. 9 (2), 1020-1031 (2010).
- Haslene-Hox, H., et al. Increased WD-repeat containing protein 1 in interstitial fluid from ovarian carcinomas shown by comparative proteomic analysis of malignant and healthy gynecological tissue. Biochimica Biophysica Acta. 1834 (11), 2347-2359 (2013).
- Wang, T. H., et al. Stress-induced phosphoprotein 1 as a secreted biomarker for human ovarian cancer promotes cancer cell proliferation. Molecular & Cellular Proteomics. 9, 1873-1884 (2010).
- Gromov, P., et al. Up-regulated proteins in the fluid bathing the tumour cell microenvironment as potential serological markers for early detection of cancer of the breast. Molecular Oncology. 4 (1), 65-89 (2010).

