Extracellular Glucose Depletion as an Indirect Measure of Glucose Uptake in Cells and Tissues Ex Vivo
Summary
Extracellular depletion of fluorescently labeled glucose correlates with glucose uptake and could be used for high-throughput screening of glucose uptake in excised organs and cell cultures.
Abstract
The ongoing worldwide epidemic of diabetes increases the demand for the identification of environmental, nutritional, endocrine, genetic, and epigenetic factors affecting glucose uptake. The measurement of intracellular fluorescence is a widely used method to test the uptake of fluorescently-labeled glucose (FD-glucose) in cells in vitro, or for imaging glucose-consuming tissues in vivo. This assay assesses glucose uptake at a chosen time point. The intracellular analysis assumes that the metabolism of FD-glucose is slower than that of endogenous glucose, which participates in catabolic and anabolic reactions and signaling. However, dynamic glucose metabolism also alters uptake mechanisms, which would require kinetic measurements of glucose uptake in response to different factors. This article describes a method for measuring extracellular FD-glucose depletion and validates its correlation with intracellular FD-glucose uptake in cells and tissues ex vivo. Extracellular glucose depletion may be potentially applicable for high-throughput kinetic and dose-dependent studies, as well as identifying compounds with glycemic activity and their tissue-specific effects.
Introduction
The demand for measuring glucose uptake rises together with the critical need to address an epidemic increase in a multitude of diseases dependent on glucose metabolism. Underlying mechanisms of degenerative metabolic diseases, neurological and cognitive disorders1, inflammatory2 and infectious diseases3, cancer4,5, as well as aging6, depend on glucose metabolism for energy and its storage, anabolic processes, protein, and gene modification, signaling, regulation of genes, and nucleic acids synthesis and replication7,8,9. Diabetes mellitus (DM) is directly related to malfunction of glucose uptake regulation. DM is a spectrum of chronic diseases such as type-1, -2, and -3 diabetes mellitus, gestational diabetes, maturity-onset diabetes of the young, and other types of this disease induced by environmental and/or genetic factors. In 2016, the first WHO Global report on diabetes demonstrated that the number of adults living with the most widespread DM has almost quadrupled since 1980 to 422 million adults10, and this number of DM patients has been rising exponentially for the last few decades. In 2019 alone, an estimate of 1.5 million deaths was directly caused by DM10. This dramatic upsurge is due to the rise in type-2 DM and the conditions driving it, including overweight and obesity10. The COVID-19 pandemic revealed a two-fold increase in mortality in patients with DM compared to the general population, suggesting the profound yet poorly understood role of glucose metabolism in the immune defense3. Prevention, early diagnosis, and treatment of DM, obesity, and other diseases require optimization of measurements of glucose uptake by different tissues, and identification of environmental11, nutritional12, endocrine13, genetic14, and epigenetic15 factors affecting glucose uptake.
In research, intracellular and/or tissue uptake of glucose is commonly measured by fluorescently-labeled glucose (FD-glucose) in vitro16,17,18 and in vivo19. FD-glucose became a preferred method compared to more precise methods using radioactively-labeled glucose20, analytical mass spectroscopy analysis21, metabolomics22, nuclear magnetic resonance methods23, and positron emission tomography/computed tomography (PET/CT)5,24. Unlike FD-glucose uptake, analytical methods requiring more biological material may involve a multi-step sample preparation, expensive instruments, and complex data analysis. Effective and inexpensive measurements of FD-glucose uptake in cell cultures have been utilized in proof-of-concept experiments and may require validation by other methods.
The basis of FD-glucose application for glucose uptake studies is the reduced metabolism of FD-glucose compared to endogenous glucose25. Nonetheless, both endogenous glucose and FD-glucose are dynamically distributed among all cellular compartments for use in anabolic, catabolic, and signaling processes. The compartmentalization and time-dependent processing25 of FD-glucose interfere with the fluorescence measurements, and represent the major limiting factors for the use of this assay in high-throughput screening experiments, kinetic analysis, 3D cell culture, co-cultures, and tissue explant experiments. Here, we provide data demonstrating a high correlation between the extracellular depletion of FD-glucose and its intracellular uptake, suggesting the extracellular depletion of FD-glucose as a surrogate measurement for intracellular glucose uptake. The measurement of extracellular depletion of glucose was applied to validate tissue-specific differences in glucose uptake in mice treated with insulin and an experimental drug18 to provide a proof-of-principle of this method.
The current protocol describes intracellular and extracellular (Figure 1) measurements of FD-glucose uptake in 3T3-L1 cells. Protocol sections 1-7 explain the culture and growth of cells for 48 h; cell starvation, stimulation, and baseline extracellular measurements; and post-stimulation measurements of extracellular FD-glucose and intracellular measurements of FD-glucose and protein. Protocol section 8 describes the ex vivo measurement of extracellular uptake of FD-glucose in tissues dissected from ob/ob mice in the presence and absence of insulin and amino acid compound 2 (AAC2) described elsewhere18.
Protocol
Animal studies were approved by the Institutional Animal Care and Use Committee of The Ohio State University (OSU, protocol 2007A0262-R4).
NOTE: All procedures must be done in a class II biosafety cabinet with the blower on and the lights off.
1. Preparation of materials
NOTE: All materials are listed in the Table of Materials.
- Prepare Medium 1, centrifugation Medium 2, and storage and working solutions of fluorescent 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]-D-glucose (2-NBDG or FD-glucose) according to Table 1 in a Class II biosafety cabinet. Protect FD-glucose from light throughout the experiment by turning off all the lights under the hood.
- Use ready-to-use cell culture reagents: Trypsin-EDTA, phosphate-buffered saline (PBS), and glucose-free and phenol red-free Dulbecco's Modified Eagle Medium (hereafter, glucose-free DMEM).
- Use 20 mL of radioimmunoprecipitation assay (RIPA) lysis buffer.
NOTE: In the following sections, extracellular depletion and intracellular uptake of different FD-glucose doses are compared in 3T3-L1 fibroblasts with and without insulin stimulation (Figure 2).
2. 3T3-L1 cell culture and maintenance
- Culture 3T3-L1 mouse fibroblasts by plating 1 x 106 cells diluted in 20 mL of Medium 1 in two 96-well plates. Plate 100 μL of cell suspension per well, ensuring to maintain homogeneity of this suspension by mixing. Grow cells for 48 h in a 37 °C incubator (5% CO2 and 95% humidity) without changing media until about 70%-80% confluency.
- Maintain cells confluent and fasted by culturing them in the same medium for 48 h. Vary the incubation time from 24-72 h depending on the desired fasting catabolic state.
3. Starvation of 3T3-L1 cells
- Decant media from cells in the 96-well plates. Absorb the remaining fluid using sterile paper towels.
- Rinse the 96-well plate with 100 μL of PBS per well and absorb the remaining fluid with sterile paper towels.
- Add 100 μL of glucose-free DMEM per well and incubate for 40 min. Adjust the time of incubation depending on cell type, stimuli, and desired level of fasting.
NOTE: The time of incubation may require optimization depending on the season.
4. Preparation of FD-glucose solutions with different concentrations
NOTE: The experiment in Figure 2 examines extracellular and intercellular media with and without stimulation with insulin.
- Use eight replicates (one row in a 96-well plate) for each stimulation condition. Therefore, prepare 1 mL for each stimulation condition (specified in Table 2 and below).
- Use eight stimulation conditions without insulin: FD-glucose of 2.5 μg/mL, 1 μg/mL, 0.5 μg/mL, 0.2 μg/mL, 0.1 μg/mL, 0.05 μg/mL, and 0 μg /mL (control without FD-glucose) in one 96-well plate.
- Use eight stimulation conditions with insulin (10 μg/mL, final concentration): FD-glucose of 2.5 μg/mL, 1 μg/mL, 0.5 μg/mL, 0.2 μg/mL, 0.1 μg/mL, 0.05 μg/mL, and 0 μg/mL (control without FD-glucose) in another 96-well plate.
- To prepare stimulation conditions, dilute 1 μL of 5 mg/mL FD-glucose working solution (Table 1) in 999 μL of glucose-free DMEM to obtain 1 mL of working stock solution (1:1000 dilution or 5 μg/mL).
- Using this working stock solution, prepare 1:2000, 1:5000, 1:10,000, 1:25,000, 1:50,000, and 1:100,000 dilutions of FD-glucose to obtain 2.5 μg/mL, 1 μg/mL, 0.5 μg/mL, 0.2 μg/mL, 0.1 μg/mL, and 0.05 μg/mL (Table 2) in 2 mL tubes immediately before experiments in a biosafety cabinet without lights.
- Repeat step 4.4 and add 1 μL of insulin (10 mg/mL, final concentration) to each of the conditions specified above.
NOTE: The final concentration of insulin in these samples is 10 μg/mL = 1.7 nmol/mL. To achieve homogenization, add insulin to the tubes before adding other solutions.
5. Treating starved 3T3-L1 cells
- After 40 min of incubation, decant the glucose-free DMEM from each well in 96-well plates and absorb the remaining fluid with sterile paper towels.
- Add 100 μL (per well) each from the various treatment conditions described above to wells along one column, and 100 μL (per well) from the same treatment condition to the wells along the row (eight replicates) in both 96-well plates. Label the plates that utilized the conditions with and without insulin. Add glucose-free DMEM alone to the control wells (n = 8).
- Incubate the plate for 40 min in a cell culture incubator (37 °C, 5% CO2, and 95% humidity) in the dark.
6. Extracellular and intercellular measurements for stimulated 3T3-L1 cells
- After the cells have been stimulated for 40 min, transfer the stimulation media from both the plates into new 96-well plates maintaining the same experimental layout.
- Decant any remaining solution from the original stimulated 96-well plates with cells on sterile paper towels.
- Optionally, wash cells with PBS. Remove PBS and decant any remaining solution on sterile paper towels.
- Add 100 μL of the RIPA lysis buffer to each well. Optionally, add protease inhibitor to the RIPA buffer to protect proteins. Place plates containing RIPA in a shaker for 30 min.
- Using a microplate reader (Table of Materials), measure fluorescence at excitation (Ex) and emission (Em) wavelengths of 485 and 535 nm, respectively, first in the medium containing extracellular FD-glucose, and then, the plate with RIPA-lysed cells at the end of 30 min incubation (see step 6.4).
7. Protein-based normalization of intracellular glucose uptake
NOTE: Intracellular FD-glucose uptake depends on the cell number. Protein levels in cellular lysates are proportional to the cell numbers that allow normalizing intracellular FD-glucose levels to the number of cells in each well.
- Perform BCA protein assay according to manufacturer's instructions (see Table of Materials). Quantify the protein concentrations in each well containing RIPA-lysed cells.
- Use 10 μL of the RIPA homogenate and measure protein concentrations in triplicate to increase the accuracy of measurements in 96-well plates.
- Quantify protein based on protein standards (0, 10, 20, 30, 40, 50, and 60 μg/mL protein) analyzed together with samples on each 96-well plate.
- Measure the absorbance at 562 nm and quantify protein concentrations in each sample based on the linear regression formula obtained for the protein standard.
- Normalize the levels of intracellular FD-glucose by using fluorescent value/protein concentration in each well. Extracellular FD-glucose depletion does not require normalization.
- Optionally, use the average baseline values in control samples for additional normalization (100%).
8. Ex vivo measurement of extracellular FD glucose depletion in organs
- Pretreat mice with compounds stimulating glucose metabolism before the organ dissection, as follows.
- Use nine-week-old Lepob male mice (n = 11). Feed all mice with a regular chow diet before the experiment.
- Assign mice randomly into three groups for intraperitoneal (i.p.) injections.
- Inject mice from the control Lepob group with 0.1 mL of sterile PBS (n = 4).
- Inject mice from the insulin Lepob group with 0.1 mL of sterile PBS, containing 12 IU human insulin per gram of body weight (BW) (n= 3).
- Inject mice from the AAC2 Lepob group with 0.1 mL of sterile PBS, containing 0.1 nmol AAC2 per gram of body weight (BW) (n = 4).
- After 15 min, subject mice to inhalation of 5% isoflurane and exsanguinate blood by cardiac puncture from the anesthetized animals26.
- Dissect visceral epidydimal white adipose tissue (200 mg), liver (200 mg), and whole-brain from each animal. Use a Class II biosafety hood for tissue handling.
- Prepare FD-glucose (0.29 mM) working solution in glucose-free DMEM in a Class II Biosafety cabinet without light.
- Measure the fluorescence of FD-glucose working solution (0.29 mM) at excitation and emission wavelengths of 485 and 535 nm, respectively, using a microplate reader.
- Incubate the harvested tissues/organs in a 6-well plate containing PBS for 1 min. Handle each tissue or organ in a separate well.
- After 1 min, place each tissue on a sterile paper towel to absorb PBS. Transfer tissues/organs into a separate 6-well plate containing 4,000 µL of glucose-free DMEM and incubate for 2 min.
- After 2 min, remove and transfer the tissues into wells of a 6-well plate containing 0.29 mM FD-glucose working solution (1 mL/well). Incubate the 6-well plates containing tissues in FD-glucose working solution at 37 °C (5% CO2 and 95% humidity).
- Collect 100 µL of FD-glucose working solution from each well after 0, 10, 20, 30, 40, 60, 90, and 120 min of incubation, to analyze the kinetics of extracellular FD glucose depletion. Shake before and after collection.
- Transfer 100 µL of FD-glucose working solution into 96-well plates to measure the fluorescence at excitation and emission wavelengths of 485 and 535 nm, respectively, using a microplate reader.
- Normalize fluorescence to the 0 min value (100%) for each organ in each animal (Figure 3).
Representative Results
Intracellular intake and extracellular glucose depletion were measured in 3T3-L1 preadipocytes, in response to different concentrations of FD-glucose (Figure 2) with and without insulin stimulation. Figure 2A demonstrates a dose-dependent increase in the intracellular uptake of FD-glucose, which was significantly increased in the presence of insulin. The concomitant decrease in extracellular FD-glucose in the same cells is shown in Figure 2B, where insulin stimulation led to significantly decreased extracellular FD-glucose levels compared to the samples without insulin stimulation. The intracellular intake of FD-glucose correlated with the extracellular depletion of FD-glucose in a dose-dependent manner in samples with and without insulin (Figure 2C). Thus, extracellular depletion of FD-glucose can measure a change in glucose uptake with comparable accuracy (R2 = 0.99, P < 0.001) as intracellular FD-glucose uptake.
Next, an experimental setting was developed to validate extracellular FD-glucose uptake application in kinetic studies examining different tissues upon stimulation with canonic and experimental inducers of glucose uptake (Figure 3). We selected the Lepob mouse model of insulin resistance in peripheral tissues, including visceral white adipose tissue27,28. The well-established responses to insulin in these mice were compared with effects of novel compound AAC2, acting on both peripheral and nervous tissues via leptin receptor and glucose transporter GLUT1 dependent mechanisms18. Lepob mice were i.p. injected with insulin or AAC2. Organs were dissected 15 min after injection. Then, the kinetics of extracellular FD-glucose depletion was studied in visceral fat, liver, and brain ex vivo (Figure 3). In agreement with the reported insulin resistance in peripheral tissues, extracellular FD-glucose was not depleted in non-stimulated control visceral fat during 120 min of incubation (Figure 3A). In contrast, pretreatment of mice with insulin or AAC2 before the dissection led to significant depletion of extracellular FD-glucose within a time interval of 30 min and 60 min, respectively.
Liver uptake of glucose utilizes glucose transporters, including GLUT2, that is independent of insulin stimulation29,30. Accordingly, extracellular FD-glucose was initially not depleted in insulin-stimulated liver explants compared with non-stimulated liver explants (Figure 3B), although significant depletion of FD-glucose was observed after 120 min of incubation in liver explant stimulated with insulin compared with the initial levels (0 min). On the other hand, extracellular FD glucose was significantly decreased in a time-dependent manner in liver explants pretreated with AAC2.
In the brain, the main transporter is GLUT1 among other transporters31. The FD-glucose depletion kinetics was strikingly different in the brain compared to other tissue (Figure 3C). The significant depletion of glucose was observed in the extracellular medium containing the non-treated brain after 60 min of incubation (from 100% at 0 min to 78%). The medium incubated with brains from insulin-treated Lepob mice have shown moderate but a significant linear decrease in FD-glucose (from 100% at 0 min to 95%). AAC2-stimulated brains lead to a profound rapid decrease of extracellular FD-glucose during the first 20 min (from 100% at 0 min to 67.4%). Together, measurements of extracellular glucose depletion support the previously reported differences in glucose uptake in these tissues in vivo32.
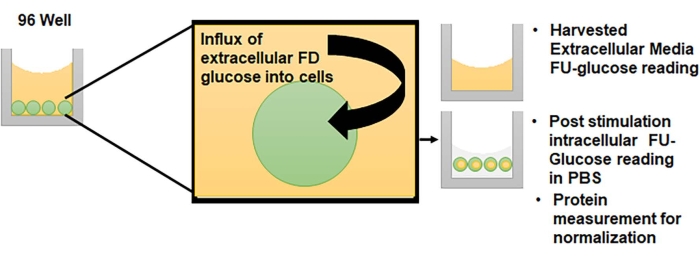
Figure 1: Principle of the extracellular FD-glucose depletions method. Cells cultured in 96-well format are suitable for this assay. To survive, cells take up glucose and this influx decreases the levels of extracellular glucose. The replacement of glucose with FD-glucose allows monitoring of these changes. Please click here to view a larger version of this figure.
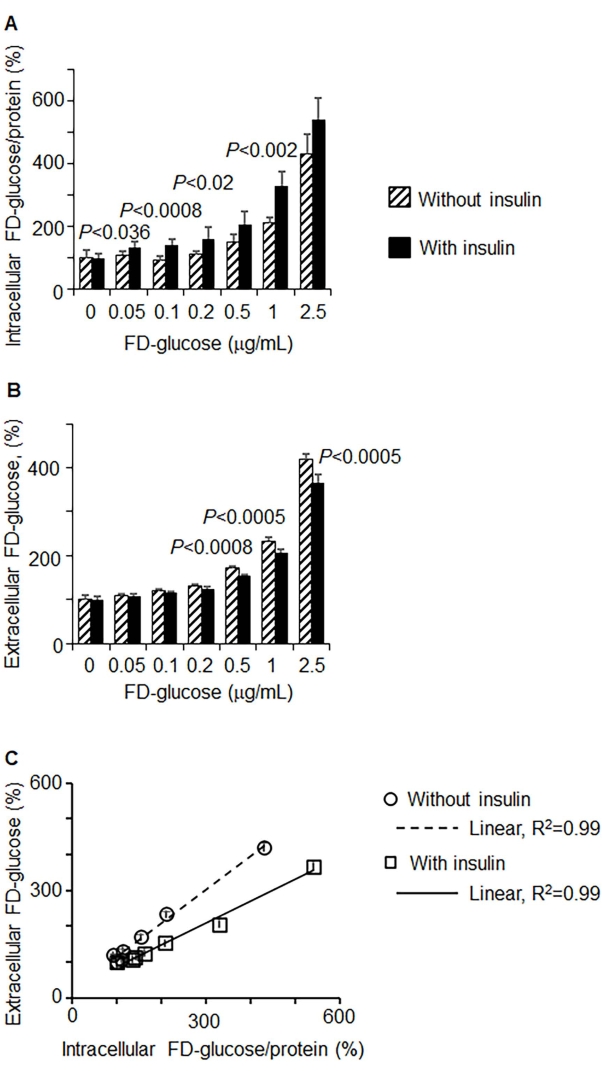
Figure 2: Depletion of extracellular FD-glucose correlated with the intracellular uptake in vitro. 3T3-L1 preadipocytes were incubated in a glucose-free medium for 40 min before incubation with different concentrations of FD-glucose with and without insulin (1.7 mM; 40 min incubation). (A, B) Dose-dependent uptake of FD-glucose (A) and extracellular FD-glucose depletion (B) in control (i.e., without insulin) (hatched bars) and insulin-stimulated cells (black bars). FD-glucose uptake was normalized by protein concentrations. Data are shown as % of the value measured in control incubated without FD-glucose (mean SD, n = 8 per condition). Significance was examined by unpaired t-test. (C) Correlation between intracellular and extracellular FD-glucose (%) measures with (squares) or without (circles) insulin in experiments described in (A) and (B). Pearson correlation, P < 0.001. Please click here to view a larger version of this figure.
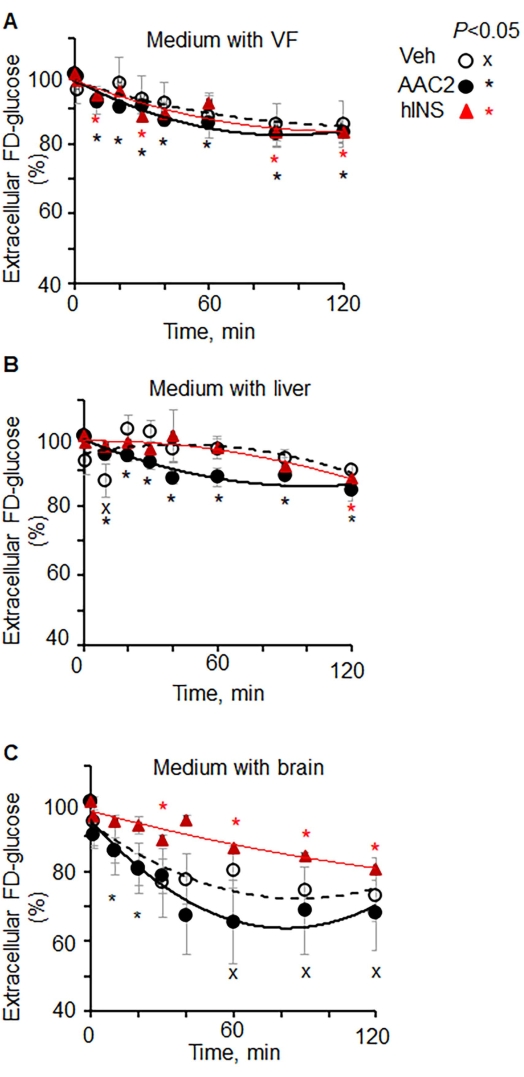
Figure 3: Kinetics of extracellular FD-glucose depletion in different organs ex vivo. (A–C) Lepob mice were injected with vehicle (PBS, n = 4), insulin (12 IU/kg BW, n = 3), and AAC2 (1 nmol/g BW, n = 3). After 15 min, tissues were dissected and isolated. Explants of (A) visceral fat, (B) liver, and (C) brain were incubated in FD-glucose (0.29 mM). The kinetics of extracellular glucose depletion was measured in aliquots of the medium at different time points. Data (mean SD) are shown as % of fluorescence in each organ at 0 min of incubation. Significance P < 0.05, unpaired t-test. Please click here to view a larger version of this figure.
| Solution/Medium | Components |
| Ethanol:DMSO (1:1/v/v) | Ethanol (200 µL) and DMSO (200 µL) in 1-1.5 mL tube; use cell culture grade ethanol and DMSO |
| Medium 1 | DMEM (89 mL), Penicillin/streptomycin (1%) (1 mL) and Calf serum (10%) (10 mL) in sterile 50 mL tube |
| Centrifugation Medium 2 | DMEM (89 mL), Penicillin/streptomycin (1%) (1 mL) and Bovine serum (10%) (10 mL) in sterile 50 mL tube |
| Storage FD-glucose solution 5 mg/mL (14.5 mM) | FD glucose (1 mg) and Ethanol:DMSO (1:1/v/v) (200 µL) in 0.5 mL tube; store at -80 °C, preferentially under argon or nitrogen atmosphere |
| Working FD-glucose solution 5 µg/mL (14.5 mM) | Storage FD glucose solution 5 mg/mL (1 µL), Glucose free DMEM (999 µL); prepare immediately before experiment. |
Table 1: Preparation of culture media and FD-glucose solutions.
| Dilution | Control | 1:2×103 | 1:5×103 | 1:1×104 | 1:2.5×104 | 1:5×104 | 1:1×105 |
| Concentration (µg/mL) | 0 | 2.5 | 1 | 0.5 | 0.2 | 0.1 | 0.05 |
| Glucose-free DMEM (µL) | 1000 | 500 | 800 | 900 | 960 | 980 | 990 |
| FD Glucose 5 µg/mL (µL) | 0 | 500 | 200 | 100 | 40 | 20 | 10 |
Table 2: Preparation of FD-glucose solutions with different concentrations for experiments shown in Figure 2.
Discussion
The direct comparison of extracellular FD-glucose depletion with normalized intracellular glucose uptake in cells culture showed a high correlation, suggesting that extracellular glucose depletion could be a surrogate measurement for glucose uptake assessment. The measurement of extracellular FD-glucose can use a broad range of FD glucose concentrations, also 0.5-2.5 μg FD-glucose/mL appear to provide the optimal range. Extracellular FD-glucose does not require normalization to cell number or protein concentrations necessary for intracellular FD-glucose uptake. The anticipated limitation of extracellular FD-glucose measurements is the investigation of reactive oxygen species-inducing agents and the traces of blood within explants that can quench fluorescence. Other environmental factors affecting fluorescence, such as light, should also be considered. Prolonged handling time required while using multiple tissue explants can compromise tissue viability and glucose metabolism. This could be an additional limitation. The excised tissues need to be in a small size range (50-300 mg) to allow the permeability of glucose inside the tissue. Simultaneous handling of tissues by multiple operators can reduce handling time. Although we did not measure the glucose uptake within the tissue explants, the accumulated observations in cultured tissue explants suggest that they survive in the glucose-containing medium for up to 17 days with the progressive loss of functionality33. In this protocol, the short incubation time for explants allows monitoring glucose metabolism in relatively functional tissues. In the tissues with complex cellular organization, such as the brain, the size presents an unresolvable limitation, because the disintegration of complex tissue and resulting cell death influences the glucose uptake. However, the incubation of the brain for 24 h has been previously used for identification of endogenous regulatory mechanism, supporting relative physiological functionality of excised mouse brain in glucose-containing medium34. Regardless, the simplified procedure and easy accessibility of extracellular FD-glucose can allow using this assay in a high-throughput format. The comparison of basic and insulin-stimulated extracellular FD-glucose depletion suggests that this assay could be applied to identify humoral, nutritional, pathogenic, environmental factors, or pharmaceutical drugs regulating glucose uptake. Although this assay simplifies the discovery, the findings required validation with the quantitative radiolabeling20 analytical methods21,22,23 and/or imaging methods5,24 for rigorous mechanistic elucidation of the pathways.
The critical steps in this protocol include optimization of confluence and fasting time that influence both basal and stimulated FD-glucose uptake. These parameters could also be affected by season based on the authors' observations. Moreover, different cell types use specific mechanisms for glucose uptake including different glucose transporters and regulatory hormone/receptor interactions32,35,36. Therefore, positive controls other than insulin need to be considered for the cell cultures representing tissues other than muscle or adipose tissue regulated by insulin/GLUT4 axes. The troubleshooting also includes adjusting cell confluence and optimizing time for fasting/refeeding with FD-glucose. In addition, the sensitivity of extracellular glucose measurement could be improved by reducing the levels of extracellular FD-glucose, taking into consideration the threshold levels required for glucose uptake. The experiment described in Figure 2 can help researchers to customize this assay for use with other cell cultures or troubleshooting.
The intrinsic variability of glucose metabolism also required a higher sample size (n = 5-8). Although extracellular measurements of FD-glucose loss exhibited the same accuracy as intracellular uptake of FD-glucose normalized by protein, the normalization can reduce assay variability. In addition to the measurement of protein concentrations, the measurement of DNA content in the cell can be another reliable surrogate assessment of cell number37.
Extracellular FD-glucose is accessible for kinetic measurements. Here, a simple procedure of kinetic measurements of extracellular FD-glucose has been developed to identify organ-specific responses to mediators of glucose uptake. These ex vitro studies can directly test organ response to various stimuli in vivo that is combined with the exposure of organs to FD-glucose and ex vivo kinetic measurements, as shown in Figure 3. Although ex vivo studies do not fully resemble in vivo conditions, they have multiple advantages. Organ explants maintain complex multicellular primary cell structure, unlike immortal cell cultures exhibiting altered gene expression. Modern drug development utilizes model organisms, indirect assessment of glucose uptake based on transcriptome or metabolome22, comprehensive insulin clamp technique38, or expensive imaging32. Although measurement of extracellular FD-glucose in explanted organs will not replace these technologies, it will provide a rapid assessment of compound, metabolite, and genes to facilitate the discovery of novel therapeutic targets regulating glucose uptake in a tissue-specific manner. The development of safe therapies addressing glucose metabolism in specific tissues can offer a solution for cancer, dementia, aging, diabetes, autoimmune diseases, obesity, and other related applications.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The project was supported by Ralph and Marian Falk Medical Research Catalyst Award and Kathleen Kelly Award. Other supports included the National Center for Research Resources UL1RR025755 and NCI P30CA16058 (OSUCCC), the NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not represent the official views of the National Center for Research Resources or the NIH.
Materials
| 3T3-L1 mouse fibroblasts | ATCC | CL-173 | Cell line |
| 96-well plates | Falcon | 353227 | Plastic ware |
| B6.V-Lepob/J male mice | Jackson Laboratory | stock number 000632 | Mice |
| BioTek Synergy H1 modular multimode microplate reader (Fisher Scientific, US) | Fisher Scientific, US | B-SHT | Device |
| Bovine serum | Gibco/ThermoFisher | 161790-060 | Cell culture |
| Calf serum | Gibco/ThermoFisher | 26010-066 | Cell culture |
| Cell incubator | Forma | Series II Water Jacket | Device |
| Diet (mouse/rat diet, irradiated) | Envigo | Teklad LM-485 | Diet |
| Dimethylsulfoxide (DMSO) | Sigma LifeScience | D2650-100mL | Reagent |
| Dulbecco's Modified Eagle Medium | Gibco/ThermoFisher | 11965-092 | Cell culture |
| Ethanol | Sigma Aldrich | E7023-500mL | Reagent |
| Fluorescent 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]-D-glucose) | Sigma | 72987-1MG | Assay |
| Glucose-free and phenol red-free DMEM | Gibco/ThermoFisher | A14430-01 | Cell culture |
| Human insulin 10 mg/mL | MilliporeSigma, Cat N 91077C | Cat N 91077C | Reagent |
| Isoflurane, 5% | Henry Schein | NDC 11695-6776-2 | Anestaetic |
| Penicillin/streptomycin (P/S) | Gibco/ThermoFisher | 15140-122 | Cell culture |
| Phosphate buffered solution | Sigma-Aldrich | DA537-500 mL | Cell culture |
| Pierce bicinchoninic acid (BCA) protein assay | ThermoFisher | Cat N23225 | Assay |
| Radioimmunoprecipitation assay lysis buffer | Santa Cruz Biotechnology | sc-24948 | Assay |
| Trypsin-EDTA (0.05%) | Gibco/ThermoFisher | 25300-054 | Cell culture |
References
- Kyrtata, N., Emsley, H. C. A., Sparasci, O., Parkes, L. M., Dickie, B. R. A systematic review of glucose transport alterations in Alzheimer’s disease. Frontiers in Neuroscience. 15, 568 (2021).
- Garcia-Carbonell, R., et al. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatology. 68 (7), 1614-1626 (2016).
- Kumar, A., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Review. 14 (4), 535-545 (2020).
- Lee, J. H., et al. Different prognostic impact of glucose uptake in visceral adipose tissue according to sex in patients with colorectal cancer. Scientific Reports. 11 (1), 21556 (2021).
- Miner, M. W. G., et al. Comparison of: (2S,4R)-4-[(18)F]Fluoroglutamine, [(11)C]Methionine, and 2-Deoxy-2-[(18)F]Fluoro-D-Glucose and two small-animal PET/CT systems imaging rat gliomas. Frontiers in Oncology. 11 (18), 730358 (2021).
- Gumbiner, B., Thorburn, A. W., Ditzler, T. M., Bulacan, F., Henry, R. R. Role of impaired intracellular glucose metabolism in the insulin resistance of aging. Metabolism. 41 (10), 1115-1121 (1992).
- Ebrahimi, A. G., et al. Beta cell identity changes with mild hyperglycemia: Implications for function, growth, and vulnerability. Molecular Metabolism. 35, 100959 (2020).
- Ruberto, A. A., et al. KLF10 integrates circadian timing and sugar signaling to coordinate hepatic metabolism. Elife. 10, 65574 (2021).
- Stocks, B., Zierath, J. R. Post-translational modifications: The signals at the intersection of exercise, glucose uptake, and insulin sensitivity. Endocrinology Reviews. , (2021).
- World Health Organization. Global report on diabetes. World Health Organization. , (2016).
- Kolb, H., Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Medicine. 15 (1), 131 (2017).
- Galicia-Garcia, U., et al. Pathophysiology of type 2 diabetes mellitus. International Journal of Molecular Science. 21 (17), 6275 (2020).
- Petrov, M. S., Basina, M. DIAGNOSIS OF ENDOCRINE DISEASE: Diagnosing and classifying diabetes in diseases of the exocrine pancreas. European Journal of Endocrinology. 184 (4), 151-163 (2021).
- Sirdah, M. M., Reading, N. S. Genetic predisposition in type 2 diabetes: A promising approach toward a personalized management of diabetes. Clinical Genetics. 98 (6), 525-547 (2020).
- Ramos-Lopez, O., Milagro, F. I., Riezu-Boj, J. I., Martinez, J. A. Epigenetic signatures underlying inflammation: an interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflammation Research. 70 (1), 29-49 (2021).
- Yamamoto, N., et al. Measurement of glucose uptake in cultured cells. Current Protocols in Pharmacology. 71 (1), 12-14 (2015).
- Yang, L., et al. A sensitive and simple HPLC-FLD-based method for the measurement of intracellular glucose uptake. Food Chemistry. 372, 131218 (2021).
- Lee, A., et al. Amino acid-based compound activates atypical PKC and leptin receptor pathways to improve glycemia and anxiety like behavior in diabetic mice. Biomaterials. 239, 119839 (2020).
- Shukla, S. K., Mulder, S. E., Singh, P. K. Hypoxia-mediated in vivo tumor glucose uptake measurement and analysis. Methods in Molecular Biology. 1742, 107-113 (2018).
- Jakson, I., Ujvari, D., Brusell Gidlof, S., Linden Hirschberg, A. Insulin regulation of solute carrier family 2 member 1 (glucose transporter 1) expression and glucose uptake in decidualizing human endometrial stromal cells: an in vitro study. Reproductive Biology and Endocrinology. 18 (1), 117 (2020).
- Saparbaev, E., et al. Identification and quantification of any isoforms of carbohydrates by 2D UV-MS fingerprinting of cold ions. Analytical Chemistry. 92 (21), 14624-14632 (2020).
- Schulz, A., et al. Targeted metabolomics of pellicle and saliva in children with different caries activity. Scientific Reports. 10 (1), 697 (2020).
- Shulman, R. G. Nuclear magnetic resonance studies of glucose metabolism in non-insulin-dependent diabetes mellitus subjects. Molecular Medicine. 2 (5), 533-540 (1996).
- Cochran, B. J., et al. In vivo PET imaging with [(18)F]FDG to explain improved glucose uptake in an apolipoprotein A-I treated mouse model of diabetes. Diabetologia. 59 (18), 1977-1984 (2016).
- Lloyd, P. G., Hardin, C. D., Sturek, M. Examining glucose transport in single vascular smooth muscle cells with a fluorescent glucose analog. Physiological Research. 48 (6), 401-410 (1999).
- Beeton, C., Garcia, A., Chandy, K. G. Drawing blood from rats through the saphenous vein and by cardiac puncture. Journal of Visualized Experiments. (7), e266 (2007).
- DiSilvestro, D. J., et al. Leptin production by encapsulated adipocytes increases brown fat, decreases resistin, and improves glucose intolerance in obese mice. PLoS One. 11 (4), 0153198 (2016).
- Friedman, J. M. Leptin and the endocrine control of energy balance. Nature Metabolism. 1 (8), 754-764 (2019).
- Guillam, M. T., Burcelin, R., Thorens, B. Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proceedings of the National Academy of Sciences. 95 (21), 12317-12321 (1998).
- Guillam, M. T., et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nature Genetics. 17 (3), 327-330 (1997).
- Barros, L. F., et al. Kinetic validation of 6-NBDG as a probe for the glucose transporter GLUT1 in astrocytes. Journal of Neurochemistry. 109, 94-100 (2009).
- Sprinz, C., et al. Effects of blood glucose level on 18F-FDG uptake for PET/CT in normal organs: A systematic review. PLoS One. 13 (2), 0193140 (2018).
- Johnson, T. V., Martin, K. R. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Investigative Ophthalmology & Visual Science. 49 (8), 3503-3512 (2008).
- de Urquiza, A. M., et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 290 (5499), 2140-2144 (2000).
- Olson, A. L., Pessin, J. E. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annual Review of Nutrition. 16 (1), 235-256 (1996).
- Muhanna, D., Arnipalli, S. R., Kumar, S. B., Ziouzenkova, O. Osmotic adaptation by Na(+)-dependent transporters and ACE2: correlation with hemostatic crisis in COVID-19. Biomedicines. 8 (11), 460 (2020).
- Ligasova, A., Koberna, K. DNA dyes-highly sensitive reporters of cell quantification: comparison with other cell quantification methods. Molecules. 26 (18), 5515 (2021).
- DeFronzo, R. A., Tobin, J. D., Andres, R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. American Journal of Physiology. 237 (3), 214-223 (1979).

