Normothermic Ex Vivo Liver Machine Perfusion in Mouse
Summary
A normothermic ex vivo liver perfusion (NEVLP) system was created for mouse livers. This system requires experience in microsurgery but allows for reproducible perfusion results. The ability to utilize mouse livers facilitates the investigation of molecular pathways to identify novel perfusate additives and enables the execution of experiments focused on organ repair.
Abstract
This protocol presents an optimized erythrocytes-free NEVLP system using mouse livers. Ex vivo preservation of mouse livers was achieved by employing modified cannulas and techniques adapted from conventional commercial ex vivo perfusion equipment. The system was utilized to evaluate the preservation outcomes following 12 h of perfusion. C57BL/6J mice served as liver donors, and the livers were explanted by cannulating the portal vein (PV) and bile duct (BD), and subsequently flushing the organ with warm (37 °C) heparinized saline. Then, the explanted livers were transferred to the perfusion chamber and subjected to normothermic oxygenated machine perfusion (NEVLP). Inlet and outlet perfusate samples were collected at 3 h intervals for perfusate analysis. Upon completion of the perfusion, liver samples were obtained for histological analysis, with morphological integrity assessed using modified Suzuki-Score through Hematoxylin-Eosin (HE) staining. The optimization experiments yielded the following findings: (1) mice weighing over 30 g were deemed more suitable for the experiment due to the larger size of their bile duct (BD). (2) a 2 Fr (outer diameter = 0.66 mm) polyurethane cannula was better suited for cannulating the portal vein (PV) when compared to a polypropylene cannula. This was attributed to the polyurethane material's enhanced grip, resulting in reduced catheter slippage during the transfer from the body to the organ chamber. (3) for cannulation of the bile duct (BD), a 1 Fr (outer diameter = 0.33 mm) polyurethane cannula was found to be more effective compared to the polypropylene UT – 03 (outer diameter = 0.30 mm) cannula. With this optimized protocol, mouse livers were successfully preserved for a duration of 12 h without significant impact on the histological structure. Hematoxylin-Eosin (HE) staining revealed a well-preserved morphological architecture of the liver, characterized by predominantly viable hepatocytes with clearly visible nuclei and mild dilation of hepatic sinusoids.
Introduction
Liver transplantation represents the gold standard treatment for individuals with end-stage liver disease. Regrettably, the demand for donor organs surpasses the available supply, leading to a significant shortage. In 2021, approximately 24,936 patients were on the waiting list for a liver graft, while only 9,234 transplants were successfully performed1. The significant disparity between the supply and demand of liver grafts highlights the pressing necessity to investigate alternative strategies to broaden the donor pool and enhance the accessibility of liver grafts. One way of expanding the donor pool is to use marginal donors2. Marginal donors include those with advanced age, moderate or severe steatosis. Although the transplantation of marginal organs may yield favorable outcomes, the overall results remain suboptimal. As a result, the development of therapeutic strategies aimed at enhancing the function of marginal donors is currently underway3,4.
One of the strategies is to use machine perfusion, especially normothermic oxygenated machine perfusion, to improve the function of these marginal organs5. However, there is still a limited understanding of the molecular mechanisms that underlie the beneficial effects of normothermic oxygenated machine perfusion (NEVLP). Mice, with their abundant availability of genetically modified strains, serve as valuable models for investigating molecular pathways. For instance, the significance of autophagy pathways in mitigating hepatic ischemia-reperfusion injury has been increasingly recognized6,7. One important molecular pathway in the hepatic ischemia-reperfusion injury is the miR-20b-5p/ATG7 pathway8. Currently, there are a number of ATG knockout and conditional knock-out mouse strains available but no corresponding rat strains9.
Based on this background, the aim was to generate a miniaturized NEVLP platform for mouse liver grafts. This platform would facilitate the exploration and evaluation of potential genetically modified strategies aimed at improving the functionality of the donor's liver. Additionally, it was essential for the system to be suitable for long-term perfusion, enabling the ex vivo treatment of the liver, commonly referred to as "organ repair."
Considering the limited availability of relevant in vitro data on mouse liver perfusion, the literature review focused on studies conducted in rats. A systematic search of literature spanning from 2010 to 2022 was performed using keywords such as "normothermic liver perfusion," "ex vivo or in vitro," and "rats". This search aimed to identify optimal conditions in rodents, allowing us to determine the most appropriate approach.
The perfusion system consists of a sealed water-jacketed glass buffer reservoir, a peristaltic roller pump, an oxygenator, a bubble trap, a heat exchanger, an organ chamber, and a closed cycling tubing system (Figure 1). The system ensures precise maintenance of a constant perfusion temperature of 37 °C using a dedicated thermo-static machine. The peristaltic roller pump drives the flow of the perfusate throughout the circuit. The perfusion circuit initiates at the insulated water-jacketed reservoir. Subsequently, the perfusate is directed through the oxygenator, which receives a gas mixture of 95% oxygen and 5% carbon dioxide from a dedicated gas bottle. Following oxygenation, the perfusate passes through the bubble trap, wherein any entrapped bubbles are redirected back to the reservoir by the peristaltic pump. The remaining perfusate flows through the heat exchanger and enters the organ chamber, from where it returns to the reservoir.
Here, we report our experiences establishing a NEVLP for mouse livers and share the promising results of a pilot experiment performed using the oxygenated medium without oxygen carriers.
Protocol
Animal experiments were performed according to the current German regulations and guidelines for animal welfare and the ARRIVE guidelines for Reporting Animal Research. The animal experiment protocol was approved by the Thüringer Landesamt für Verbraucherschutz, Thuringia, Germany (Approval-Number: UKJ – 17 – 106).
NOTE: Male C57BL/6J mice weighing 34 ± 4 g (mean ± standard error of the mean [SEM]) were used as liver donors. They were maintained under controlled environmental conditions (50% humidity and 18 – 23 °C) with free access to standard mouse chow and water. Throughout the surgical procedure, a respiratory rate exceeding 60 breaths/min was maintained, and body temperature was kept above 34 °C.
1. Preparation
- Setting up the operation table
- Autoclave all surgical instruments and consumables for sterilization purposes.
- Turn on all equipment, including the warming board and electrocoagulation.
- Place one 50 mL syringe with 25 mL heparinized (2,500 U/L) saline in a warm incubator (37 °C).
- Place the surgical instruments, the 6 – 0 silk suture, sterile small cotton applicator, veterinary saline (500 mL), and non-woven gauze sponges (10 cm x 10 cm) on the operation table appropriately.
- Place a 26 G needle on the operation table to create a small hole in the lid of the 0.5 mL microcentrifuge tube to receive the biliary tube for bile collection.
- Place the cannula (1 Fr polyurethane cannula or UT – 03 polyethylene cannula) and a sterilized 0.5 mL microcentrifuge tube for bile collection on the operation table.
- Self-made portal vein cannula
- Hold the 2 Fr cannula with forceps and puncture the wall with a 30 G needle at a 1 cm distance from the end of the cannula. Push the needle through the cannula until the tip of the needle becomes visible.
- Trim the tip of the cannula resulting in a sharp triangle.
- Preparation of heparinized saline
- Prepare 25 mL of heparinized saline with a final concentration of 2,500 IU/mL.
- Remove all air bubbles and place the syringe in the 40 °C incubator.
- Demonstration of the perfusion system
- See Figure 1 for the main components of the machine perfusion system.
- Set up of the organ chamber
- See Figure 2 for the layout of the organ chamber.
- Set up of the perfusion system
- Turn on the lab chart program for pressure monitoring.
- Connect the pressure calibrator and pressure sensor at the organ chamber level.
- Adjust the pressure calibrator to read 0 mmHg and check the corresponding value on the pressure control software.
- Adjust the pressure calibrator to read 20 mmHg and again check the corresponding value on the pressure control software.
- Turn on the water bath, and prewarm the organ chamber to 40 °C.
- Flush the entire plumbing system twice with distilled deionized water for 30 min each, ensuring complete removal of the sanitizing solution.
- Initiate the circulation of the disinfection solution throughout the entire system for a duration of 20 min to ensure thorough disinfection.
- Turn on the gas mixture (95% oxygen (O2) and 5% carbon dioxide (CO2).
- Perfusate filling
- Supplement 250 mL of Williams' E medium with 50 mL of fetal bovine serum, 3 mL of penicillin/ streptomycin (1 mg/mL), 0.17 mL of insulin (100 IE/mL), 0.34 mL of heparin (5000 U/mL), and 0.07 mL of hydrocortisone (100 mg/2 mL) to prepare the complete Williams' E medium.
- Add equal volumes (150 mL) of perfusate to the reservoir and the organ chamber to prime the system.
NOTE: Special attention must be paid to maintain sterility during the filling process. The perfusate is constantly pumped through these two key components of the closed recirculating machine perfusion. - Turn on the peristaltic pump at medium speed (15 mL/min) to prime the perfusion system with the oxygenated medium.
2. Liver explantation
- Pre-surgery preparation
- Weigh the animal. Prepare the analgesic buprenorphine (0.3 mg/mL) (0.05 mg/kg body weight).
- Connect the induction chamber with the wall socket. Turn oxygen to 0.5 L/min. Turn isoflurane to 3%.
- Place the animal in the chamber until deep anesthesia (righting reflex positive) is reached.
- Use a micro syringe to apply body weight-adapted dose of analgesia subcutaneously.
- Use an electric shaver to trim the fur on the abdominal skin.
- Transfer the mouse to the operation table and turn on the isoflurane vaporizer to 2.5% to maintain anesthesia. Confirm the depth of anesthesia by testing the interdigital toe reflex.
- Preparation of the mouse abdomen
- Place the mouse in a supine position.
- Test interdigital reflex to double confirm the appropriate depth of anesthesia. Fix all four limbs with tape.
- Disinfect both sides of the abdomen to the mid-axillary line using three consecutive rounds of iodine-alcohol. Use non-woven sterilized gauze to cover the area around the surgical field.
- Make a 3 cm transverse incision 1 cm below the xiphoid in the abdominal area of the mouse using Metzenbaum baby scissors and surgical forceps.
- Extend the skin incision bilaterally to the midaxillary line on both sides.
- Carefully make a 2 cm longitudinal incision along the linea alba using spring scissors.
- Cut through the abdominal muscle layer with electrocoagulation and Vannas spring scissors.
- Carefully place a piece of wet gauze to protect the liver from electrocoagulation.
- Use a 6 – 0 silk suture with the round needle to retract the xiphoid process for better exposure of the coronary ligament.
- Use two rib retractors to fully expose the abdominal cavity of the mouse.
- Carefully move the small intestine out of the abdominal cavity with a wet cotton swab to fully expose the hilum.
- Common bile duct preparation
- Transect the falciform, phrenic, and gastrohepatic ligaments with sharp scissors.
- Carefully free the common bile duct using fine curved forceps without teeth.
NOTE: The common bile duct is very easily damaged and breaks. Once it breaks, it cannot be cannulated. Due to the direction of the anatomical position, curved forceps are better to be used. - Place two 6 – 0 silk suture loops over the common bile duct in preparation for the next step.
- Common bile duct cannulation
- Carefully puncture the bile duct with a 30 G needle. Use pointed curved forceps to enlarge the small hole to fit the bile duct cannulation.
- Use vessel cannulation forceps to grasp the bile duct cannula and push it into the bile duct.
- Double secure the cannula with the preset 6 – 0 suture loops.
NOTE: During the cannulation, resistance by bile is felt. If the force is not well controlled, the cannula will be pushed out of the biliary tract by the pressure of bile outflow. Carefully adjust the depth of the cannula. If it is too deep, it may damage the bile duct, and if it is not deep enough, it may slip out. - Observe the bile flow in the cannula after successful cannulation.
- Portal vein preparation
- Clamp the portal vein with flat forceps and carefully free the connective tissue with curved forceps. Do not pull hard to avoid causing tearing of the portal vein. Once the portal vein is damaged, it is difficult to re-cannulate the portal vein.
- Dissect the PV just superior to the bifurcation and place the first suture loop using 6 – 0 silk suture on PV close to the confluence for later use.
- Place the second suture loop for later fixation of the PV as close to the hepatic hilum as possible.
- Portal vein cannulation
- Use an arterial clip to close the distal portal vein.
- Very carefully, puncture the portal vein with one of the above portal vein cannulas. Blood flow can be clearly observed within the cannula after a successful puncture.
- Secure the PV cannula with the pre-placed 6 – 0 suture loop.
- Liver flushing
- Increase isoflurane to 5% and euthanize the mouse with an overdose of isoflurane inhalation.
- Take prewarmed heparin saline solution from the incubator. Remove all air bubbles formed inside the heparinized saline.
- Fix the syringe with prewarmed heparinized saline into the syringe pump.
- Connect the extension tube of the syringe pump to the cannula of the portal vein, adjust the speed to 2 mL/min, and start the liver flushing.
- Observe the color of the liver at the end of the flushing procedure. Excise the liver once the color turns to a homogenous yellow.
- Transect the diaphragm, suprahepatic inferior vena cava, infra hepatic vena cava, hepatic artery, distal portal vein, and any remaining connective tissue.
- Place the liver into the Petri dish.
3. Liver and chamber connection
- Liver transfer
- Carefully transfer the liver into the organ chamber using a Petri dish.
- Keep a small amount of saline in the Petri dish to prevent the liver from drying out.
NOTE: Portal vein and bile duct can easily be twisted during this procedure, which may affect liver perfusion and bile collection.
- Portal vein cannula connection
- Slowly infuse normal saline into the portal vein cannula with a syringe to evacuate the air bubbles in the cannula.
- Connect the portal vein cannula into the perfusate outflow tube in the organ chamber.
- Bile duct cannula connection
- Guide the mouse bile duct cannula through the valve of a rubber cap which is connected to the organ chamber.
- Insert the bile duct cannula into a pre-prepared 0.5 mL microtube with a small hole in the lid.
- Place the microtube on clay outside of the organ chamber.
4. Adjust the flow rate according to PV pressure
- Turn on the peristaltic pump from 1 mL/min.
- Check the portal vein pressure reading to adjust the flow rate.
- Maintain the portal vein pressure in the physiological range between 7 – 10 mmHg by adjusting the flow rate.
NOTE: Nominal flow rate can vary slightly depending on the usage and positioning of tubes.
5. Sample collection
- Obtain inlet perfusate samples from the portal vein inflow tube and outlet perfusate samples from the organ chamber in 3h intervals.
- Collect samples from all liver lobes for histological analysis at the end of the perfusion period of 12 h.
Representative Results
Establishment of surgical procedure
A total of 17 animals were utilized for this experiment: 14 mice were employed for optimizing the organ procurement process, including cannulation of the portal vein (PV) and bile duct (BD), while 3 mice were used to validate the procedure (Table 1). Histological results (Figure 3) were compared to facilitate the identification of the optimal perfusion condition.
Selection of perfusate
A previously utilized hepatocyte culture medium was selected for this study10,11. William's E medium was initially designed by Williams and Gunn as a serum-reduced medium for prolonged in vitro cultivation of mature rat liver epithelial cells12. Nonetheless, it has also found utility in supporting the growth and maintenance of rodent hepatocytes13. Fetal bovine serum (FBS) is a widely employed cell culture supplement due to its rich composition of essential nutrients and growth factors that facilitate cell growth, proliferation, and viability14. Complete William's E medium was used as the perfusate (Table 2), which is supplemented with 20% fetal bovine serum, 1 % penicillin/streptomycin, 5,000 U/L heparin, 50 U/L insulin, and 0.010 g/L hydrocortisone.
Selection of cannula
The cannulation procedure involved first cannulating the bile duct (BD) for bile fluid collection, followed by the cannulation of the portal vein (PV). For BD cannulation, a UT-03 polypropylene tube with an outer diameter of 0.3 mm and an inner diameter of 0.18 mm was initially used. However, due to concerns regarding potential BD damage and the higher risk of unintended catheter displacement associated with the trimmed and rigid UT-03 tip, a preference was given to the 1 Fr polyurethane tubes. The polyurethane tubes, with their softer material and reduced slipperiness, were considered more suitable for BD cannulation.
Initially, a 26 G polypropylene intravenous needle cannula was used for cannulating the portal vein (PV). However, the removal of the needle and subsequent attachment of the cannula to the perfusion tube resulted in the formation of bubbles, which had the potential to obstruct the intrahepatic sinusoids. To overcome this issue, an indwelling cannula was constructed using a 30 G needle inserted into the distal 1 cm of a 2 Fr polyurethane cannula. This self-made "needle-guided cannula" was then inserted into the distal PV above the confluence. As the catheter was positioned within the PV, the needle was slowly withdrawn while simultaneously advancing the tube. The end of the self-made cannula was connected to the perfusion tube inside the chamber. Using a soft material in this cannulation technique provided an advantage by reducing the risk of injury to the vessel's back wall.
Validation studies
Inlet and outlet perfusate samples were subjected to the determination of pH and potassium levels. The obtained results (Figure 4) were then compared to the findings reported in recent publications15,16,17. Three mouse livers were perfused with oxygenated and supplemented with William's E medium for 12 h. Throughout this period, a stable perfusion pressure of 7 – 10 mmHg was continuously recorded. The mean pH was relatively stable throughout the 12 h perfusion and ranged between 7.3 and 7.7. Mean potassium levels were also stable throughout the perfusion period and ranged between 5.9 and 6.8 mmol/L (Figure 4). The PV flow rate was maintained within a range of 0.8 – 1.2 mL/min/g depending on the usage and positioning of pump tubes during the experimental procedures. All results observed in mouse liver perfusion demonstrated similarities with previously reported observations in rat liver perfusion (Table 3).
Tissue samples were collected from three livers (N = 3) and were subjected to 12 h perfusion using the optimized protocol for HE-staining, followed by whole slide scanning. Each liver lobe was scored using a modified Suzuki score (Table 4). The classic Suzuki score18 was augmented by incorporating three additional parameters: pyknosis of nuclei, detachment of vessels, and presence of erythrocytes in sinusoids and large vessels. Each parameter was graded as absent (0), mild (1), moderate (2), and severe (3). A final score of 0 – 7 was considered to reflect good preservation, 8 – 14 was taken as moderate preservation, and 14 – 21 indicated bad preservation.
Preservation of the mouse livers was assessed based on the modified Suzuki score. Two medical experts conducted an independent assessment of the morphology of the seven lobes of the three livers (Figure 5, Figure 6, Figure 7). The average of the seven lobe scores for each liver lobe was calculated; lower scores indicate a better-preserved liver. The evaluations rendered by the experts exhibited a high degree of concordance. Notably, while slight discrepancies in the scores assigned by the two experts were observed in the assessment of pyknosis of nuclei, these variations did not impact the overall scoring outcomes significantly.
At best, the liver parenchyma was relatively intact with a well-preserved typical lobular structure, hardly distinguishable from a normal liver. Hepatocytes appeared viable with clearly visible cell membranes and round nuclei. However, some nuclei underwent pyknosis, and some hepatic sinusoids were slightly dilated, resulting in a score of 4. (Figure 3, Figure 6)
At worst, the lobular structure was distorted, with vessels detached from the parenchyma and confluent parenchymal necrosis. On the cellular level, cellular vacuolization, and nuclei pyknosis became obvious, especially in the pericentral region. Furthermore, mild to moderate vacuolization was observed. Up to 30 % of the hepatocytes were undergoing necrosis resulting in a maximal score of 14. (Figure 3, Figure 7)
Table 1: Step-by-step establishment of a mouse liver perfusion model. Various complications were observed during the establishment process due to differences in cannula size, material, and positioning. Please click here to download this Table.
Table 2: Comparison of in vitro organ preservation methods15,17,19,20,21,22. The optimization of perfusate selection, oxygen carrier selection, and nutritional component comparison is crucial under various storage conditions. Please click here to download this Table.
Table 3: Hemodynamics and blood gas analysis in normal rats and rat NEVLP11,15,16,17,18,20,21,23,24,25,26,27,28,29,30,31. The provided information selectively describes the hemodynamic features of rat liver and key parameters of normothermic perfusion in vitro. Specifically, the rat liver perfusion can be considered optimal when the PV pressure typically ranges from 4 to 10 mmHg, and the partial pressure of oxygen in the perfusate entering the PV ranges from 80 to 550 mmHg, meeting the necessary criteria for successful in vitro rat liver perfusion. Please click here to download this Table.
Table 4: Modified Suzuki score. The modified Suzuki score used in this study expands upon the classic Suzuki score by incorporating three additional parameters: pyknosis of nuclei, detachment of vessels, and the presence of erythrocytes in sinusoids and large vessels. Each parameter was assigned a grade on a scale of 0 (absent), 1 (mild), 2 (moderate), or 3 (severe). The total score, ranging from 0 to 8, indicates good preservation; 9 to 16 suggests moderate preservation, and 17 to 24 indicates poor preservation. Please click here to download this Table.
Table 5: Selection of perfusate medium, perfusate volume, and perfusion pressure based on a literature workup of NEVLP in rats (2010-2022)3,8,10,14,17,19,27,30,31,32,33,34,35,36,37,38,
39,40,41,42,43,44,45. Normothermic machine perfusion in rat liver can exhibit variability across studies in terms of the specific type and volume of perfusate utilized, as well as the duration of perfusion. Different studies may employ varying approaches, such as dialysis or high-volume perfusion, for extended periods. However, despite these differences, parameters such as portal pressure, portal vein flow rate, and partial pressure of oxygen in the perfusate generally demonstrate minimal variation across the various methods employed. Please click here to download this Table.
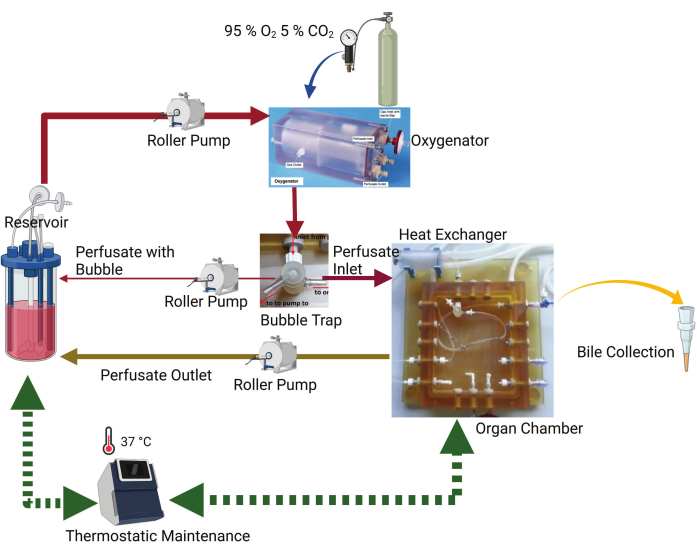
Figure 1: Schematic diagram of the perfusion system. The key components are the organ chamber, thermostatic machine, roller pump, oxygenator, and reservoir. The perfusate is pumped from the reservoir by a peristaltic pump into an oxygenator. There is an uninterrupted gas flow of 95% O2 and 5% CO2 in the oxygenator. The perfusate passes through the bubble trap, where any air bubbles present in the perfusate are captured and pumped back to the reservoir. The remaining perfusate flows to the organ chamber, where the tube is connected to the portal vein. The perfusate outflow from the organ chamber is directed back to the reservoir by the peristaltic pump. A bile drainage tube is connected to the organ chamber to collect bile. Please click here to view a larger version of this figure.
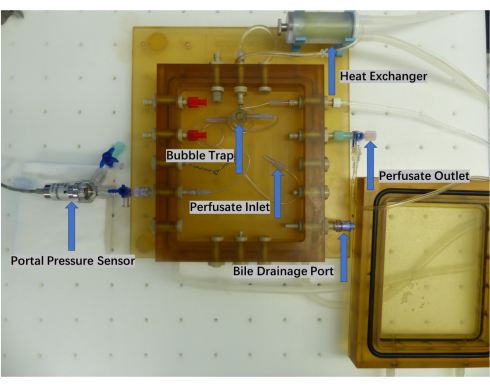
Figure 2: Close-up of the perfusion chamber. The organ perfusion chamber comprises a perfusate inlet and outlet, a bubble trap, a heat exchanger, and a bile collection port. Real-time monitoring of the perfusate pump pressure is accomplished using a pressure sensor. Please click here to view a larger version of this figure.
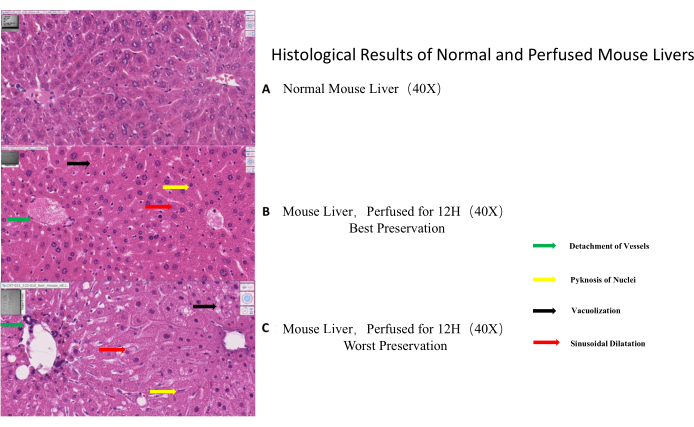
Figure 3: Histological results of normal and perfused mouse livers. (A) Normal mouse liver morphology (control). (B) Example of best-preserved morphology as visualized by HE-staining after 12 h of perfusion. (C). Example of worst-preserved morphology as visualized by HE-staining after 12 h of perfusion. The black arrow indicates vacuolization, the red arrow points to sinusoidal dilatation, the yellow arrow shows pyknosis of nuclei and the green arrow specifies vascular detachment. Please click here to view a larger version of this figure.
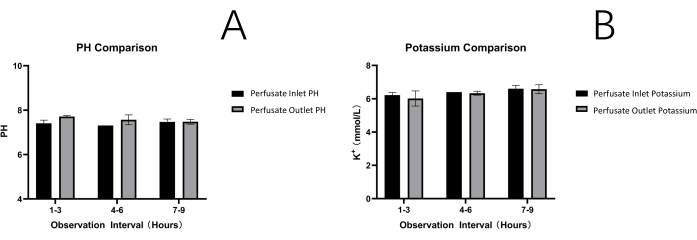
Figure 4: Perfusate analysis. Ph (A) and potassium levels (B). Both parameters are stable throughout the observation times of 12 h, indicating constant perfusion conditions Please click here to view a larger version of this figure.
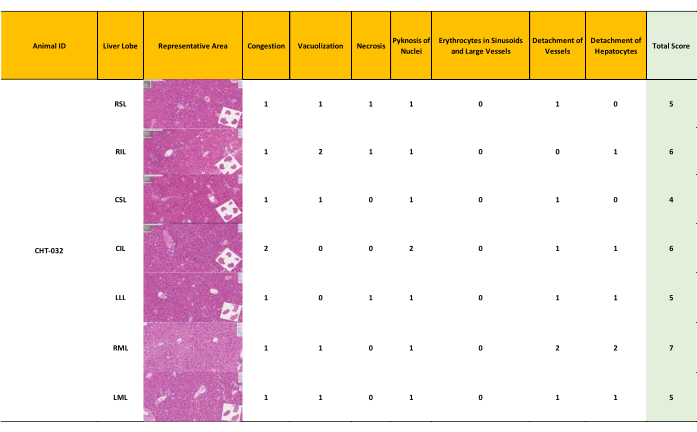
Figure 5: Minor liver damage resulting in a modified Suzuki score 4-7. After 12 hours of NEVLP, a semiquantitative assessment of liver morphology was conducted. Samples from each liver lobe were assessed and graded separately, resulting in a range and a mean score for each liver, with the highest possible score being 4. Well-preserved liver morphology with a score ranging from 4-7 depending on the liver lobe (mean = 5) (Score 0-7: well-preserved liver morphology, score 8-14 moderately preserved morphology, score 15-21: ill-preserved liver morphology) Please click here to view a larger version of this figure.
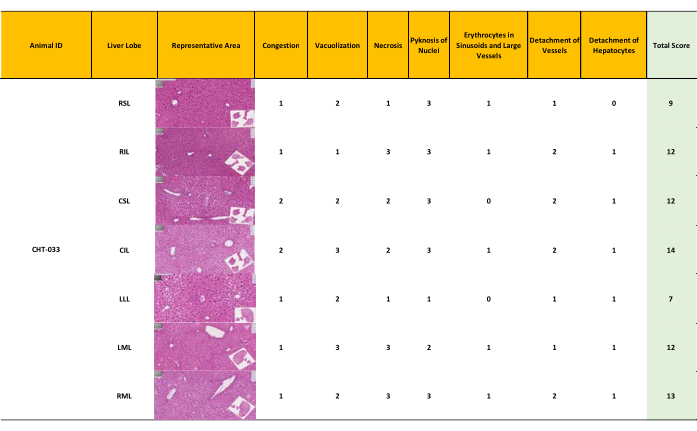
Figure 6: Moderate liver damage resulting in a modified Suzuki score 7-14. Semiquantitative assessment of liver morphology after 12 hours of normothermic oxygenated machine perfusion according to the modified Suzuki score. Moderately preserved morphology with a score ranging from 7 to 14 (mean = 11). (Score 0-7: well-preserved liver morphology, score 8-14 moderately preserved morphology, score 15-21: ill-preserved liver morphology) Please click here to view a larger version of this figure.
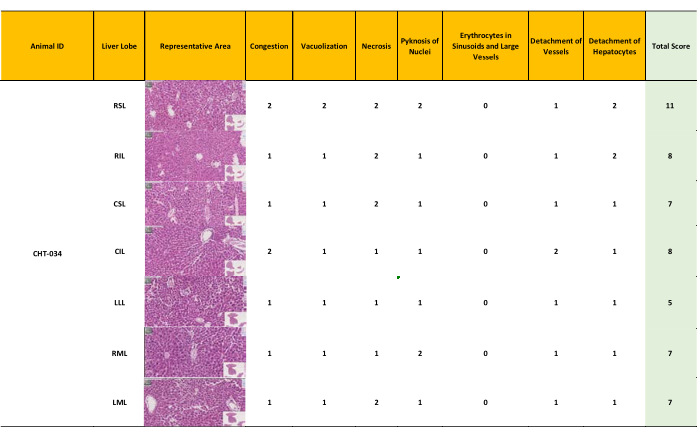
Figure 7: Minor – moderate liver damage resulting in a modified Suzuki score 5-11. The inhomogeneous perfusion resulted in minor to moderate preserved morphology in different liver lobes, with scores ranging from 5 to 11 (mean = 8). (Score 0-7: well-preserved liver morphology, score 8-14 moderately preserved morphology, score 15-21: ill-preserved liver morphology) Please click here to view a larger version of this figure.
Discussion
Critical steps in the protocol
The two crucial steps in liver explantation are the cannulation of the portal vein (PV) and the subsequent cannulation of the bile duct (BD). These steps are of paramount importance in ensuring successful organ retrieval and subsequent perfusion or transplantation procedures.
Challenges and solutions
PV cannulation presents three challenges: injury of the vessel wall, displacement of the catheter, and practicability of the insertion process. The delicate nature of the PV vessel wall makes it susceptible to puncture and subsequent bleeding if not handled carefully during cannulation. Moreover, any blood loss from the PV reduces portal pressure, making inserting the PV cannula more challenging. Furthermore, an injury to the portal venous wall may introduce air emboli into the intrahepatic vascular system. Therefore, exercising precision and caution during the PV cannulation process is crucial to minimize the risk of complications.
During PV cannulation, the risk of catheter displacement is a common concern, particularly when using cannulas with slippery materials. In comparison to polypropylene, the use of polyurethane cannulas is more favorable for PV cannulation. The soft and pliable nature of polyurethane significantly reduces the risk of catheter displacement or loss, potentially attributed to its material properties. Additionally, using a soft cannula minimizes the likelihood of damaging the endothelial layer of blood vessels. In the study, three types of vascular access cannulas, namely 24 G polypropylene, 26 G polypropylene, and 2 Fr polyurethane, were compared for PV cannulation. Among these options, both the 26 G and 2 Fr polyurethane cannulas demonstrated better compatibility with the size of the mouse PV. While the 26 G cannula exhibited better anatomical compatibility, the 2 Fr polyurethane cannula with an outer diameter of 0.66 mm was deemed suitable for the intended application due to its unique material properties, effectively minimizing the risk of unintentional catheter dislodgement from the PV.
Regarding the practicability of insertion, different techniques were tested. One way is to clamp the proximal and distal ends of the PV, cut a small hole with fine scissors, and insert the PV cannula. This technique necessitates the involvement of an additional person due to the complexity and requirement for simultaneous actions. Consequently, it is not feasible for a single microsurgeon to perform the procedure independently. Therefore, a catheter with an inner needle and an outer cannula is needed. As described before, using the commercially available stiff and smooth 26 G polypropylene cannula carries the risk of slipping out and risking the formation of air bubbles when connecting the cannula to the flushing system. To address these concerns, a self-constructed "needle-guided tube system" was devised by inserting a 26 G needle from a needle catheter into a lengthy and pliable polyurethane 2 Fr cannula. This approach offers three key advantages: (1) a catheter-insertion system, (2) a long and flexible tube, and (3) favorable material properties. However, during this step, it is crucial to exercise caution to prevent the needle tip from contacting the PV wall while advancing, as this could cause irreversible damage to the vessel wall.
BD cannulation presented the same three challenges: injury of the vessel wall, displacement of the catheter, and practicability of the insertion process. Ultimately, the 1 Fr polyurethane cannula was deemed more suitable than the UT – 03 polypropylene cannula due to its favorable material properties. Despite the slightly smaller outer diameter of the UT – 03 cannula (0.30 mm) compared to the 1 Fr thermo-sensitive polyurethane tube (0.33 mm), the polyurethane material is rigid and less prone to bending. On the other hand, the 1 Fr cannula, being soft and flexible, offers easier insertion and thus became our preferred choice. However, in cases where the BD size is exceptionally small and unable to accommodate the larger 2 Fr cannula, the UT – 03 cannula remains a viable alternative. In such instances, special attention must be given to prevent the displacement of the cannula from the BD.
As summarized in the literature review (Table 5), most of the experimenters19,41,42,46 used a portal flow rate of 1 – 3 mL/min/g liver. However, the flow rate should be adjusted to maintain the physiological portal pressure in the range of 4 – 10 mmHg28. High PV pressure may cause sinusoidal dilatation and vascular detachment. Low PV pressure and low PV flow rate might result in a low flow hypo-oxygenation with subsequent mid-zonal to pericentral necrosis. The manipulation of the PV flow rate serves the function of regulating PV pressure, which may vary according to the cannula used and the size of the liver, thus necessitating minor adjustments to the PV flow rate. So, in this study, perfusion was started at a flow rate of 1 mL/min/g liver, and at the same time, the PV pressure was monitored using a blood pressure transducer to maintain physiological PV pressure by adjusting the flow rate.
During the development NEVLP model, an attempt was made to further improve the oxygen supply to the perfused liver by adding washed red blood cells to the perfusate. Nevertheless, significant sedimentation of erythrocytes was observed in the large chamber and reservoir, thereby reducing the delivery of oxygen carriers to the organ during perfusion. Furthermore, damage to erythrocytes was caused by the mechanical action of the peristaltic perfusion pump, which drives the perfusate by compressing a silicon tube. Both reasons facilitated our decision not to use red blood cells in this experiment. Perfluorocarbon-based oxygen carriers may be able to solve these problems47.
To assess the viability of the liver graft during perfusion, mouse bile fluid was collected at 3 h intervals. Nevertheless, it is difficult to collect bile via the catheter due to the high viscosity of mouse bile fluid. This is initially compensated by the capillary forces induced by the fine 1 Fr polyurethane catheter placed in the BD. However, only about 20 µL of bile fluid could be collected within the first hour of the experiment. Instead of draining through the cannula, retrograde filling of the gallbladder was observed.
At the end of the 12 h perfusion period, the gallbladder was filled with clear bile fluid suggesting active bile production during machine perfusion. This can be potentially counteracted by placing a larger tube into the gall bladder.
In the meantime, the histological damage of the hepatic cellular and lobular structure was assessed to determine the result of preservation. It was observed that even within the same liver, preservation was inhomogeneous. The inhomogeneity of the structural changes suggests that the perfusion was heterogeneous throughout the liver (Figure 5, Figure 6, Figure 7). Moreover, the histological findings provide evidence that it is viable to preserve the structural integrity of the mouse liver graft for a minimum duration of 12 hours during machine perfusion. However, the presence of intact liver histology can only assist in the assessment but cannot definitively determine the function and viability of the liver. It should be noted that necrosis, as the ultimate manifestation of cellular damage, may not be readily observable until a later stage. Thus, relying solely on histological assessment may not provide a comprehensive understanding of the functional status and viability of the liver. Other complementary assays and evaluations are required to ascertain the overall condition of the liver, including functional assays, biochemical markers, and assessment of metabolic activity.
Good preservation was achieved after 12 hours of perfusion. However, further extending the perfusion time as possibly needed for organ repair requires addressing some issues. Firstly, it should be noted that achieving long-term perfusion durations exceeding 12 hours would necessitate maintaining sterile conditions rather than just clean conditions. However, in these initial experiments, the focus was on maintaining clean conditions rather than sterile conditions, as ensuring sterility would introduce additional complexities to the procedure. Secondly, longer perfusion would possibly require adding a dialysis unit, as described by Herman Tolboom22, to remove accumulating toxic metabolic waste products. They used a system with a total volume of 55 – 60 mL to perfuse a rat liver of 10 g for 4 h. In this study, a relatively large reservoir with a total volume of 300 mL was utilized despite the small size of the mouse liver, which weighs approximately 1 g. This configuration resulted in an additional dilution factor of 50 times. Remarkably, no detrimental effects were observed during the 12 hour perfusion period with this setup. Thirdly, longer perfusion would also require the addition of oxygen carriers, at best in the form of artificial hemoglobin to ensure adequate oxygen supply, as described by Dondossola et al.47 and Jägers et al.48.
The development of "non-ischemic" liver transplantation based on NEVLP has undoubtedly brought new ideas and methods towards solving or even preventing the problem of ischemia-reperfusion injury. However, NEVLP is a very promising concept for improving organ preservation and its potential application to extend organ preservation to organ repair47.
Currently, three different techniques of machine perfusion are used experimentally and clinically. The key difference is the working temperature: hypothermic machine perfusion, subnomothermic machine perfusion, and normothermic machine perfusion (Table 2). Other differences include the choice of preservation solution, perfusate solution, and addition of oxygen carriers (Table 5).
NEVLP is principally of advantage because (1) the organ is maintained at its normal temperature, (2) it is oxygenated and (3) it is metabolically fully supplied. The system provides an excellent platform for diagnostic evaluation, intervention therapy, and ultimately organ repair49. However, NEVLP poses a great challenge to the ex vivo organ support technology. The challenge for NEVLP is to mirror the near-physiological condition. Until recently, due to the small size and lack of standard evaluation criteria, only a limited number of rodent NEVLP studies were performed25,26,27,28,29,30,31.
It has been demonstrated here that the mouse model is a valid model that allows a preservation time of 12 hours. In comparison, most rat studies have reported perfusion times of 6 hours or less28,33. In addition, the use of small animals is of advantage for molecular studies compared to large animals due to the availability of abundant reagents and the lower experimental costs. For instance, mice are currently a preferable option for testing knockout models of the ATG gene family, especially for studying signaling pathways of ischemia-reperfusion injury in the liver8,50.
Experiments on rat livers regarding NEVLP have revealed that normothermic machine perfusion preservation is associated with reduced hepatocellular damage and improved early post-transplantation survival compared to cold preservation31,40,51,52,53,54. NEVLP using rat livers is also suitable for studying the additions of drugs or cells to the perfusate. An impressive example is the study of Xuan Tian26, who used heme oxygenase-1 modified mesenchymal stem cells combined with normothermic machine perfusion to improve the quality of liver grafts via the Wnt signaling pathway. Haojie Wang39 has confirmed in his recent study that adding bone marrow mesenchymal stem cells to the perfusate can greatly improve the quality of NEVLP in rats for short-time perfusion. In their study using DCD livers, bone marrow mesenchymal stem cells combined with NEVLP inhibited hepatic sinusoidal congestion and endothelial injury. The addition of mesenchymal stem cells prevented intrahepatic macrophage activation and intercellular adhesion. Furthermore, the addition of mesenchymal stem cells regulated the endothelin-1 / endothelial nitric oxide balance to improve liver perfusion and microcirculation.
Mice offer a distinct advantage over rats when it comes to NEVLP, particularly in the context of molecular studies focused on transgenic or genetically modified livers. However, due to the small size of the animal, the procedure poses a greater but manageable challenge to the microsurgeon37.
Grading system for histological assessment
The histological assessment is decisive for determining the impact of perfusion conditions on the morphological integrity of the graft.
While the Suzuki score is commonly utilized in studies assessing liver pathology, it has been noted that this scoring system may not adequately capture the specific findings observed in ex-vivo liver preservation. To address this limitation, four additional criteria were introduced to enhance the comprehensive evaluation of preserved liver tissue (Table 4). Firstly, the inclusion of nuclear pyknosis assessment was implemented, as it serves as a valuable parameter indicative of cellular damage. Secondly, the evaluation of vessel and hepatocyte detachment, which signifies damage to the hepatic lobule, was incorporated as an additional criterion. Lastly, the grading of erythrocytes' presence in sinusoids was utilized as an indicator of heterogeneous flushing and perfusion. By incorporating these supplementary criteria, a more nuanced and accurate assessment of the preserved liver tissue's condition was achieved, providing a deeper understanding of the effects of ex-vivo liver preservation.
Hepatocyte vacuolization55 occurs after alterations in substrate use, energy expenditure, microtubule disintegration, and inhibition of protein synthesis. The nucleus of the hepatocyte is forced to shift toward the periphery of the cell by large vacuoles. This process is frequently accompanied by nuclear pyknosis. In this study, 12 h of normothermic machine perfusion led to different degrees of vacuolization of hepatocytes compared to the control suggesting inhomogeneous perfusion.
The presence of dilated sinusoids between hepatocyte cords was notable in this study. This phenomenon primarily arises from hepatic venous outflow obstruction, leading to vascular stasis and congestion within the hepatic parenchyma. In this mouse liver model, the challenges associated with maintaining a consistent portal perfusion pressure due to the organ's small size may contribute to the observed dilation of hepatic sinusoids.
Necrotic changes typically manifest in cell clusters, regional areas, or specific zones. The well-perfused periportal area exhibited relatively better preservation of hepatocytes compared to the pericentral area. Dual-vessel liver perfusion, as demonstrated in rat livers20, presents a greater challenge in mouse livers due to the small size of the hepatic artery. Consequently, the observed inhomogeneous perfusion of the mouse liver may be attributed, at least in part, to this limitation.
These observations are not exclusive to mouse livers undergoing NEVLP but can also be visualized in histological images from other NEVLP studies, although they may not be explicitly described.
Importance and Potential Applications of Mouse NEVLP
NEVLP of mouse livers is a challenging but feasible procedure. Further efforts are needed to make the best use of this technology to elucidate the mechanism underlying the beneficial effect of NEVLP. Enhancing our knowledge will facilitate the progressive evolution of this technology, transitioning it beyond organ preservation toward the realm of "organ repair.".
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Throughout the writing of this paper, I have received a great deal of support and assistance. I would particularly like to acknowledge my teammate XinPei Chen for his wonderful collaboration and patient support during my operation.
Materials
| 0.5 ml Micro Tube PP | Sarstedt | 72699 | |
| 1 Fr Rubber Cannula | Vygon | Sample Cannula | |
| 10 µL Micro Syringe | Hamilton | 701N | |
| 2 Fr Rubber Cannula | Vygon | Sample Cannula | |
| 24 G Butterfly Cannula | Terumo | SR+OF2419 | |
| 26 G Butterfly Cannula | Terumo | SR+DU2619WX | |
| 30 G Hypodermic Needle | Sterican | 100246 | |
| 50 ml Syringe Pump | Braun | 110356 | |
| 6-0 Perma-Hand Seide | Ethicon | 639H | |
| Arterial Clip | Braun | BH014R | |
| Autoclavable Moist Chamber | Hugo Sachs Elektronik | 73-4733 | |
| Big Cotton Applicator | NOBA Verbandmittel Danz GmbH | 974018 | |
| Bubble Trap | Hugo-Sachs-Elektronik | V83163 | |
| Buprenovet (0.3 mg / ml) | Elanco | / | |
| CIDEX OPA solution (2 L) | Cilag GmbH | 20391 | |
| Electrosurgical Unit for Monopolar Cutting VIO® 50 C | ERBE | / | |
| Fetal Bovine Serum(500 ml) | Sigma-Aldrich | F7524-500ML | |
| Gas Mixture (95 % oxygen & 5 % carbon dioxide) | House Supply | / | |
| Heating Circulating Baths | Harvard-Apparatus | 75-0310 | |
| Heparin 5000 (I.E. /5 ml) | Braun | 1708.00.00 | |
| Hydrocortisone (100 mg / 2 ml) | Pfizer | 15427276 | |
| Insulin(100 IE / ml) | Sigma | I0516-5ML | |
| Iris Scissors | Fine Science Instruments | 15000-03 | |
| Isofluran (250 ml) | Cp-Pharma | 1214 | |
| Membrane Oxygenator | Hugo Sachs Elektronik | T18728 | |
| Microsurgery Microscope | Leica | M60 | |
| Mouse Retractor Set | Carfil Quality | 180000056 | |
| NanoZoomer 2.0 HT | Hamamatsu | / | |
| Non-Woven Sponges | Kompressen | 866110 | |
| Penicillin Streptomycin (1 mg / ml) | C.C.Pro | Z-13-M | |
| Perfusion Extension Tube (30 cm) | Braun | 4256000 | |
| Peristaltic Pump | Harvard-Apparatus | P-70 | |
| Petri Dishc 100×15 mm | VWR® | 391-0578 | |
| Povidon-Jod (Vet-Sep Spray) | Livisto | 799-416 | |
| Pressure Transducer Simulator | UTAH Medical Products | 650-950 | |
| Reusable Blood Pressure Transducers | AD Instruments | MLT-0380/D | |
| S & T Vessel Cannulation Forceps | Fine Science Instruments | 00608-11 | |
| Small Cotton Applicator | NOBA Verbandmittel Danz GmbH | 974116 | |
| Straight Forceps 10 cm | Fine Science Instruments | 00632-11 | |
| Suture Tying Forceps | Fine Science Instruments | 11063-07 | |
| Syringe 50ml Original Perfusor | Braun | 8728810F-06 | |
| UT – 03 Cannula | Unique Medical, Japan | / | |
| Vannas Spring Scissors | Fine Science Instruments | 15018-10 | |
| Veterinary Saline (500 ml) | WDT | 18X1807 | |
| Water Jacketed Reservoir 2 L | Harvard-Apparatus | 73-3441 | |
| William's E Medium (500 ML) | Thermofischer Scientific | A1217601 |
Riferimenti
- Kwong, A. J., et al. OPTN/SRTR 2021 Annual data report: liver. American Journal of Transplantation. 23 (2), S178-S263 (2023).
- Linares, I., Hamar, M., Selzner, N., Selzner, M. Steatosis in Liver Transplantation: Current Limitations and Future Strategies. Transplantation. 103 (1), 78-90 (2019).
- Cheng, N., et al. Pharmacological activating transcription factor 6 activation is beneficial for liver retrieval with ex vivo normothermic mechanical perfusion from cardiac dead donor rats. Frontiers in Surgery. 8, 665260 (2021).
- Porte, R. J. Improved organ recovery after oxygen deprivation. Nature. 608 (7922), 273-274 (2022).
- Goumard, C., et al. Ex-Vivo Pharmacological Defatting of the Liver: A Review. Journal of Clinical Medicine. 10 (6), 1253 (2021).
- Mao, B., Yuan, W., Wu, F., Yan, Y., Wang, B. Autophagy in hepatic ischemia-reperfusion injury. Cell Death Discovery. 9 (1), 115 (2023).
- Hale, A. N., Ledbetter, D. J., Gawriluk, T. R., Rucker, E. B. Autophagy: regulation and role in development. Autophagy. 9 (7), 951-972 (2013).
- Tang, B., Bao, N., He, G., Wang, J. Long noncoding RNA HOTAIR regulates autophagy via the miR-20b-5p/ATG7 axis in hepatic ischemia/reperfusion injury. Gene. 686, 56-62 (2019).
- Kuma, A., Komatsu, M., Mizushima, N. Autophagy-monitoring and autophagy-deficient mice. Autophagy. 13 (10), 1619-1628 (2017).
- van der, V. a. l. k. . J. Fetal bovine serum-A cell culture dilemma. Science. 375 (6577), 143-144 (2022).
- Haque, O., et al. Twenty-four hour ex-vivo normothermic machine perfusion in rat livers. Technology (Singapore World Science). 8 (1-2), 27-36 (2020).
- Op den Dries, S., et al. Normothermic machine perfusion reduces bile duct injury and improves biliary epithelial function in rat donor livers. Liver Transplantation. 22 (7), 994-1005 (2016).
- Izamis, M. L., et al. Machine perfusion enhances hepatocyte isolation yields from ischemic livers. Cryobiology. 71 (2), 244-255 (2015).
- Gassner, J. M. G. V., et al. Improvement of normothermic ex vivo machine perfusion of rat liver grafts by dialysis and kupffer cell inhibition with glycine. Liver Transplantation. 25 (2), 275-287 (2019).
- Casado, J., et al. Rat splanchnic net oxygen consumption, energy implications. The Journal of Physiology. 431, 557-569 (1990).
- Tolboom, H., et al. A model for normothermic preservation of the rat liver. Tissue Engineering. 13 (8), 2143-2151 (2007).
- Yamada, S., et al. Effects of short-term normothermic and subnormothermic perfusion after cold preservation on liver transplantation from donors after cardiac death. Transplantation Proceedings. 52 (6), 1639-1642 (2020).
- Behrends, M., et al. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. Journal of Gastrointestinal Surgery. 14 (3), 528-535 (2010).
- Tolboom, H., et al. Sequential cold storage and normothermic perfusion of the ischemic rat liver. Transplant Proceeding. 40 (5), 1306-1309 (2008).
- Daemen, M. J., et al. Liver blood flow measurement in the rat. The electromagnetic versus the microsphere and the clearance methods. Journal of Pharmacological Methods. 21 (4), 287-297 (1989).
- Koo, A., Liang, I. Y. Microvascular filling pattern in rat liver sinusoids during vagal stimulation. The Journal of physiology. 295, 191-199 (1979).
- Beal, E. W., et al. [D-Ala2, D-Leu5] Enkephalin improves liver preservation during normothermic ex vivo perfusion. Journal of Surgical Research. 241, 323-335 (2019).
- Birnie, J. H., Grayson, J. Observations on temperature distribution and liver blood flow in the rat. The Journal of Physiology. 116 (2), 189-201 (1952).
- Silitonga, M., Silitonga, P. M. Haematological profile of rats (Rattus norvegicus) induced BCG and provided leaf extract of Plectranthus amboinicus Lour Spreng). AIP Conference Proceedings. 1868, 090008090008 (2017).
- Jacob Filho, W., et al. Reference database of hematological parameters for growing and aging rats. Aging Male. 21 (2), 145-148 (2018).
- Tian, X., et al. Heme oxygenase-1-modified bone marrow mesenchymal stem cells combined with normothermic machine perfusion repairs bile duct injury in a rat model of DCD liver transplantation via activation of peribiliary glands through the Wnt pathway. Stem Cells International. 2021, 9935370 (2021).
- Yang, L., et al. Normothermic machine perfusion combined with bone marrow mesenchymal stem cells improves the oxidative stress response and mitochondrial function in rat donation after circulatory death livers. Stem Cells Development. 29 (13), 835-852 (2020).
- Wang, L., He, H. W., Zhou, X., Long, Y. Ursodeoxycholic Acid (UDCA) promotes lactate metabolism in mouse hepatocytes through cholic acid (CA) – farnesoid x receptor (FXR) pathway. Current Molecular Medicine. 20 (8), 661-666 (2020).
- Akateh, C., Beal, E. W., Whitson, B. A., Black, S. M. Normothermic ex-vivo liver perfusion and the clinical implications for liver transplantation. Journal of Clinical and Translational Hepatology. 6 (3), 276-282 (2018).
- Westerkamp, A. C., et al. Metformin preconditioning improves hepatobiliary function and reduces injury in a rat model of normothermic machine perfusion and orthotopic transplantation. Transplantation. 104 (9), e271-e280 (2020).
- Nösser, M., et al. Development of a rat liver machine perfusion system for normothermic and subnormothermic conditions. Tissue Engineering. Part A. 26 (1-2), 57-65 (2020).
- Yao, J., et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB Journal. 33 (2), 1695-1710 (2019).
- Haque, O., et al. The effect of blood cells retained in rat livers during static cold storage on viability outcomes during normothermic machine perfusion. Scientific Reports. 11 (1), 23128 (2021).
- Gillooly, A. R., Perry, J., Martins, P. N. First report of siRNA uptake (for RNA interference) during ex vivo hypothermic and normothermic liver machine perfusion. Transplantation. 103 (3), e56-e57 (2019).
- Beal, E. W., et al. A small animal model of ex vivo normothermic liver perfusion. Journal of visualized experiments. (136), e57541 (2018).
- Claussen, F., et al. Dual versus single vessel normothermic ex vivo perfusion of rat liver grafts using metamizole for vasodilatation. PLoS One. 15 (7), (2020).
- Yang, L., et al. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell and Tissue Research. 381 (2), 239-254 (2020).
- Wu, L., et al. Bone marrow mesenchymal stem cells modified with heme oxygenase-1 alleviate rejection of donation after circulatory death liver transplantation by inhibiting dendritic cell maturation in rats. International Immunopharmacology. 107, 108643 (2022).
- Lonati, C., et al. Quantitative Metabolomics of Tissue, Perfusate, and Bile from Rat Livers Subjected to Normothermic Machine Perfusion. Biomedicines. 10 (3), (2022).
- Oldani, G., et al. The impact of short-term machine perfusion on the risk of cancer recurrence after rat liver transplantation with donors after circulatory death. PLoS One. 14 (11), e0224890 (2019).
- Abraham, N., et al. Two compartment evaluation of liver grafts during acellular room temperature machine perfusion (acRTMP) in a rat liver transplant model. Frontiers in Medicine (Lausanne). 9, 804834 (2022).
- Scheuermann, U., et al. Sirtuin-1 expression and activity is diminished in aged liver grafts. Scientific Reports. 10 (1), 11860 (2020).
- Scheuermann, U., et al. Damage-associated molecular patterns induce inflammatory injury during machine preservation of the liver: potential targets to enhance a promising technology. Liver Transplantation. 25 (4), 610-626 (2019).
- Carnevale, M. E., et al. The novel N, N-bis-2-hydroxyethyl-2-aminoethanesulfonic acid-gluconate-polyethylene glycol-hypothermic machine perfusion solution improves static cold storage and reduces ischemia/reperfusion injury in rat liver transplant. Liver Transplantation. 25 (9), 1375-1386 (2019).
- Von, C., Horn, H., Zlatev, J., Pletz, B., Lüer, T., Minor, Comparison of thermal variations in post-retrieval graft conditioning on rat livers. Artificial Organs. 46 (2), 239-245 (2022).
- Tomizawa, M., et al. Oncostatin M in William’s E medium is suitable for initiation of hepatocyte differentiation in human induced pluripotent stem cells. Molecular Medicine Reports. 15 (5), 3088-3092 (2017).
- Dondossola, D., et al. Human red blood cells as oxygen carriers to improve ex-situ liver perfusion in a rat model. Journal of Clinical medicine. 8 (11), (2019).
- Jägers, J., Wrobeln, A., Ferenz, K. B. Perfluorocarbon-based oxygen carriers: from physics to physiology. European Journal of Physiology. 473 (2), 139-150 (2021).
- Jia, J., et al. A promising ex vivo liver protection strategy: machine perfusion and repair. Surgery and Nutrition. 8 (2), 142-143 (2019).
- Jennings, H., et al. The immunological effect of oxygen carriers on normothermic ex vivo liver perfusion. Frontiers in Immunology. 13, 833243 (2022).
- Kim, J. S., et al. Carbamazepine suppresses calpain-mediated autophagy impairment after ischemia/reperfusion in mouse livers. Toxicology and Applied Pharmacology. 273 (3), 600-610 (2013).
- Imber, C. J., et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 73 (5), 701-709 (2002).
- Tolboom, H., et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation. 87 (2), 170-177 (2009).
- Rigo, F., Navarro-Tableros, V., De Stefano, N., Calleri, N., Romagnoli, A. Ex vivo normothermic hypoxic rat liver perfusion model: an experimental setting for organ recondition and pharmacological intervention. Methods in Molecular Biology. 2269, 139-150 (2021).
- van Dyk, J. C., Pieterse, G. M., van Vuren, J. H. Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicology and Environmental Safety. 66 (3), 432-440 (2007).

