小龙虾的游泳足系统:实用指南为神经线及电机模式外记录的解剖
Summary
Here we describe the dissection of the crayfish abdominal nerve cord. We also demonstrate an electrophysiological technique to record fictive locomotion from swimmeret motor neurons.
Abstract
在这里,我们证明小龙虾腹部神经索的解剖。该制剂包含的最后两个胸神经节(T4,T5)和腹腔神经节的链(A1到A6)。这种链节包括中枢神经系统(CNS),其驱动所述腹肢(游泳足)的协调运动的部分:游泳足系统。它是已知的,在小龙虾每个游泳足由它自己的独立模式生成内核生成节奏交替活动1-3从动超过五十年。运动神经元支配每个游泳足的肌肉组织包括两个解剖学和功能上不同的人群4。一个是负责对游泳足的缩回(动力行程,PS)。其他驱动游泳足的前伸(回击,RS)。所述游泳足系统的运动神经元都能够自发产生假想马达模式,这是相同的记录在体内图案</EM> 1。
本报告的目的是介绍一个有趣和方便的模型系统研究的节奏生成网络和协调学生的实践课程,实验室独立的微电路。所提供的协议包括一步一步的说明小龙虾的腹神经索的解剖,神经节钉扎的孤立链,desheathing的神经节,并从隔离神经系统记录游泳足虚构的运动模式细胞外。
此外,我们还可以监视从树突细胞内记录的游泳足的神经元的活性。在这里,我们还简要介绍这些技术,并提供了一些例子。此外,游泳足神经元的形态,可以使用各种染色技术进行评估。在这里,我们提供的细胞内(通过离子电渗疗法)染料填充神经元和游泳足运动神经元的池的回填的例子。在我们的实验室我们使用这种制剂来研究微电路之间假想运动,感觉反馈对CNS的活性的效果,和协调的基本功能在细胞水平上。
Introduction
小龙虾的游泳足发球局中姿态控制功能,并有节奏地敲打当动物往前游,通风的洞穴或女性充气鸡蛋5,6,信号小龙虾, 螯leniusculus的游泳足,是成对出现的,从第二到第五腹段,用一肢上腹部7的每一侧。对中枢神经系统产生自身的节律马达图案驱动在完整的动物,以及在分离的神经索制备的游泳足运动。当没有感官反馈或降序输入本公司生产的节奏马达模式被称为假想运动1,2,在游泳足系统该电动机模式中不从在完整动物测得的游泳足的活性的任何参数不同。
每个游泳足的运动是通过将位于和限制为一个c-微电路驱动orresponding hemiganglion 1 – 3在每个微电路是有规律生成内核包括五个确定非扣球的interneurons。它们可以在功能特征为两种动力冲程抑制剂(IPS)或回击抑制剂(IRS)8。这些IPS和IRS的interneurons不是内生的振荡器,而他们的交流活动是由交互抑制9驱动。由于这些的interneurons直接抑制游泳足运动神经元,交替的PS-RS机芯生成10。运动然而,这不仅需要活动的产生,同时也协调不同的独立微电路。在游泳足系统,这种协调是通过协调微电路可确保四肢活跃在正确的时间建立。此微电路由三个识别神经元中的每个段11-15构建的。
该协议规定,日Ë首次一步一步夹层导向分离神经节(T4至A6, 图1)的链。我们展示了如何针隔离腹部神经索和desheathe每节。在这种孤立的神经系统的准备,负责游泳足的运动神经元是准备在电生理及形态学实验。此协议的第二部分展示了游泳足运动模式的主要特点。这包括了一步一步的指导,细胞外记录的游泳足的运动神经元的活动。 RS运动神经元的轴突通过神经N1的前支伸出,而PS的运动神经元的轴突通过同一神经的后支( 图1)突出4。因此它们的活性可以从这些分支具有差分针电极进行记录。
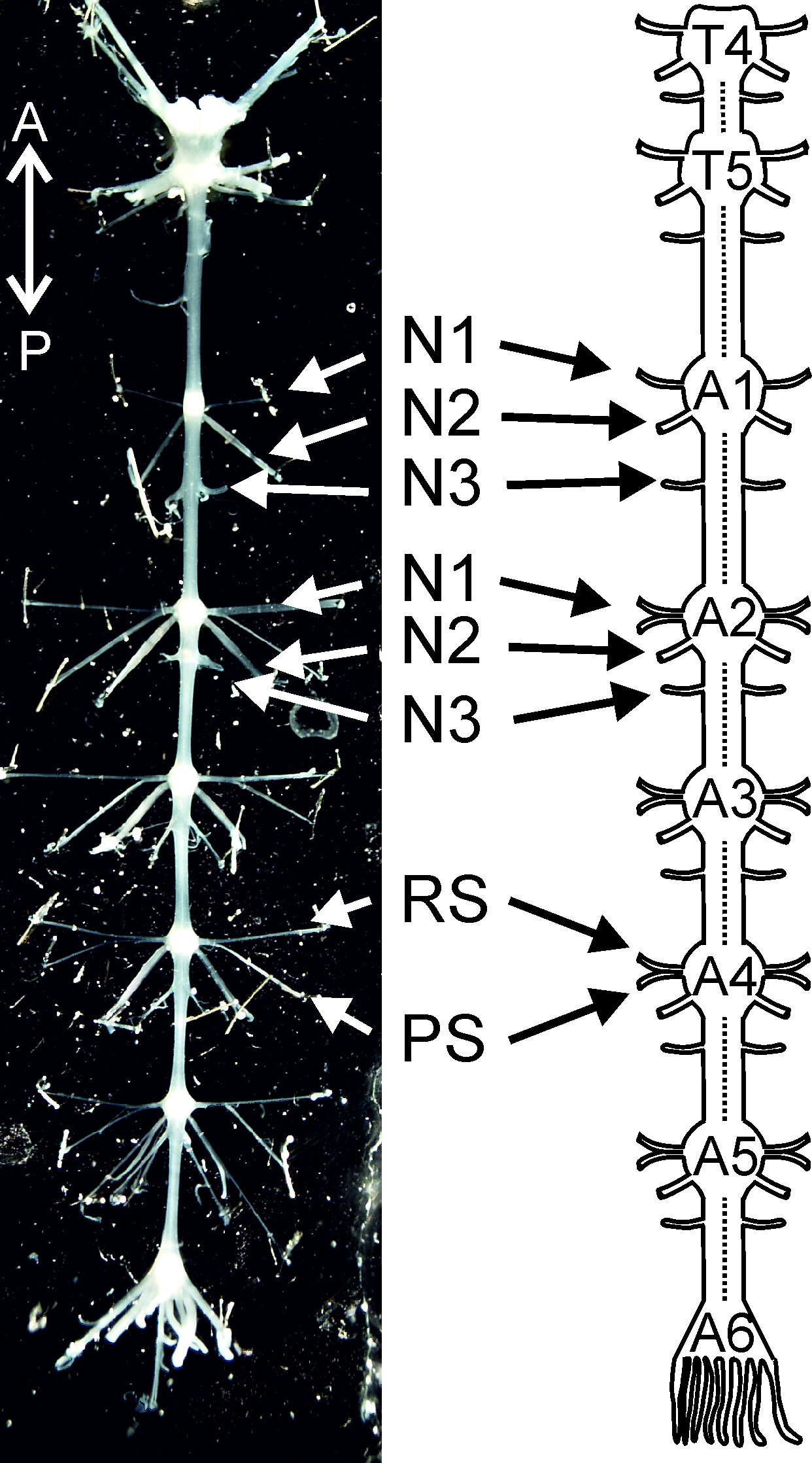 <br/> 图1:隔离从胸神经节4(T 4),以腹神经节6(A6)和它 T4 的示意图神经系统:胸神经节4; T5:胸神经节5; A1,A2 …… A6腹神经节1,腹神经节2 …腹神经节6; N1:神经N1; N2:神经N2; N3:神经N3; PS:电源冲程; RS:返回行程。定向缩写: A =前; P =后路。
<br/> 图1:隔离从胸神经节4(T 4),以腹神经节6(A6)和它 T4 的示意图神经系统:胸神经节4; T5:胸神经节5; A1,A2 …… A6腹神经节1,腹神经节2 …腹神经节6; N1:神经N1; N2:神经N2; N3:神经N3; PS:电源冲程; RS:返回行程。定向缩写: A =前; P =后路。
这解剖程序和电技术展示是方便本科生和可能的生理补充学生实践课程。神经节的分离链已被用在大量的实验以研究神经系统的功能,协调性,或游泳足微电路6的调制以及在运动16,17适应性行为的神经元控制,小龙虾游泳足系统因此提供了一个巨大的量有趣的教学或T下雨,所有开始与小龙虾和假想马达图案的细胞外记录的腹神经索的解剖机会。
Protocol
Representative Results
Discussion
小龙虾的解剖和它们的腹神经节以前已经描述5,18,19,20和建议之前夹层熟悉它们,以避免的重要神经切割。
关键是要保持的制备在温度低于23℃,以防止分离的神经索的降解。这可以容易地通过更换浴液每20-30分钟用冷小龙虾盐水来实现。在这些情况下,可用于电生理实验长达12小时神经节的链。
有时候,游泳足运动神经元处于非活动状态…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
我们感谢乔斯Burgert帮助有一些数字。我们感谢英戈SELBACH(和组“Edelkrebsprojekt北威州”),为他的努力提供实验室与实验动物。我们感谢安娜·C·施奈德校对书稿的第一个版本。这项研究是由埃米诺特DFG授予SM 206 / 3-1和科隆大学女教师启动资助。
Materials
| Name of Material/ Equipment | Tipo | Company | Catalog Number | Comments/ Description |
| 4-channel extracellular amplifier: MA 102 | Amplifier | Elektroniklabor, Zoologie, Universität zu Köln, Germany | for extracellular recording | |
| air-table | Technical Manufacturing Corporation (TMC) a unit of AMETEK Ultra Precision Technologies, Peabody, MA, USA |
63-534 | for intracellular recording | |
| Axon Digidata 1440A | Digitizer | Axon Instruments, Molecular Devices Design, Union City, CA | DD1440A | digitizes recorded signals |
| big bucket | filled with ice | |||
| Clampex & Clampfit | pClamp 10, recording and analysis software | Molecular Devices Design, Union City, CA | pClamps 10 Standard | for extracellular recording |
| cold lamp source | with flexible light guide (fiber optic bundle) | Euromex microscopes holland, Arnhem, BD | LE.5211 & LE.5235 | |
| computer and monitor | equipped with recording software | for extracellular recording | ||
| container and pipette for liquid waste | ||||
| crayfish saline | contains (in mM): 5.4 KCl, 2.6 MgCl2, 13.5 CaCl2, and 195 NaCl, buffered with 10mM Tris base and 4.7mM maleic acid; aerated for 3 hours. Adjust at pH of 7.4. | always keep at temperatures ~ 4° C | ||
| dextran, Texas Red (3000MW, lysine fixable) | fluorescent dye, lysine fixable | Life Technologies GmbH, Darmstadt, Germany | D3328 | for intracellular dyefill of neurons |
| differential pin electrodes | made from stainless steel ɸ 0.2 mm | for extracellular recording | ||
| dissection dish | (l x w x h) 15x7x5 cm; linned with black silicone | used in the gross disection | ||
| faraday cage | for extracellular recording | |||
| fixing pins | for pinning the specimen | |||
| forceps (biology, Dumont #5) | Forceps: Biology, tip 0.05 x 0.02 mm, length 11cm, INOX | Fine Science Tools (FST), Germany | 11252-20 | fine forceps: used to pick nerves |
| forceps (biology, Dumont #55) | Forceps: Biology, tip 0.05 x 0.02 mm, length 11cm, INOX | Fine Science Tools (FST), Germany | 11255-20 | extra fine forceps: used for desheathing |
| forceps (electronic, Dumont #5) | Forceps: Standard, tip 0.1 x 0.06 mm, length 11cm, INOX | Fine Science Tools (FST), Germany | 11251-20 | coarse forceps: used to grab specimen and pins |
| intracellular electrode | Borosilicate glass capillaries (outer/inner diameter: 1mm/0.5mm), with filament | Sutter Instruments, Novato, CA | BF100-50-10 | for intracellular recording and dyefill of neurons |
| Leica S8 Apo StereoZoom | Dissection Microscope Zoom 1x – 8x | Leica, Germany | 10446298 | for extracellular recording |
| microscope table | for extracellular recording | |||
| mirror | to illuminate preparation from below | for extracellular recording | ||
| modeling clay | for extracellular recording | |||
| Olympus SZ61 | Dissection Microscope Zoom 0.67x – 4.5x | Olympus, Germany | for the dissection | |
| petri dish | 94 x 16 mm; lined with clear silicone | Greiner bio-one, Germany | 633180 | used to pin the isolated chain of ganglia |
| ring scissors | ThoughCut, cutting edge: sharp/blunt, straight: 13cm | Fine Science Tools (FST), Germany | 14054-13 | for gross dissection (steps 2.1 – 2.11) |
| saline dispenser with a 16 gauge needle (outer ɸ 1.6mm) attached via a flexible tube. | Volume ~ 60ml, | used for exsanguination | ||
| spring scissors or alternative: Vannas spring scissors | cutting edge: 8 mm, tip diameter: 0.2mm, straight: 10cm or cutting edge 2.5 mm, tip diameter 0.075 mm, straight: 8cm | Fine Science Tools (FST), Germany | 15024-10 or 15000-08 | for desheathing |
| stainless steel wire ɸ 0.125 mm | to cut pins of 4-7 mm length | Goodfellow GmbH, Bad Nauheim, Germany | for pinning of the nerve cord | |
| student Vannas spring scissors or alternative: Moria Spring Scissors | cutting edge: 5mm, tip diameter: 0.35mm, straight: 9cm or cutting edge: 5mm, tip diameter 0,1 mm, straight: 8 cm | Fine Science Tools (FST), Germany | 91500-09 or 15396-00 | for gross and fine disection (steps 2.11 – 3.14) |
| sylgard | 184 Silicone Elastomer Base and Curing Agent; for black sylgard add activated carbon | Dow Corning, Midland, MI, USA | ||
| syringe filled with petroleum jelly and equipped with a 20 gauche needle with rounded tip | for extracellular recording |
Riferimenti
- Hughes, G. M., Wiersma, C. A. G. The Co-Ordination of Swimmeret Movements in the Crayfish, Procambarus-Clarkii (Girard). J Exp Biol. 37 (4), 657-670 (1960).
- Mulloney, B., Smarandache, C. Fifty Years of CPGs: Two Neuroethological Papers that Shaped the Course of Neuroscience. Front Behav Neurosci. 4, 45 (2010).
- Murchison, D., Chrachri, A., Mulloney, B. A Separate Local Pattern-Generating Circuit Controls the Movements of Each Swimmeret in Crayfish. J Neurophys. 70 (6), 2620-2631 (1993).
- Mulloney, B., Hall, W. M. Functional organization of crayfish abdominal ganglia. III. Swimmeret motor neurons. J Comp Neurol. 419 (2), 233-243 (2000).
- Davis, W. J. Lobster Righting Responses and Their Neural Control. Proc R Soc Ser B-Bio. 170 (1021), 435-456 (1968).
- Mulloney, B., Smarandache-Wellmann, C. Neurobiology of the crustacean swimmeret system. Prog Neurobiol. 96 (2), 242-267 (2012).
- Huxley, T. H. . The crayfish: An introduction to the study of zoology. , (1980).
- Smarandache-Wellmann, C., Weller, C., Wright, T. M., Mulloney, B. Five types of nonspiking interneurons in local pattern-generating circuits of the crayfish swimmeret system. J Neurophys. 110 (2), 344-357 (2013).
- Skinner, F. K., Mulloney, B. Intersegmental coordination of limb movements during locomotion: mathematical models predict circuits that drive swimmeret beating. J Neurosci. 18 (10), 3831-3842 (1998).
- Mulloney, B. During fictive locomotion, graded synaptic currents drive bursts of impulses in swimmeret motor neurons. J Neurosci. 23 (13), 5953-5962 (2003).
- Smarandache-Wellmann, C., Grätsch, S. Mechanisms of coordination in distributed neural circuits: Encoding coordinating information. J Neurosci. 34 (16), 5627-5639 (2014).
- Mulloney, B., Hall, W. M. Local commissural interneurons integrate information from intersegmental coordinating interneurons. J Comp Neurol. 466 (3), 366-376 (2003).
- Mulloney, B., Harness, P. I., Hall, W. M. Bursts of information: Coordinating interneurons encode multiple parameters of a periodic motor pattern. J Neurophys. 95 (2), 850-861 (2006).
- Smarandache, C., Hall, W. M., Mulloney, B. Coordination of Rhythmic Motor Activity by Gradients of Synaptic Strength in a Neural Circuit That Couples Modular Neural Oscillators. J Neurosci. 29 (29), 9351-9360 (2009).
- Smarandache-Wellmann, C., Weller, C., Mulloney, B. Mechanisms of Coordination in Distributed Neural Circuits: Decoding and Integration of Coordinating Information. J Neurosci. 34 (3), 793-803 (2014).
- Chrachri, A., Neil, D., Mulloney, B. State-Dependent Responses of 2 Motor Systems in the Crayfish, Pacifastacus leniusculus. J Comp Physiol A. 175 (3), 371-380 (1994).
- Chrachri, A., Neil, D. M. Interaction and Synchronization between 2 Abdominal Motor Systems in Crayfish. J Neurophys. 69 (5), 1373-1383 (1993).
- Skinner, K. The Structure of the 4th Abdominal-Ganglion of the Crayfish, Procambarus-Clarki (Girard) II. Synaptic Neuropils. J Comp Neurol. 234 (2), 182-191 (1985).
- Skinner, K. The Structure of the 4th Abdominal-Ganglion of the Crayfish, Procambarus-Clarki (Girard) I. Tracts in the Ganglionic Core. J Comp Neurol. 234 (2), 168-181 (1985).
- Mulloney, B., Tschuluun, N., Hall, W. M. Architectonics of crayfish ganglia. Microsc Res Techniq. 60 (3), 253-265 (2003).
- Braun, G., Mulloney, B. Cholinergic modulation of the swimmeret motor system in crayfish. J Neurophys. 70 (6), 2391-2398 (1993).
- Davis, W. J. Motoneuron Morphology and Synaptic Contacts – Determination by Intracellular Dye Injection. Science. 168 (3937), 1358-1360 (1970).
- Altman, J. S., Tyrer, N. M., Strausfeld, N. J., Miller, T. A. Filling Selected Neurons with Cobalt through Cut Axons. Neuroanatomical Techniques. , 373-402 (1980).

