Summary
Here, we describe a cardiac surgical procedure to implant engineered tissue in the atrioventricular (AV)-groove of an adult Lewis rat.
Abstract
Protocol
Part 1: Induction of general anesthesia
- A 200-250 g Lewis rat is anesthetized with 50 mg/kg Ketamine and 4 mg/kg Xylazine injected with a 30 G needle into the intraperitoneal cavity. The upper abdomen and right chest are clipped to remove hair and the incision site thoroughly cleaned with 70% ethanol and then a povidone-iodine topical antiseptic solution.
- The animal is intubated endotracheally using a 16 G intravenous catheter connected to a small animal respirator (Harvard Apparatus). The respirator is connected to a vaporizer to supply 0.5 to 1.0% Isoflurane (Baxter) mixed with 100% oxygen to maintain general anesthesia. Respiration rate and tidal volume is adjusted according to the body weight of the rat. The animal is placed on a heating pad (Hood Thermo-Pad) to maintain body temperature during the surgical procedure.
- To monitor heart rate and oxygen saturation throughout the surgical procedure a pulsoximeter device (Nellcor) is clipped onto one of the back limbs.
Part 2: Thoracotomy and engineered tissue implantation
- The surgical site is prepared with sterile surgical draping that is held in place with towel clamps.
- An incision is made from the antero-lateral to the lateral wall of the right chest, just above the 5th intercostal space. The interior of the chest is accessed through the 5th intercostal space and a small retractor (Roboz) is positioned to maximize the view.
- The pericardium is cut just above the appendix of the right atrium and right ventricle from the base toward the apex of the heart. Afterward, the AV groove is identified and the epicardial layer adjacent to the aorto-atrio-ventricular triangle is gently teased apart.
- A 7-0 Prolene suture (Ethicon) is placed through the basal shoulder of the right ventricle. A 2 x 2 x 2 mm piece is dissected from the engineered tissue construct and positioned within the AV groove and gently tied down with the Prolene suture.
- The surgical field is checked for bleeding and two 4-0 Vicryl sutures (Ethicon) are placed between the upper and lower intercostal space for closure of the rib-cage.
- Any apparent pneumothorax is excavated by expanding the lungs by way of the ventilator.
- The chest wall is then closed in layers.
Part 3: Recovery
- While the animal recovers from the surgery, the incision site is infiltrated with 5 mL 0.25% Bupivacaine[2].
- The animal is extubated following weaning from the respirator and is returned to a cage with fresh bedding once it is fully awake and sternal. The rat is subsequently administered 0.01 mg/kg Buprenorphine analgesia subcutaneously every 12 hours for 3 days.
Part 4: Representative results
If performed properly, the described surgical implantation of engineered tissue in the AV-groove of the heart (Figure 1) results in greater than 90% recovery of the animals.
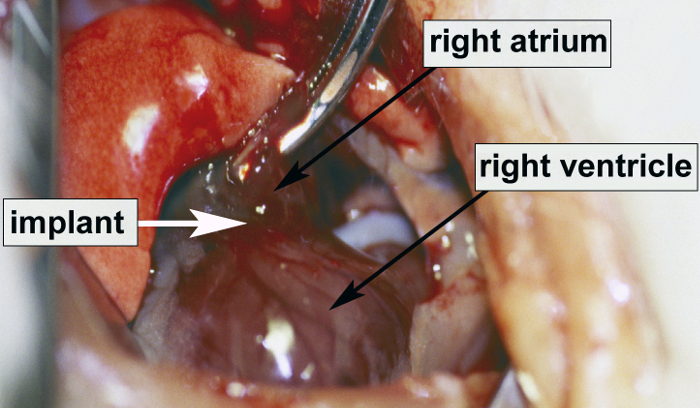
Figure 1. A photograph of the exposed heart showing the site of implantation of engineered tissue.
Discussion
For a favorable outcome to the surgical procedure, it is critical to properly intubate and invasively ventilate the animal. If the rat is simply sedated and allowed to spontaneously breathe, both lungs will inevitably collapse while the thorax is opened due to common pleura in rodents. Before closing the chest, any pneumothorax needs to be completely eliminated and the lungs fully expanded to reduce atelectasis. Otherwise, weaning from the respirator and recovery will be unsuccessful and the animal will become hypercapnic and/or hypoxic[3]. Removal of epicardial tissue within the AV groove is potentially hazardous and could result in massive venous bleeding from the right atrium due to the delicate nature of the rat atrial wall. While placing the Prolene suture in step 2.4, it is essential to be aware of and avoid the epicardial coronary branches. The animal should be kept warm on a heading pad during the recovery period or its body temperature will fall abruptly.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by research grants from the National Institutes of Health (HL068915; HL088206) and contributions to the Cardiac Conduction Fund at Children’s Hospital Boston.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| 7×4 Thermo Pad 4/box | Equipment | Hood Thermo-Pad, Summerland, BC Canada | MODEL 704R | |
| Inspira ASV Ventilator | Equipment | Harvard Apparatus, Holliston, MA 01746 USA | ||
| Handheld Pulse Oximeter | Equipment | Nellcor, Boulder, CO 80301 USA | N20PA | |
| Retractor | Equipment | Roboz, Gaithersburg, MD 20898 USA | RS-6514 | |
| Isoflurane (Forane) | Reagent | Baxter | 1001936040 | |
| Ketamine HCl | Reagent | HOSPIRA Inc., Lake Forest, IL 60045 | RL-0065 | |
| Xylazine (AnaSed) | Reagent | Lloyd | LB15705A | |
| Buprenorphine HCL Inject | Reagent | Bedford Labs, Bedford, OH 44146 USA | Div-BUP-P00 | |
| Bupivacaine 0.25% | Reagent | HOSPIRA Inc., Lake Forest, IL 60045 |
References
- Choi, Y. H. Cardiac conduction through engineered tissue. Am J Pathol. 169, 72-85 (2006).
- Shin, J. W. Low-dose systemic bupivacaine prevents the development of allodynia after thoracotomy in rats. Anesth Analg. 107, 1587-1591 (2008).
- Horstick, G. Surgical procedure affects physiological parameters in rat myocardial ischemia: need for mechanical ventilation. Am J Physiol. 276, H472-H479 (1999).

