人类诱导多能干细胞衍生心肌细胞中的单细胞光学作用潜在测量
Summary
在这里,我们使用高速模块化光测量系统描述从诱导多能干细胞衍生心肌细胞中获得的光学采集和作用潜力的特征。
Abstract
传统的细胞内微电极技术,量化心肌细胞电生理学是非常复杂的,劳动密集型,通常进行低吞吐量。诱导多能干细胞(iPSC)技术的快速和持续扩展为心血管研究提出了新的标准,现在需要替代方法,以提高单个细胞水平的电生理数据吞吐量。VF2.1Cl 是一种最近衍生的电压敏感染料,它提供快速的单通道,对膜电位波动的高级响应。它具有优于其他现有电压指标的动力学,并提供与传统微电极技术相当的功能数据。在这里,我们展示了简化的,非侵入性的行动潜在特征在外部节奏的人类iPSC衍生心肌细胞使用模块化和高度负担得起的光测量系统。
Introduction
心肌细胞的电生理建模和心脏药物筛查高效平台的建设对于制定各种心律失常的治疗策略至关重要。诱导多能干细胞(iPSC)技术的迅速扩展,利用分离的患者衍生心肌细胞(iPSC-CM)在人类疾病建模和药理学研究方面取得了可喜的成效。通过贴片夹(电流夹)对这些细胞进行电生理特征的”黄金标准”技术可以量化作用潜力(AP)的形态和持续时间,然而,这种方法极其复杂和缓慢,不适合高吞吐量数据采集1。iPSC-CMs 经常被报告有增加的舒张膜潜力和增加泄漏电流相比,成人本地心肌细胞2。有人建议,在iPSC-CMs中观察到的更小的细胞大小和更小的膜电容在使用电流夹技术时可能会产生一些系统性错误,或许可以解释这些偏差3。为了最大限度地提高 iPSC-CM 平台的效用,在 iPSC-CM 中单个单元格级别上对跨膜电压变化进行描述时,采用另一种方法具有提高吞吐量和确保数据准确性的宝贵价值。
电压敏感染料 (VSD) 长期以来一直是一种建议的方法,提供更快,非侵入性和等效的心脏AP动能分析相比,传统技术4。最近的一项研究已经证明了比率电压敏感探针光度测量是否适合精确量化心脏AP5。此外,能够轻松扩展光学光度测量方法,使这项技术能够扩展到在治疗药物开发中至关重要的大型心毒性屏幕(例如 CiPA)。利用微电极阵列和电压感应光学技术进行盲目多现场研究,开发标准化心毒性协议,证明了这种方法的关键价值。
许多强力计量染料在商业上都有售,而新探针的不断合成开发显示出在各种心脏和神经结构中简化其有效性令人振奋的潜力。理想的VSD将具有增强的动能和灵敏度,同时显示电容负荷降低、光出血和细胞毒性。最近合成的VF2.1Cl(FluoVolt)表达了许多这些有益的属性,这主要是因为其新的基于电线的分子结构,由新的电压流体(VF)家族7的其他成员共享。与普通电色VSD(在这种电色中,简单的探头在分子和电学上与等离子膜结合)不同,这种染料由一条被动插入的膜跨膜合成线组成,该线将富含电子的捐赠者与经过改良的荧光素氟磷(FITC)配对。机械细节在 图1中提供。这种染料表现出对膜电压波动的极高灵敏度,显示每100 mV的发射强度变化27%,而其他常见探针以可比速度7的±10%变化。此外,基于电线的PET系统不会直接与细胞电场相互作用,而蜂窝电场产生的电干扰最小,细胞电容负荷的变化也微乎其微。
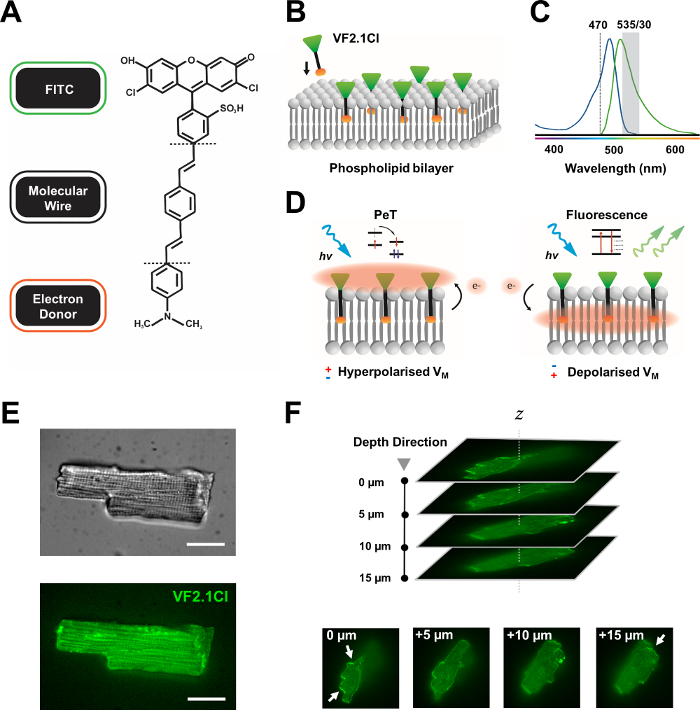
图1:VF2.1Cl染料的化学、光谱和机械参数。(A) VF2.1Cl.分子特征的化学结构需要注意,包括苯乙烯分子线内的多个烷基组,这有利于插入等离子体膜。与FITC探针结合的负电压硫酸组可确保细胞外表面的氟醇稳定,并有助于相对于脂质双层的电场接近垂直插入。(B) 垂直VF2.1Cl嵌入目标细胞等离子膜的简化示意图。(C) VF2.1Cl 染料的吸收和发射光谱。光谱与标准 FITC 和 GFP 探头相同。(D) VF2.1Cl的机械动作模式的描述。在休息条件下(超极化),负细胞内电压将自由电子驱动到荧光管。电子丰度确保光诱导电子转移(PET)被青睐为在光学激发后走出兴奋状态的途径,有效抑制荧光。相比之下,去极化膜潜在影响向下电子运动,有利于荧光对光学激发。由此产生的荧光反应与膜电压有线性关系,可精确利用它来收集有关细胞电生理动力学的详细时间信息。(E) 代表亮场(上)和荧光在470纳米(下)图像的麻风病心肌细胞加载VF2.1Cl.(F)Z堆栈的单载心肌细胞。箭头表示 VF2.1Cl 到细胞膜的清晰定位区域。图像是用一个由X光v3旋转盘共焦头和50μm针孔图案组成的旋转圆盘共焦系统获得的;LDI-7 照明器;总理95B相机和普莱纳波兰姆达100倍的目标。缩放栏:20μm。请单击此处查看此图的较大版本。
FITC 探头与 VF2.1Cl 结合,可确保在标准和 GFP 滤清器配置下有效使用,并且只需要一个通道采集系统,这两个系统都是荧光成像平台的共同功能。最近有报道说,这种染料的致密人类iPSC-CM单层分析有8、9、10、11。我们的协议与这些研究不同,因为我们对单个、孤立的 iPSC-CMs 进行了调查,不受密集同步单层的电气和参数影响,以及我们使用经济实惠且可定制的光度测量系统,而不是复杂的共聚焦或广域成像安排。
在这里,我们描述了我们的协议,快速获取和分析强大的光学APs从孤立的人类iPSC衍生心肌细胞和原生心肌细胞(见 补充文件)。我们使用 VF2.1Cl 以及可定制的单细胞光度测量技术平台。这些实验方案已得到哥廷根大学医学中心伦理委员会(第10/9/15号)的批准。
Protocol
Representative Results
Discussion
在这里,我们描述了一个基本协议,以轻松获得详细的AP配置文件从孤立的iPSC-CMs适合电生理建模和心脏药物筛查。我们从我们稀疏的种子 iPSC-CM 中检测出定期的、坚固的 APs,这表明指标功能和方法保真度。
由于iPSC重新编程的商业方法范围广泛,心脏分化协议缺乏标准化,基于iPSC的模型在功能和形态16方面具有巨大的变异性。这也会妨碍心毒性研究的有效?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
作者要感谢凯恩研究有限公司为支付本出版物制作成本而作出的善意财政贡献。此外,我们感谢伊内斯·穆勒女士和斯特凡妮·凯斯特尔女士给予的出色技术支持。
作者的研究得到了德国心血管研究中心(DZHK)的支持,德国福松斯格梅因沙夫特(DFG, 德国研究基金会,VO 1568/3-1,IRTG1816 RP12,SFB1002 TPA13和德国卓越战略 – EXC 2067/1-390729940)和埃尔塞-克雷纳-弗雷塞纽斯·斯蒂夫通(EKFS 2016_A20)。
Materials
| Reagents | |||
| 0.25 Trypsin EDTA | Gibco | 25200056 | |
| B27 Supplement | Gibco | 17504044 | |
| CaCl2 | Carl Roth | HN04.2 | |
| D(+)-Glucose anhydrous BioChemica | ITW Reagents | A1422 | |
| Fetal Bovine Serum | Gibco | 10270-106 | |
| FluoVolt Membrane Potential Kit | Invitrogen | F10488 | |
| HEPES | Carl Roth | HN77.4 | |
| KCl | Sigma-Aldrich | 6781.1 | |
| Lamanin | Sigma-Aldrich | 114956-81-9 | |
| Matrigel | BD | 354230 | |
| NaCl | Sigma-Aldrich | 9265.2 | |
| Nifedipine | Sigma-Aldrich | 21829-25-4 | |
| Penicillin/Streptomycin | Invitrogen | 15140 | |
| ROCK Inhibitor Y27632 | Stemolecule | 04-0012-10 | |
| RPMI 1640 Medium | Gibco | 61870010 | |
| Versene EDTA | Gibco | 15040033 | |
| Equipment | |||
| 495LP Dichroic Beamsplitter | Chroma Technology | ||
| Axopatch 200B Amplifier | Molecular Devices | ||
| Circle Coverslips, Thickness 0 | Thermo Scientific | CB00100RA020MNT0 | |
| Digidata 1550B | Molecular Devices | ||
| Dual OptoLED Power Supply | Cairn Research | ||
| ET470/40x Excitation Filter | Chroma Technology | ||
| ET535/50m | Chroma Technology | ||
| Etched Neubauer Hemacytometer | Hausser Scientific | ||
| Filter Cubes | Cairn Research | ||
| IX73 Inverted Microscope | Olympus | ||
| MonoLED | Cairn Research | ||
| Multiport Adaptors | Cairn Research | ||
| Myopacer Cell Stimulator | IonOptix | ||
| Optomask Shutter | Cairn Research | ||
| Optoscan System Controller | Cairn Research | ||
| PH-1 Temperature Controlled Platform | Warner Instruments | ||
| Photomultiplier Detector | Cairn Research | ||
| PMT Amplifier Insert | Cairn Research | ||
| PMT Supply Insert | Cairn Research | ||
| RC-26G Open Bath Chamber | Warner Instruments | ||
| SA-OLY/2AL Stage Adaptor | Olympus | ||
| T565lpxr Dichroic Beamsplitter | Chroma Technology | ||
| T660lpxr Dichroic Beamsplitter | Chroma Technology | ||
| TC-20 Dual Channel Temperature Controller | npi Electronic | ||
| UPLFLN 40X Objective | Olympus | ||
| USB 3.0 Colour Camera | Imaging Source | ||
| Software | |||
| Clampex 11.1 | Molecular Devices | ||
| Clampfit 11.1 | Molecular Devices | ||
| IC Capture 2.4 | Imaging Source | ||
| Prism 8 | Graphpad |
References
- Miller, E. W. Small molecule fluorescent voltage indicators for studying membrane potential. Current Opinion in Chemical Biology. 33, 74-80 (2016).
- Liang, P., et al. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 127 (16), 1677-1691 (2013).
- Horváth, A., et al. Low resting membrane potential and low inward rectifier potassium currents are not inherent features of hiPSC-derived cardiomyocytes. Stem Cell Reports. 10 (3), 822-833 (2018).
- Salama, G., Morad, M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 191 (4226), 485-487 (1976).
- Hortigon-Vinagre, M., et al. The use of ratiometric fluorescence measurements of the voltage sensitive dye Di-4-ANEPPS to examine action potential characteristics and drug effects on human induced pluripotent stem cell-derived cardiomyocytes. Toxicological Sciences. 154 (2), 320-331 (2016).
- Blinova, K., et al. International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Reports. 24 (13), 3582-3592 (2018).
- Miller, E. W., et al. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proceedings of the National Academy of Sciences. 109 (6), 2114-2119 (2012).
- Bedut, S., et al. High-throughput drug profiling with voltage- and calcium-sensitive fluorescent probes in human iPSC-derived cardiomyocytes. American Journal of Physiology-Heart and Circulatory Physiology. 311 (1), 44-53 (2016).
- McKeithan, W. L., et al. An automated platform for assessment of congenital and drug-induced arrhythmia with hiPSC-derived cardiomyocytes. Frontiers in Physiology. 8, 766 (2017).
- Duncan, G., et al. Drug-mediated shortening of action potentials in LQTS2 human induced pluripotent stem cell-derived cardiomyocytes. Stem Cells and Development. 26 (23), 1695-1705 (2017).
- Asakura, K., Hayashi, S., Ojima, A., Taniguchi, T., Miyamoto, N. Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes. Journal of Pharmacological and Toxicological Methods. 75, 17-26 (2015).
- Lian, X., et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nature Protocols. 8 (1), 162-175 (2013).
- Burridge, P. W., et al. Chemically defined generation of human cardiomyocytes. Nature methods. 11 (8), 855-860 (2014).
- Kleinsorge, M., Cyganek, L. Subtype-directed differentiation of human iPSCs into atrial and ventricular cardiomyocytes. STAR Protocols. , 100026 (2020).
- Knollmann, B. C., Katchman, A. N., Franz, M. R. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. Journal of Cardiovascular Electrophysiology. 12 (11), 1286-1294 (2001).
- Leopold, J. A., Loscalzo, J. Emerging role of precision medicine in cardiovascular disease. Circulation Research. 122 (9), 1302-1315 (2018).
- Voigt, N., Zhou, X. B., Dobrev, D. Isolation of human atrial myocytes for simultaneous measurements of Ca2+ transients and membrane currents. Journal of Visualized Experiments. (77), e50235 (2013).
- Voigt, N., et al. Enhanced sarcoplasmic reticulum Ca2+ Leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 125 (17), 2059-2070 (2012).
- Voigt, N., et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 129 (2), 145-156 (2014).
- Fakuade, F. E., et al. Altered atrial cytosolic calcium handling contributes to the development of postoperative atrial fibrillation. Cardiovascular Research. , 162 (2020).
- Gross, E., Bedlack, R. S., Loew, L. M. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophysical Journal. 67 (1), 208-216 (1994).
- Matiukas, A., et al. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm. 4 (11), 1441-1451 (2007).
- Mutoh, H., et al. Spectrally-resolved response properties of the three most advanced fret based fluorescent protein voltage probes. PLoS One. 4 (2), 4555 (2009).
- Hochbaum, D. R., et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nature Methods. 11 (8), 825-833 (2014).
- Huang, Y. L., Walker, A. S., Miller, E. W. A photostable silicon rhodamine platform for optical voltage sensing. Journal of the American Chemical Society. 137 (33), 10767-10776 (2015).
- Deal, P. E., Kulkarni, R. U., Al-Abdullatif, S. H., Miller, E. W. Isomerically pure tetramethylrhodamine voltage reporters. Journal of the American Chemical Society. 138 (29), 9085-9088 (2016).
- Fluhler, E., Burnham, V. G., Loew, L. M. Spectra, membrane binding, and potentiometric responses of new charge shift probes. 생화학. 24 (21), 5749-5755 (1985).
- Fromherz, P., Muller, C. O. Voltage-sensitive fluorescence of amphiphilic hemicyanine dyes in neuron membrane. Biochimica et Biophysica Acta. 1150 (2), 111-122 (1993).
- Salama, G., et al. Properties of new, long-wavelength, voltage-sensitive dyes in the heart. Journal of Membrane Biology. 208 (2), 125-140 (2005).
- Jin, L., et al. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 75 (5), 779-785 (2012).
- Kralj, J. M., Douglass, A. D., Hochbaum, D. R., MacLaurin, D., Cohen, A. E. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nature Methods. 9 (1), 90-95 (2012).
- Tsutsui, H., Karasawa, S., Okamura, Y., Miyawaki, A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nature Methods. 5 (8), 683-685 (2008).
- Lundby, A., Mutoh, H., Dimitrov, D., Akemann, W., Knöpfel, T. Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS One. 3 (6), 2514 (2008).
- Bradley, J., Luo, R., Otis, T. S., DiGregorio, D. A. Submillisecond optical reporting of membrane potential in situ using a neuronal tracer dye. The Journal of neuroscience. 29 (29), 9197-9209 (2009).
- Herron, T. J., Lee, P., Jalife, J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circulation Research. 110 (4), 609-623 (2012).
- Kappadan, V., et al. High-resolution optical measurement of cardiac restitution, contraction, and fibrillation dynamics in beating vs. blebbistatin-uncoupled isolated rabbit hearts. Frontiers in Physiology. 11, 464 (2020).
- Kettlewell, S., Walker, N. L., Cobbe, S. M., Burton, F. L., Smith, G. L. The electrophysiological and mechanical effects of 2,3-butane-dione monoxime and cytochalasin-D in the Langendorff perfused rabbit heart. Experimental Physiology. 89 (2), 163-172 (2004).
- Képiró, M., et al. para-Nitroblebbistatin, the non-cytotoxic and photostable Myosin inhibitor. Angewandte Chemie International Edition. 53 (31), 8211-8215 (2014).

