Primary Culture of Neurons Isolated from Embryonic Mouse Cerebellum
Summary
Conducting in vitro experiments to reflect in vivo conditions as adequately as possible is not an easy task. The use of primary cell cultures is an important step toward understanding cell biology in a whole organism. The provided protocol outlines how to successfully grow and culture embryonic mouse cerebellar neurons.
Abstract
The use of primary cell cultures has become one of the major tools to study the nervous system in vitro. The ultimate goal of using this simplified model system is to provide a controlled microenvironment and maintain the high survival rate and the natural features of dissociated neuronal and nonneuronal cells as much as possible under in vitro conditions. In this article, we demonstrate a method of isolating primary neurons from the developing mouse cerebellum, placing them in an in vitro environment, establishing their growth, and monitoring their viability and differentiation for several weeks. This method is applicable to embryonic neurons dissociated from cerebellum between embryonic days 12–18.
Introduction
For several decades, cell lines have been widely used as a high throughput tool in preclinical studies and biological research. Cost-effectiveness, fast growth, and reduction of live animal use are some benefits of using these cells. However, genetic alterations and phenotypical changes accumulate after several passages in vitro1. Misidentification of cell lines and genetic dissimilarity from primary cells can lead to irreproducible experiments and false conclusions2,3,4,5. Therefore, in spite of some similarities to differentiated cells such as neurons (e.g., neurotransmitters, ion channels, receptors, and other neuron-specific proteins), neuronal cell lines cannot replicate the full phenotype of neurons. Using mature neurons is another option; however, these cells are non-dividing postmitotic cells that are difficult to propagate in culture. Moreover, re-entry into the cell cycle may precipitate apoptosis6.
Three-dimensional (3D) cell culture, organotypic slice cultures, and organoid cultures have been developed to provide an environment in which cells can arrange into a 3D form that mimics the in vivo setting. Thus, cell-to-cell communication, migration, invasion of tumor cells into surrounding tissues, and angiogenesis can be studied7. However, additional costs of using extra cellular matrix (ECM) proteins or synthetic hydrogels as a bedding, difficulty in imaging, and compatibility with high-throughput screening instruments are considerable drawbacks of 3D cell culturing. A major disadvantage of organotypic tissue slice culture is the use of a large number of animals and the adverse effects of axotomy, which leads to inaccessibility of targets and growth factors for axons, and consequently neuronal death8.
Therefore, an alternate approach, which avoids the problems with cell lines, the difficulty of growing mature cells, and complexity of tissues, is in vitro maturation of immature primary cells. Primary cells are derived directly from human or animal tissue and dissociated using enzymatic and/or mechanical methods9. The main principles of isolation, seeding, and maintenance in culture medium are similar regardless of the tissue source. However, the trophic factors necessary to promote proliferation and maturation are highly cell specific6.
Knowing the ‘birthdate’ of each cerebellar cell type is a prerequisite for designing a primary culture experiment. In general, Purkinje cells (PCs) and the neurons of the cerebellar nuclei (CN), are born before the smaller cells, including interneurons (e.g., basket, stellate cells) and granule cells. In mice, PCs emerge between embryonic day (E)10–E13, whereas CN neurons at approximately E9–E1210.
Other cerebellar neurons are born much later. For example, in mice, the Golgi subpopulation of interneurons are generated from VZ at (~E14−E18) and the remaining interneurons (basket cell and stellate cells) located in the molecular layer emerge from dividing progenitor cells in the white matter between early postnatal (P)0–P711. Granule cells are generated from the external germinal zone (EGZ), a secondary germinal zone that is derived from the rostral rhombic lip and goes through terminal division after birth. But before their precursors arise from the rhombic lip from E13–E16, the cells have already migrated rostrally along the pia surface to make a thin layer of cells on the dorsal surface of the cerebellum anlage. Nonneuronal macroglial cells such as astrocytes and oligodendrocytes, which originate from the ventricular neuroepithelium, are born at E13.5−P0 and P0−P7 respectively11,12,13,14,15. Microglia are derived from yolk-sac primitive myeloid progenitor cells between E8–E10 and after invasion into the central nervous system can be detected in the mouse brain by E916.
The method presented in this article is based on the one originally developed by Furuya et al. and Tabata et al.17,18, which was optimized for primary culturing of Purkinje cells derived from Wistar rat cerebella. We have now adapted this method and carefully modified it to study the growth of mouse cerebellar neurons19. Unlike in our new protocol, cold dissection medium is the main washing buffer used during dissection and dissociation steps before adding seeding medium in Furuya’s protocol17. This buffer lacks the nutrition, growth factors, and hormones (all in Dulbecco’s modified Eagle medium:nutrient mixture F-12 [DMEM/F12]) that are necessary to support cell growth and survival during the aforementioned steps. In addition, based on our extensive experience with murine primary cerebellar cultures, we have used 500 μL of culture medium in each well (instead of 1 mL) and increased the tri-iodothyronine concentration to 0.5 ng/mL, which improves growth of neuronal cells, in particular those with a Purkinje cell phenotype, and promotes the outgrowth of dendritic branches in culture. The principal method featured in this article can be broadly applied to other small rodents (e.g., squirrels and hamsters) during embryonic development and can be used to study cerebellar neurogenesis and differentiation in the various embryonic stages, which differ between species.
Protocol
All animal procedures were performed in accordance with institutional regulations and the Guide to the Care and Use of Experimental Animals from the Canadian Council for Animal Care and has been approved by local authorities (“the Bannatyne Campus Animal Care Committee”). All efforts were made to minimize the number and suffering of animals used. Adequate depth of anesthesia was confirmed by observing that there was no change in respiratory rate associated with manipulation and toe pinch or corneal reflex.
1. Preparation
NOTE: Schedule the provision of timed pregnant mice, based on the research plan, for post-conception E12–E18. Choice of the timing depends on the desired cell characteristics (see below). Prepare the cover slips and plates at least 2 days before the experiment. Poly-L-ornithine is used as a coating material to enhance cell attachment to the cover slips.
- Coat the cover slips 2 days before cell isolation.
- Place the round cover slips in a 24 well plate, under sterile conditions in a biosafety cabinet. Leave a gap between each cover slip to avoid any contamination.
- Add 90 µL of poly-L-ornithine (PLO, 500 µg/mL) to the center of each cover slip. Close the cap and gently place the plate in a 37 °C/5% CO2 incubator for 2 days.
NOTE: A greater volume may spill off the cover slips during incubation. The cover slip does not need to be completely covered with PLO after placing a drop. During overnight incubation, PLO distributes equally to the edge of the cover slips.
- One day before cell isolation, prepare the culture medium I (DMEM/F-12 containing putrescine 100 µM, sodium selenite 30 nM, L-glutamine 3.9 mM, gentamicin 3.5 µg/mL, tri-iodothyronine (T3) 0.5 ng/mL, and N3 supplements [progesterone 4 µM, insulin 20 μg/mL, transferrin 20 mg/mL]) and seeding medium (culture medium I [without N3 and T3] containing 10% fetal bovine serum [FBS]) and store them in 4 °C. Prepare the trypsin working solution (0.25%) in DMEM/F12 and keep it at 4 °C.
NOTE: The medium compositions are provided in Table 1. - On the day of cell isolation, place culture medium I and seeding medium in the incubator at 37 °C.
NOTE: Before starting the cerebellum isolation, make sure that all the tools and working surfaces are sterile. - Wash cover slips.
- Take the 24 well plate out of the incubator.
- Wash the cover slips in the 24 well plate 3x with double distilled water (DDW) in a biosafety cabinet under sterile conditions. Each time let the cover slips soak in DDW for 5 min before starting aspiration.
- Leave the cover slips in a biosafety cabinet for at least 2 h to completely dry.
2. Cerebellum collection
- Prepare three 10 cm sterile plastic Petri dishes filled with ice-cold 1x phosphate-buffered saline (PBS), 3 Petri dishes filled with ice-cold 1x Hank’s balanced salt solution (HBSS), and approximately 5 Petri dishes filled with ice-cold dissection medium (1x HBSS containing gentamicin 10 μg/mL). Keep them on ice.
- Anesthetize the E18 CD1 pregnant mouse with 40% isoflurane. Perform cervical dislocation on the mouse. Sterilize the abdomen with 70% ethanol solution.
- Use a pair of scissors to make a skin incision from the pubic symphysis to the xiphoid process. Then hold the skin with forceps and open the abdominal cavity.
- Excise the uterine horns with forceps and wash them 3x in the ice-cold 1x PBS on ice.
NOTE: The following steps should all be conducted on ice to minimize the metabolic rate and prevent tissue and cell damage.
3. Dissecting the cerebellum
- After the last washing step, using a pair of fine forceps separate the embryos from the uterus in 1x HBSS and transfer them to the ice-cold dissection medium. In the dissection medium, decapitate the embryos with scissors. Place the tissue in a clean dissection medium.
NOTE: Using a stereomicroscope for microdissection is optional. - Hold the skull with fine forceps and cut through the calvarium with a pair of small scissors from the lateral aspect of the skull in a line from the foramen magnum to the external acoustic meatus and inferior border of the orbital cavity.
NOTE: Taking this step exposes the cranial cavity at the level of the skull base and makes it easier to remove the brain. - Using a pair of fine forceps, remove the skull base and peel the skull away from the brain.
- Carefully remove the meninges on the cerebellum, starting from the lateral surface of the middle cerebellar peduncle and pons.
- Cut both cerebellar peduncles and separate the cerebellum from the rest of the brain (Figure 1).
- Immediately place the collected cerebella in a sterile 15 mL conical tube filled with 14 mL of DMEM/F12 on ice.
- Centrifuge the tube at 1,000 x g, 4 °C for 1 min, 3x. Each time gently remove the supernatant with a pipette and resuspend the pellet in fresh, ice-cold DMEM/F12.
NOTE: To avoid losing the samples, use the cabinet suction as little as possible.
4. Cerebellum dissociation
- Add 2 mL of prewarmed (37 °C) trypsin to the pellet from step 3.7 and gently pipet for adequate mixing.
- Place the tube in a 37 °C water bath for 12 min.
- After incubation, bring the tube to a biosafety cabinet and add 10 mL of DMEM/F12 to inactivate the trypsin.
- Centrifuge the mixture at 1,200 x g for 5 min. Discard the supernatant and resuspend the pellet in fresh DMEM/F12. Repeat 3x.
- Prewet a sterile plastic transfer pipet with DMEM/F12.
- After washing and the final centrifugation, add 3.5 mL of DNase working solution (1 mL of DNase I stock solution [0.05% DNase + 12 mM MgSO4 + 1x HBSS] in 500 µL of heat-inactivated FBS and 2 mL of DMEM/F12) to the pellet in the same tube.
- Triturate the tissue with a pipet at least 30x until the mixture attains a homogenous milky color.
5. Cell collection
- Add 10 mL of ice-cold DMEM/F12 to the mixture.
- Centrifuge the sample at 1,200 x g, 4 °C for 5 min.
- Carefully remove the supernatant without disturbing the pellet.
- Remove the seeding medium from the incubator.
- Add 500 µL of prewarmed seeding medium to the pellet and mix it very well using a pipet to resuspend the cells.
- Count the cells using a hemocytometer.
- Dilute the cell suspension with the seeding medium to a density of 5 x 105 cells/mL.
- Under a biosafety cabinet, add 90 µL of the diluted mixture to each well at the center of the cover slip.
NOTE: Do not load the particles that did not suspend in the DNase. - Place the plate in the incubator at 37 °C for 3−4 h.
- After incubation, add 500 µL of prewarmed culture medium I to each well and place the plate back into the incubator (37 °C).
6. Treatment of the recovered cells
- After 7 days, replace the old medium with fresh culture medium II (culture medium I supplemented with cytosine arabinoside [Ara-C, 4 μM] and 100 μg/mL bovine serum albumin [BSA]; see Table 1).
NOTE: This step is critical to avoid growth of nonneuronal cells. - Monitor the culture medium once a day. If the pH changes (indicated by a marked change in color, usually yellower), remove half (almost 250 µL) of the old medium from all wells and add 300 µL of prewarmed culture medium I to each of them to avoid nutrient loss.
NOTE: Phenol red in culture medium is a good indicator of the pH of the medium and activity of the cells. Try to minimize the exposure time of the cells to out of incubator conditions. This prevents any stress that might affect their viability.
7. Cell collection and fixation
NOTE: Depending on the experimental design, the cells can be collected at any day, at any timepoint.
- Prepare a separate 24 well plate with the corresponding numeric organization and add 100 µL of 4% paraformaldehyde (PFA) to each well.
- To harvest cells on the desired days (depending on the experimental protocol), gently remove the cover slips from the wells of the original culture plate and place them in the corresponding wells of the PFA-filled plate.
- Add PFA to the wells to completely immerse the cover slips.
NOTE: To avoid cell detachment from the cover slip, do not add the PFA directly on the cover slip. - Keep the PFA plate at 4 °C for 30−120 min.
- After incubation, restore the plate to room temperature.
- Gently wash the cover slips 3x for 5 min with 1x PBS.
- Proceed to the immunostaining process.
NOTE: In this study, a fluorescence microscope equipped with a camera was used to capture the images and assembled into montages using an image editing software application.
Representative Results
Based on the different birthdates of neuronal subtypes in the cerebellum, cultures from E12−E18 mouse embryos yielded different cell types. Large projection neurons, such as CN neurons (E9−E12) and PCs (E10−E13), emerged early during cerebellar development. In mice, granule and Golgi cells arose between ~E13–E18 and underwent terminal divisions up to postnatal week 4.
Replacing old medium I with fresh culture medium II at days in vitro (DIV) 7 will eventually prevent glial cell proliferation. The interneurons of the molecular layer, such as basket and stellate cells, differentiate after birth. Therefore, most of the cells in the culture medium at E18 were expected to be a combination of PCs, granule cells, CN, and some Golgi cells. Calbindin 1 (CALB1), a specific marker of PCs, was used to track the morphological changes during the 21-day time course. On DIV 0, the cell bodies of PCs were detectable (e.g., 10−11 h of incubation), but the outgrowth of neurite had not yet started (Figure 2A). By DIV 3, the axonal extension showed progress, while dendritic processes had just started. This status remained almost unchanged until the second week in vitro (WIV) (Figure 2B–D). On DIV 10, a few sparse branches and primary dendrites with spines started growing (Figure 2E). Sprouting of the new primary dendrites occurred continuously after DIV 14. Therefore, after DIV 14, the number of secondary and tertiary dendrites increased and developed wide branches at DIV 21 (Figure 2F,G).
It is important to know that the timing of the morphological changes in vitro may not follow the same pattern as in vivo. Following these results, in another experiment the dissociated cerebellar primordia from E12, E13, E14, and E15 were cultured for 3 weeks. The cultured PCs from E12 and E13 did not develop any dendritic outgrowth on DIV 18 except axonal extensions (Figure 3A,B). However, after the same period of time, the dissociated cerebellar primordium cultures from E14 and E15 showed dendritic outgrowth and arborization (Figure 3C,D). This growth rhythm variation among neural cells in vitro sheds light on a big change that happened in their natural environment between the early and late stage of cerebellar development, which needs to be applied in vitro for medium optimization.
Along with PCs, there are other neuronal cell types in the cerebellum that develop during dissociated primary cerebellar cultures that can be detected using specific neuronal markers. Double immunofluorescence labeling for CALB1 and a calcium-binding albumin protein, parvalbumin (PVALB), showed that CALB1 expression was exclusively restricted to PCs in primary cerebellar cultures, while PVALB was expressed in CALB1+ neurons (i.e., PCs) and PVALB+/CALB1– neurons, which are molecular layer interneurons (basket/stellate cells) (Figure 4A–C). The alpha subunit (Nav1.6) of the voltage-gated sodium channel (SCN) is a marker for granule cells and PCs in the cerebellum20. Double labeling with anti-SCN and anti-CALB1 shows the colocalization of granule cell bodies and PCs in culture medium on DIV 21 (Figure 4D–F). For other specific cerebellar cell types from dissociated primary cerebellar cultures, please see Marzban and Hawkes19.
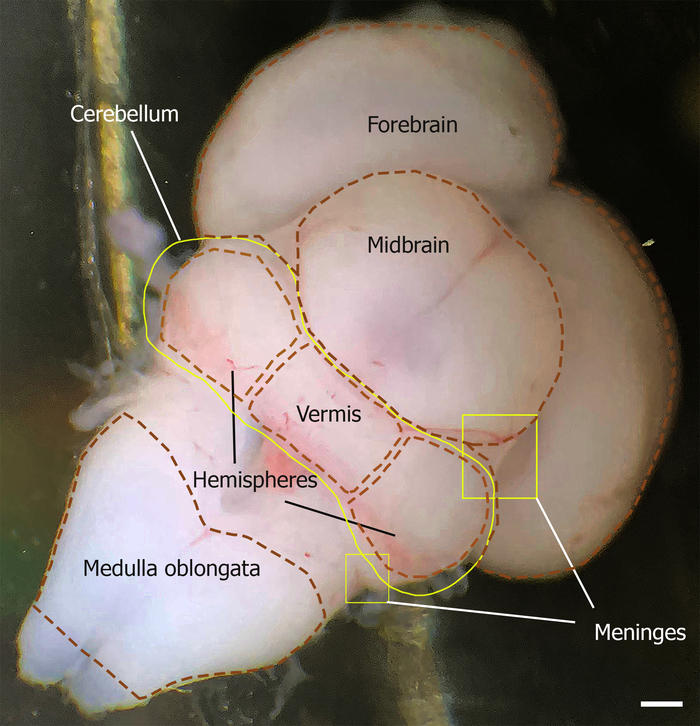
Figure 1: Dorsal aspect of the mouse brain outlining the cerebellum at E18. The meninges are indicated by yellow squares. The location of the cerebellum is outlined by the yellow line, which is limited rostrally by the midbrain and caudally by the medulla oblongata (outlined by brown dashed lines). The cerebellum vermis and hemisphere are shown by brown dashed lines. Please click here to view a larger version of this figure.
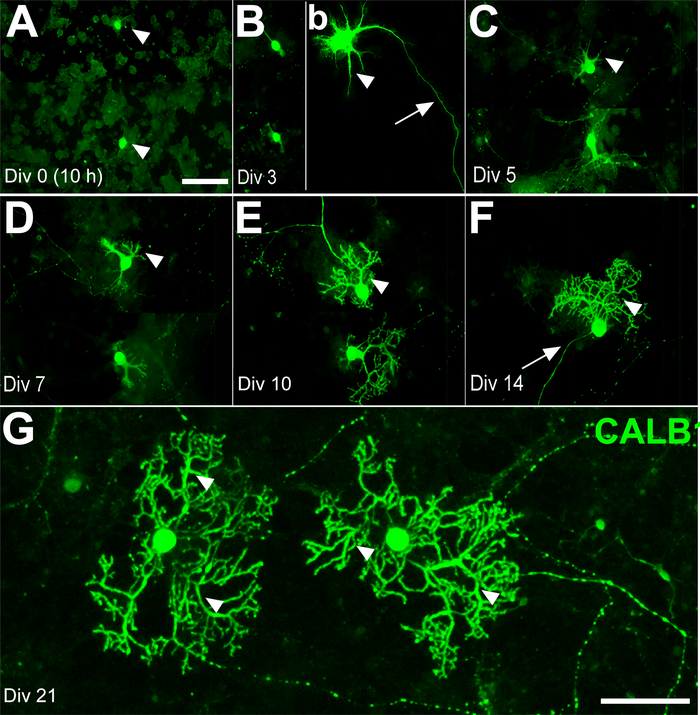
Figure 2: Development of Purkinje cells (PCs) derived from the mouse cerebellum at E18 in primary culture for days in vitro (DIV) 0–21. (A) PC somata (arrowheads) were labeled by immunofluorescence using anti-calbindin 1 (CALB1) at DIV 0 (after 10 h). (B) The first axonal extension (arrow) and early dendritic outgrowth (arrowhead) appear on DIV 3. The PCs’ dendritic outgrowth and development (arrowhead) continue on DIV 5 (C) and DIV 7 (D). The PCs’ dendritic branches are clearly distinguishable on DIV 10 (E) and DIV 14 (F) (arrowheads) and elaborate dendritic trees are detectable at DIV 21 (arrowhead) (G). Scale bars: A = 100 μm (applies to panels B, C, D, E and F); G = 50 μm. Please click here to view a larger version of this figure.
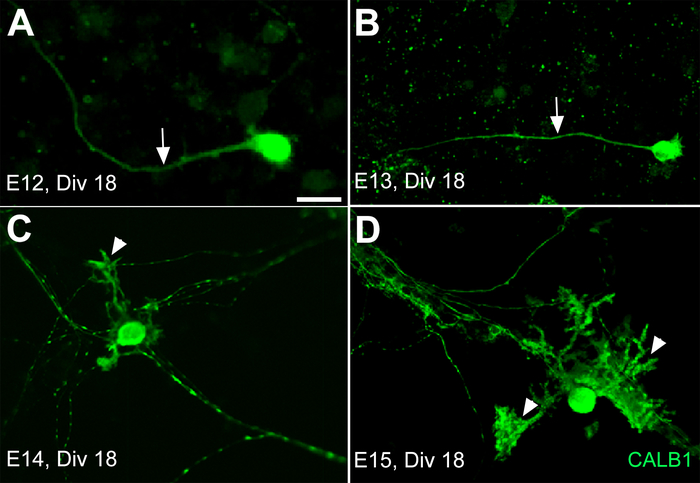
Figure 3: Development of Purkinje cells (PCs) derived from the mouse cerebellar primordium at E12, E13, E14, and E15 after 18 days in primary culture (DIV 18). The axons are the only extensions (arrow) from the PC somata in primary culture of the E12 and E13 cerebella primordium after 18 days in vitro (DIV 18) (A,B). The PCs’ dendrite outgrowth and extension develop by DIV 18 (arrowhead) only from E14 (C) and E15 (D) cerebellar primary cultures. Scale bar = 20 μm (applies to panels A−D). Please click here to view a larger version of this figure.
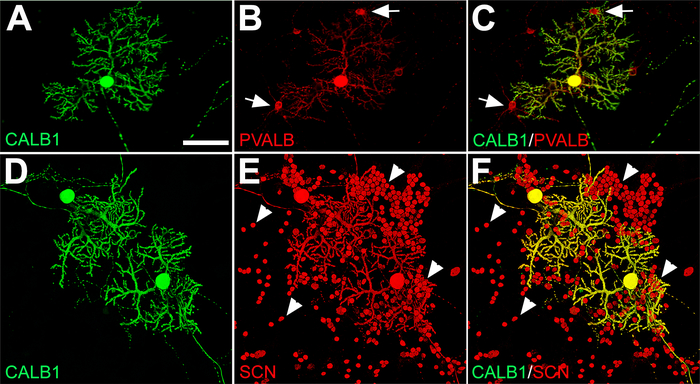
Figure 4: Immunofluorescence labeling of PCs, GABAergic interneurons (basket and stellate cells), and granule cells from E18 mouse cerebellar primary culture at DIV 21. (A−C) Double labeling with anti-CALB1 (green) and anti-PVALB (red) shows PCs and a few interneurons (arrow) in contact with PC dendritic arbors. (D−F) Voltage-gated sodium channels (SCNs) in PC bodies with elaborated dendrites and numerous small granule cell bodies (arrowhead) are labeled with anti-SCN (red) and double-labeled with anti-CALB1 (green). Abbreviations: Purkinje cells = PCs; CALB1 = calbindin 1; PVALB = parvalbumin; SCN = voltage-gated sodium channel. Scale bar = 100 μm (applies to A−F). Please click here to view a larger version of this figure.
| Name | Basic medium | Putrescine | Sodium selenite | L-glutamine | Gentamicin | T3 | N3 | BSA | Ara-C | FBS |
| Culture medium I | DMEM/F12 | 100 µM | 30 nM | 3.9 mM | 3.5 µg/mL | 0.5 ng/mL | Progesterone 4 µM, Insulin 20 μg/mL, Transferrin 20 mg/mL | – | – | – |
| Seeding medium | DMEM/F12 | 100 µM | 30 nM | 3.9 mM | 3.5 µg/mL | – | – | – | – | 10% with culture medium I |
| Culture medium II | Culture medium I | 100 μg/mL | 4 μM | – | ||||||
Table 1: Media compositions.
Discussion
The use of primary cultures is a well-known method applicable for all types of neurons17,18,19. In the presented protocol, we explain how to isolate cerebellar neurons and maintain their viability with optimum survival in vitro for a maximum of 3 weeks. Primary culture of cerebellar cells, which were isolated at E15−E18, confirms the collection of three classes of large neurons: PCs, Golgi cells, and CNs. The cell bodies and projections of Golgi cells (some of which emerge at E19−P5) and soma of granule cells can be detected by neurogranin (NRGN) and SMI32 antibodies, respectively, within 21 days of culture19,21.
A critical factor for the survival and maintenance of cerebellar neurons is the type of culture medium used. FBS supplemented medium has been a standard condition for cell lines and primary culture for decades. However, ethical concerns, variability in serum composition, and its potential to be a source of contamination, have led to some caution22. Recently, supplement-enriched DMEM/F-12 medium was found to reduce the gap between physiological conditions and in vitro neuronal models. The medium suggested by Furuya et al. enhanced survival rate of PCs and improved their dendrite differentiation compared with the widely used basal medium eagle (BME)-based serum-free medium17,18,23. This medium has become the basis of chemically defined media implemented in cerebellum cell primary culture.
Primary cell culturing of neurons has limitations. As in any in vitro culture, cells can survive for a limited period of time. Without exception, primary neuronal cells cultured in vitro can only be passaged a limited number of times. Excessive passaging will affect cellular health, function, and phenotype, and as such render variable experimental results. Therefore, neuronal/nonneuronal primary culture-based experiments usually take place within the first 3 weeks of starting the culture24. The in vitro conditions have not yet been sufficiently optimized to serve all cells of the nervous system simultaneously, in a way similar to their natural environment. For example, using Ara-C to inhibit proliferation of nonneuronal cells is quite common in studies where achieving a higher survival rate of neuronal cells is a priority. Thus, the quality of primary cultures relies on methods that artificially rebalance the in vitro cell population in favor of the cell types of interest25,26. Despite its limitations, primary cell culture is an excellent approach to study cellular mechanisms, signaling pathways, and pheno- and genotypic changes under conditions where optimized cellular growth (subject to the specific research aims) is carefully characterized and compared to the in vivo situation. In addition, primary cerebellar culture as a simplified model system provides a controlled microenvironment to investigate the plethora of morphological and physiological effects mediated by the numerous molecules affecting neuronal cells27,28. However, to what extent the physiological properties of these cells are preserved outside of the in vivo setting remains to be fully elucidated.
The presented method is a cost-effective general protocol for culturing primary cells. It provides broader options for manipulating cells and testing drugs without changing the nature of the cells, which is crucial to (pre-)clinical trials.
Declarações
The authors have nothing to disclose.
Acknowledgements
These studies were supported by grants from the Natural Sciences and Engineering Research Council (HM: NSERC Discovery Grant # RGPIN-2018-06040), and Children Hospital Research Institute of Manitoba (HM: Grant # 320035), and the ALS Canada-Brain Canada Arthur J. Hudson Translational Team Grant (JK, HM).
Materials
| Adobe Photoshop CS5 Version 12 | Adobe Inc | ||
| Anti-Sodium Channel (SCN)PN4 (Nav1.6) | Sigma | S0438 | 3 μg/mL |
| Bovine Serum Albumin | Millipore Sigma | A3608 | |
| CALB1 | Swant Swiss Antibodies (Polyclonal) | CB38 | 1/5000 dilution |
| CALB1 | Swant Swiss Antibodies (Monoclonal) | 300 | 1/1000 dilution |
| Cytosine β-D-arabinofuranoside or Cytosine Arabinoside (Ara-C) | Millipore Sigma | C1768 | |
| DNase I from bovine pancreas | Roche | 11284932001 | |
| Dressing Forceps | Delasco | DF-45 | |
| Dulbecco’s Modified Eagle’s Medium (DMEM-F12) | Lonza | 12-719F | |
| Fisherbrand Cover Glasses: Circles | fisher scientific | 12-545-81 | |
| Gentamicin | Gibco | 15710-064 | |
| Hanks’ Balanced Salt Solution (HBSS) | Gibco | 14185-052 | |
| Insulin from bovine pancreas | Millipore sigma | I5500, I6634, I1882, and I4011 | |
| Large Scissor | Stoelting | 52134-38 | |
| L-glutamine | Gibco | 25030-081 | |
| Metallized Hemacytometer | Hausser Bright-Line | 3100 | |
| Microdissection Forceps | Merlan | 624734 | |
| Pattern 5 Tweezer | Dixon | 291-9454 | |
| Phosphate Buffered Saline (PBS) | Fisher BioReagents | BP399-26 | |
| Poly-L-Ornithine | Millipore Sigma | P4638 | |
| Progesteron (P4) | Millipore sigma | P8783 | |
| PVALB | Swant Swiss Antibodies | 235 | 1/1500 dilution |
| Samll Scissor | WPI Swiss Scissors, 9cm | 504519 | |
| Sodium Selenite | Millipore Sigma | S9133 | |
| Transferrin | Millipore Sigma | T8158 | |
| Tri-iodothyronine (T3) | Millipore Sigma | T2877 | |
| Trypsin | Gibco | 15090-046 | |
| Zeiss Fluorescence microscope | Zeiss | Z2 Imager |
Referências
- Giffard, R. G., Ouyang, Y. B., Squire, L. R. Cell culture: primary neural cells. Encyclopedia of Neuroscience. , 633-637 (2009).
- Huang, Y., Liu, Y., Zheng, C., Shen, C. Investigation of Cross-Contamination and Misidentification of 278 Widely Used Tumor Cell Lines. PLoS One. 12 (1), 0170384 (2017).
- Lorsch, J. R., Collins, F. S., Lippincott-Schwartz, J. Fixing problems with cell lines. Science. 346 (6216), 1452-1453 (2014).
- Kaur, G., Dufour, J. M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis. 2 (1), 1-5 (2012).
- Capes-Davis, A., et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. International Journal of Cancer. 127 (1), 1-8 (2010).
- Frade, J. M., Ovejero-Benito, M. C. Neuronal cell cycle: the neuron itself and its circumstances. Cell Cycle. 14 (5), 712-720 (2015).
- Antoni, D., Burckel, H., Josset, E., Noel, G. Three-dimensional cell culture: a breakthrough in vivo. International Journal of Molecular Science. 16 (3), 5517-5527 (2015).
- Humpel, C. Organotypic brain slice cultures: A review. Neurociência. 305, 86-98 (2015).
- Voloboueva, L., Sun, X., Ouyang, Y. B., Giffard, R. G. Cell culture: primary neural cells. Reference Module in Neuroscience and Biobehavioral Psychology. , (2017).
- Marzban, H., et al. Cellular commitment in the developing cerebellum. Frontiers in Cellular Neuroscience. 8, 450 (2014).
- Sudarov, A., et al. Ascl1 genetics reveals insights into cerebellum local circuit assembly. Journal of Neuroscience. 31 (30), 11055-11069 (2011).
- Rahimi-Balaei, M., et al. Zebrin II Is Ectopically Expressed in Microglia in the Cerebellum of Neurogenin 2 Null Mice. Cerebellum. 18 (1), 56-66 (2019).
- Rahimi-Balaei, M., Bergen, H., Kong, J., Marzban, H. Neuronal Migration During Development of the Cerebellum. Frontiers in Cellular Neuroscience. 12, 484 (2018).
- Buffo, A., Rossi, F. Origin, lineage and function of cerebellar glia. Progress in Neurobiology. 109, 42-63 (2013).
- Alder, J., Cho, N. K., Hatten, M. E. Embryonic Precursor Cells from the Rhombic Lip Are Specified to a Cerebellar Granule Neuron Identity. Neuron. 17 (3), 389-399 (1996).
- Reemst, K., Noctor, S. C., Lucassen, P. J., Hol, E. M. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Frontiers in Human Neuroscience. 10, 566 (2016).
- Furuya, S., Makino, A., Hirabayashi, Y. An improved method for culturing cerebellar Purkinje cells with differentiated dendrites under a mixed monolayer setting. Brain research. Brain Research Protocols. 3 (2), 192-198 (1998).
- Tabata, T., et al. A reliable method for culture of dissociated mouse cerebellar cells enriched for Purkinje neurons. Journal of Neuroscience Methods. 104 (1), 45-53 (2000).
- Marzban, H., Hawkes, R. Fibroblast growth factor promotes the development of deep cerebellar nuclear neurons in dissociated mouse cerebellar cultures. Brain Research. 1141, 25-36 (2007).
- Schaller, K. L., Caldwell, J. H. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum. 2 (1), 2-9 (2003).
- Alder, J., Cho, N. K., Hatten, M. E. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 17 (3), 389-399 (1996).
- van der Valk, J., Gstraunthaler, G. Fetal Bovine Serum (FBS) – A pain in the dish. Alternatives to Laboratory Animals. 45 (6), 329-332 (2017).
- Froud, S. J. The development, benefits and disadvantages of serum-free media. Developments in Biological Standardization. 99, 157-166 (1999).
- Kwist, K., Bridges, W. C., Burg, K. J. The effect of cell passage number on osteogenic and adipogenic characteristics of D1 cells. Cytotechnology. 68 (4), 1661-1667 (2016).
- Uysal, O., SevimLi, T., SevimLi, M., Gunes, S., Eker Sariboyaci, A., Barh, D., Azevedo, V. Cell and tissue culture: the base of biotechnology. Omics Technologies and Bio-Engineering. , 391-429 (2018).
- Seibenhener, M. L., Wooten, M. W. Isolation and culture of hippocampal neurons from prenatal mice. Journal of Visualized Experiments. (65), e3634 (2012).
- Nelson, K. B., Bauman, M. L. Thimerosal and autism. Pediatrics. 111 (3), 674-679 (2003).
- Marzban, H., Hawkes, R. Fibroblast growth factor promotes the development of deep cerebellar nuclear neurons in dissociated mouse cerebellar cultures. Brain Research. 1141, 25-36 (2007).

