Tumor Transplantation for Assessing the Dynamics of Tumor-Infiltrating CD8+ T Cells in Mice
Summary
Here, we present a tumor transplantation protocol for the characterization of tumor-inherent and periphery-derived tumor-infiltrated lymphocytes in a mouse tumor model. Specific tracing of the influx of recipient-derived immune cells with flow cytometry reveals the dynamics of the phenotypic and functional changes of these cells during antitumor immune responses.
Abstract
T cell-mediated immunity plays a crucial role in immune responses against tumors, with cytotoxic T lymphocytes (CTLs) playing the leading role in eradicating cancerous cells. However, the origins and replenishment of tumor antigen-specific CD8+ T cells within the tumor microenvironment (TME) remain obscure. This protocol employs the B16F10-OVA melanoma cell line, which stably expresses the surrogate neoantigen, ovalbumin (OVA), and TCR transgenic OT-I mice, in which over 90% of CD8+ T cells specifically recognize the OVA-derived peptide OVA257–264 (SIINFEKL) bound to the class I major histocompatibility complex (MHC) molecule H2-Kb. These features enable the study of antigen-specific T cell responses during tumorigenesis.
Combining this model with tumor transplantation surgery, tumor tissues from donors were transplanted into tumor-matched syngeneic recipient mice to precisely trace the influx of recipient-derived immune cells into transplanted donor tissues, allowing the analysis of the immune responses of tumor-inherent and periphery-originated antigen-specific CD8+ T cells. A dynamic transition was found to occur between these two populations. Collectively, this experimental design has provided another approach to precisely investigate the immune responses of CD8+ T cells in TME, which will shed new light on tumor immunology.
Introduction
CD8+ T cell-mediated immune response plays a pivotal role in controlling tumor growth. During tumorigenesis, naive CD8+ T cells get activated upon antigen recognition in an MHC class I-restricted manner and subsequently differentiate into effector cells and infiltrate into tumor mass1,2. However, within the tumor microenvironment (TME), prolonged antigen exposure, as well as immunosuppressive factors, drive infiltrated tumor-specific CD8+ T cells into a hyporesponsive state known as "exhaustion"3. Exhausted T cells (Tex) are distinct from effector or memory T cells generated in acute viral infection, both transcriptionally and epigenetically. These Tex cells are mainly characterized by the sustained and elevated expression of a series of inhibitory receptors as well as the hierarchical loss of effector functions. Further, the impaired proliferative capacity of exhausted CD8+ T cells results in decreasing numbers of tumor-specific T cells, such that the residual CD8+ T cells within the TME can barely provide sufficient protective immunity against tumor progression3. Thus, the maintenance or reinforcement of intratumoral antigen-specific CD8+ T cells is indispensable for tumor repression.
Moreover, immune checkpoint blockade (ICB) therapy is believed to reinvigorate Tex in tumors by increasing T cell infiltration and hence, T cell numbers and rejuvenating T cell functions to boost tumor repression. The widespread application of ICB treatment has changed the cancer therapy landscape, with a substantial subset of patients experiencing durable responses4,5,6. Nevertheless, the majority of patients and cancer types do not or only temporarily respond to ICB. Inadequate T cell infiltration in the TME has been postulated to be one of the underlying mechanisms accounting for ICB resistance7,8.
Several studies have demonstrated the heterogeneity of tumor-infiltrating CD8+ T cells (TILs) in both patients and mouse models9,10,11,12. It has been confirmed that a subset of CD8+ T cells expressing T cell factor-1 (TCF1) in a tumor mass exhibits stem cell-like properties, which could further give rise to terminally exhausted T cells and is responsible for the proliferation burst after ICB therapy12,13,14,15,16,17,18,19,20,21,22. However, it has been proved that only a small proportion of antigen-specific TCF1+CD8+ T cells exist in the TME and generate an expanded pool of differentiated progeny in response to ICB23,24,25,26. Whether the limited size of this population is enough to ensure the persistence of cytotoxic T lymphocytes (CTLs) to control tumor progression remains unknown, and whether there is replenishment from periphery tissues requires further investigation. Furthermore, recent research suggests the insufficient reinvigoration capacity of pre-existing tumor-specific T cells and the appearance of novel, previously non-existing clonotypes after anti-programmed cell death protein 1 treatment. This indicates that T cell response to checkpoint blockade may be due to the new influx of a distinct repertoire of T cell clones27. Together with the presence of bystander non-tumor-reactive cytotoxic T cell fraction in the TME, these findings prompted the establishment of a tumor allograft model to study the role of periphery-derived CD8+ T cells11.
Until now, several kinds of tumor implantation, as well as immune cell adoptive transfer, have been widely used in the field of tumor immunology28. TILs, peripheral blood mononuclear cells, and tumor-reactive immune cells originated from other tissues can be well-characterized using these methods. However, when studying the interactions between systemic and local antitumor immunity, these models appear inadequate to examine the interactions between immune cells derived from the periphery and the TME. Here, tumor tissues were transplanted from donors into tumor-matched recipient mice to precisely trace the influx of recipient-derived immune cells and observe the donor-derived cells in the TME concomitantly.
In this study, a murine syngeneic model of melanoma was established with the B16F10-OVA melanoma cell line, which stably expresses the surrogate neoantigen ovalbumin. TCR transgenic OT-I mice, in which over 90% of CD8+ T cells specifically recognize the OVA-derived peptide OVA257–264 (SIINFEKL) bound to the class I MHC molecule H2-Kb, enable the study of antigen-specific T cell responses developed in the B16F10-OVA tumor model. Combining this model with tumor transplantation, the immune responses of tumor-inherent and periphery-originated antigen-specific CD8+ T cells were compared to reveal a dynamic transition between these two populations. Collectively, this experimental design has provided another approach to precisely investigate the immune responses of CD8+ T cells in the TME, which sheds new light on the dynamics of tumor-specific T cell immune responses in the TME.
Protocol
All mouse experiments were performed in compliance with the guidelines of the Institutional Animal Care and Use Committees of the Third Military Medical University. Use 6-8-week-old C57BL/6 mice and naïve OT-I transgenic mice weighing 18-22 g. Use both male and female without randomization or "blinding."
1. Preparation of medium and reagents
- Prepare cell culture medium D10 as previously described29 by adding 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM L-glutamine into Dulbecco's Modified Eagle Medium.
- Prepare cell culture medium R10 by supplementing RPMI-1640 with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin.
NOTE: The culture media, D10 and R10, can remain sterile and stable for at least 2 weeks when stored at 2-4 °C. - Prepare Fluorescence-Activated Cell Sorting (FACS) buffer by supplementing 1x phosphate-buffered saline (PBS) with 2% FBS and 0.01% of sodium azide.
NOTE: With the addition of sodium azide, FACS Buffer can be stored at 2-4 °C for months. - Prepare red blood cell lysis (RBL) buffer by adding 155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM ethylenediamine tetraacetic acid (EDTA) into double-distilled water, and adjust its pH to 7.3.
NOTE: RBL buffer is stable for up to 3 months at room temperature (RT). - Prepare magnetic-activated cell sorting (MACS) buffer by supplementing PBS with 0.5% bovine serum albumin (BSA) and 2 mM EDTA.
NOTE: The solution should be passed through a 0.22 µm filter after the reagent is dissolved and preserved in asepsis. - Prepare a working solution of 2,2,2-tribromoethanol.
- Dissolve 2.5 g of 2,2,2-tribromoethanol in 5 mL of tert-amyl alcohol (2-methyl-2-butanol). Stir in a vapor-bathing, constant-temperature vibrator at 180 rpm, 40 °C overnight.
- Filter the solution through a 0.22 µm filter into a sterile container. Add double-distilled water up to a final volume of 200 mL, and mix thoroughly and continuously until the solution becomes clear and transparent.
- Determine and adjust the pH value of the solution to 7.3. Completely wrap the container with aluminum foil to exclude light and store at 4 °C.
NOTE: The final concentration of the working solution of 2,2,2-tribromoethanol is 12.5 mg/mL. A more concentrated solution is not recommended because the material is irritating at higher concentrations. Test the pH value of the working solution before each use, and discard it if the pH is less than 5.
2. Preparation of B16F10-OVA cell suspension
NOTE: Cell culture should be carried out in a biosafety hood under strict aseptic conditions.
- Thaw and culture a vial of B16F10-OVA cells with D10 in a cell culture incubator at 37 °C and 5% CO2.
- When the cells reach the confluency of about 80-90%, subculture the cells.
- Remove the culture medium with a pipettor, and rinse the cells twice using PBS.
NOTE: Do not add PBS forcefully against the adherent cells in the flask or cell culture dish. Instead, pipette the PBS toward a sidewall or add it drop-wise into the flask or dish. - Remove the PBS, and add 1-2 mL of 0.25% trypsin-EDTA solution into the flask or dish. Rock it back and forth to cover the entire cell surface. Place the flask or dish in an incubator at 37 °C for ~1 min or at RT until the cells detach.
NOTE: An inverted microscope can be used to check whether the cells have detached. - Add fresh D10 to stop the trypsinization. Pipette the suspension up and down to ensure that all the cells are dissociated from the flask or dish surface.
- Transfer the B16F10-OVA cell suspension into a 15 mL conical tube. Centrifuge the cells at 125 × g for 5-7 min at RT.
- Discard the supernatant, and resuspend the cell pellet with D10. Dispense the B16F10-OVA cell suspension into a new flask or cell culture dish containing D10 and incubate in a cell culture incubator at 37 °C and 5% CO2.
- Remove the culture medium with a pipettor, and rinse the cells twice using PBS.
- On the day of the tumor implantation, harvest B16F10-OVA cells that are ~90% confluent as described in steps 2.2.1 to 2.2.4. Discard the supernatant, and resuspend the cell pellet with 1 mL of PBS.
- Count the viable cells with a hemocytometer using 0.4% trypan blue. Adjust the cell density to 1 × 106 cells per 100 µL by adding PBS. Keep the cells on ice.
3. Ectopic tumor implantation of B16F10-OVA cells in the inguinal region of mice
- Use 6-8-week-old C57BL/6 mice weighing 18-22 g. Use both male and female without randomization or "blinding."
- Withdraw 100 µL of the prepared B16F10-OVA cell suspension into a 1 mL tuberculin syringe. Tap the barrel to move bubbles to the top, and gently push the plunger to remove air bubbles.
- Restrain the mouse and expose its abdomen. Press the left hind leg with the little finger to tighten the skin of the left inguinal region.
- Remove the mouse's hair from its left lower abdomen with an electric shaver. Use cotton soaked in 75% ethanol to clean the posterior quadrant of the left abdomen.
- Holding the syringe at a very shallow angle (0-15°) with the bevel of the needle facing upwards, insert it at the site of the left upper thigh, and advance 0.5-1 cm through the subcutaneous tissue into the inguinal region.
- Pull back on the plunger prior to injection. If there is negative pressure, depress the plunger entirely, and observe a small bolus (formation of fluid pocket) in the subcutis emerge.
NOTE: If blood is drawn back into the needle hub, withdraw and try again at another site. - Remove the needle after the injection is carried out and dispose of it appropriately. Release and place the mouse back into the cage.
- Measure tumor size on days 6-8 using a vernier scale after B16F10-OVA implantation. Select mice with a ~3 mm diameter (mung bean-sized) tumor and divide them equally and randomly into two groups.
NOTE: Mice with tumors of similar size are randomly assigned as donor and recipient mice; the matched tumor tissue excised from donor mice will be transplanted into the recipient mice. Furthermore, non-operated controls and sham-operated controls should be included to evaluate the effects of surgery on adoptive cell transfer and on the general health of mice. Thus, one group of tumor-bearing mice serves as non-operated controls, receiving either CD45.1+CD45.2+ or CD45.1+ OT-I cells but no surgery. The other group of mice serves as sham-operated controls, receiving either CD45.1+CD45.2+ or CD45.1+ OT-I cells and subsequent surgery similar to the experimental group but no allograft transplantation.
4. Adoptive transfer of congenically marked OT-I T cells into tumor-bearing mice
- On the day before the transfer, administer 4 mg of cyclophosphamide dissolved in 200 µL of PBS via intraperitoneal injection to each tumor-bearing mouse.
NOTE: Treatment with cyclophosphamide aims to induce lymphopenia in the host that produces "space" for transferred cells, promoting their survival and homing to lymphoid organs to function efficiently. - Use naïve OT-I transgenic mice with distinct congenic markers (6-8-week-old, 18-22 g, the same sex as the tumor-bearing mice). Use CD45.1+ OT-I mice and CD45.1+CD45.2+ OT-I mice to adoptively transfer OVA257-264 antigen-specific T cells into tumor-bearing donor and recipient mice, respectively.
NOTE: The origin of adoptively transferred OT-I cells can be easily identified if they display distinct congenic or fluorescent markers. For instance, inject CD45.1+ OT-I T cells into B16F10-OVA-bearing donor mice while injecting CD45.1+CD45.2+ OT-I T cells into B16F10-OVA-bearing recipient mice. CD45.1 and CD45.2 are both isoforms of the pan-lymphocyte marker CD45 (Ly5). Other commonly used congenic markers include different isoforms of CD90 (Thy1). This protocol can be used for mice carrying different congenic markers. OT-I mice should be of the same sex as the mice receiving OT-I cell transfer to avoid rejection issues. - Isolate the lymphocytes from the spleen and lymph nodes of the OT-I mouse.
NOTE: The following procedures in this step must be performed in a biosafety cabinet to maintain strict asepsis.- Prepare two 60 mm × 10 mm Petri dishes. Add 3 mL of R10 medium into one dish while adding 3 mL of RBL buffer into another dish. Place a 70 µm nylon cell strainer in the dish containing RBL buffer.
- Euthanize an OT-I mouse in an isoflurane chamber followed by cervical dislocation.
- Harvest the spleen, inguinal (subiliac), and axillary lymph nodes of the mouse and transfer them to a 60 mm × 10 mm dish with 3 mL of R10 on ice.
NOTE: The number of OT-I mice sacrificed may be adjusted depending on the number of tumor-bearing mice to be transferred. A typical yield of OT-I CD8+ T cells from a spleen and bilateral inguinal and axillary lymph nodes of OT-I CD8+ T cells is ~30-100 × 106 cells per mouse. - Using the end barrel of a 1 mL syringe, macerate the spleen in 3 mL of RBL buffer through the strainer. Incubate for 3 min at RT, and terminate the reaction by adding 3 mL of cold R10 medium.
- Mash the lymph nodes until only connective tissues remain. Rinse the filter with R10. Transfer the cell suspension into a new 15 mL conical tube. Centrifuge at 500 × g, 4 °C for 6 min.
- Decant the supernatant, and resuspend the cells in 3 mL of MACS buffer. Pass the cell suspension through a new 70 µm cell strainer to remove any flocs.
- Centrifuge the cell suspension at 500 × g for 5 min at 4 °C. Decant the supernatant.
- Use a mouse CD8+ T cell isolation kit (see the Table of Materials) to purify CD8+ T cells by negative selection, as per the manufacturer's protocol.
NOTE: When using kits from other companies, follow the manufacturer's instructions. - Keep the purified cell suspension on ice. Take a small sample of cells and mix with trypan blue to count cells using a hemocytometer.
- Determine the percentage of OT-I (live/dead–CD8+Va2+) cells by flow cytometry.
NOTE: Simultaneous staining of congenic markers and the transgenic TCR should be performed to verify the correct phenotype of the cells prior to transfer.- Add 5 × 104-1 × 105 cells into 1 mL of FACS buffer in a 1.5 mL centrifuge tube, and centrifuge the cell suspension at 350 × g, 4 °C for 3 min.
- Discard the supernatant, and disperse the cells by flicking the bottom of the tube. Place the tube on ice.
- Prepare the following conjugated antibody mixtures (diluted in 100 µL FACS buffer): anti-CD8, 1:200; anti-TCR Vα2, 1:100; anti-CD45.1, 1:200; anti-CD45.2, 1:200; and live/dead, 1:200 (refer to the Table of Materials).
- Vortex the antibody cocktail and centrifuge at 15,000 × g for 3 min to pellet antibody aggregates. Store the cocktail on ice and protect it from light.
- Resuspend the cells with 100 µL of antibody cocktail and thoroughly mix by flicking the tube. Incubate in the dark for 30 min on ice.
NOTE: Avoid disturbing the antibody aggregates at the bottom of the tube. - Wash the pellets twice with 1 mL of FACS buffer. Centrifuge at 350 × g, 4 °C for 3 min. Resuspend the cells in 200 µL of FACS buffer, and transfer the cell suspension to a FACS tube.
NOTE: To maintain the viability of the OT-I cells to be transferred, test the specimen as soon as possible. If the stained OT-I cells cannot be tested immediately, keep the cells in the dark on ice or refrigerate at 4 °C until analysis. Alternatively, the samples can be resuspended in 1-4% paraformaldehyde for extended storage (16 h) to prevent deterioration. - Run the specimen on a flow cytometer. Calculate the percentage of live/dead–CD8+Va2+ cells by dividing the number of live/dead–CD8+Vα2+ cells by the number of live/dead– cells.
- Determine the absolute number of OT-I cells (live/dead–CD8+Va2+) by multiplying the percentage of live/dead–CD8+Va2+ cells by the viable cell number obtained in step 4.3.9.
- Adjust the concentration of OT-I cells (live/dead–CD8+Va2+) to 1.5 × 106 /mL with PBS.
- Inject 3 × 105 distinct congenically marked OT-I cells (live/dead–CD8+Va2+) in 200 µL of PBS intravenously into two groups of B16F10-OVA-bearing mice (tumor-bearing mice divided into donor and recipient mice from step 3.8).
- Withdraw 200 µL of OT-I cell (live/dead–CD8+Va2+) suspension into a 100 U insulin syringe (29 G), and remove bubbles as in step 3.2.
- Place the mouse separately in a cage with an infrared lamp over the cage for 5-10 min to dilate the tail vein. Immobilize the mouse with a restraining device of appropriate size. Pull the tail to straighten it and spray with 75% ethanol to make the vein visible.
- Hold the syringe parallel to the vein and insert it into the vein at an angle of 0-15°. Pull back the plunger slightly, and if blood enters the barrel, slowly and steadily inject the suspension at a rate of no more than 1 mL/min.
NOTE: Resistance or swelling at the injection site indicates that the needle is not inside the vein; the injection site should be moved proximally. - After the injection is completed, remove the syringe, and press the insertion area gently for 3-5 s to stop bleeding. Return the mouse to the cage and closely observe it for a few minutes for adverse reactions. If it has normal mobility and nasal discharge, place it back in the company of the other mice.
5. Dissect tumor mass from tumor-bearing donor mice
NOTE: Maintain sterile conditions during surgery in sections 5 and 6. Sterilize all surgical instruments by autoclaving before and after each use. Disinfect the operating area in the biosafety cabinet with 75% ethanol followed by ultraviolet irradiation. Wear a clean gown, cap, face mask, and sterile gloves.
- Eight to ten days after the adoptive transfer, select donor mice bearing comparable tumor mass of ~5 mm diameter (soybean-sized) for transplantation surgery.
- Prepare a 100 mm × 20 mm dish in a biosafety cabinet, and add 10 mL of sterile ice-cold PBS.
- Euthanize a tumor-bearing donor mouse in an isoflurane chamber followed by cervical dislocation. Immerse the mouse in 75% ethanol for 3-5 min and transfer to the biosafety cabinet.
NOTE: The following procedures in this step must be performed in a biosafety cabinet to maintain strict asepsis. - Place the mouse on a dissection board covered with clean absorbent paper in a supine position. Restrain the mouse limbs with dissection needles.
- Cut the skin along the midline from above the urethral orifice to the xiphoid with scissors. Stretch the skin towards the left side of the mouse body with tweezers and restrain the skin with dissection needles.
- Excise the tumor, keeping its capsule as intact as possible. Carefully and gently remove the connective tissue near the tumor with surgical scissors.
NOTE: To maintain the integrity of the tumor, do not peel off the tumor capsule or cut the tumor tissue into pieces. - Place the tumor tissue in a 100 mm × 20 mm dish containing 10 mL of sterile ice-cold PBS for subsequent transplantation.
6. Subcutaneous transplantation of donor-derived tumor onto the tumor-matched recipient mice
NOTE: The allograft is supposed to be implanted into the mouse's lower flank on the same side as the previously existing tumor to make two tumors drain to the identical lymph node. In the protocol presented here, as the B16F10-OVA tumor was implanted subcutaneously on the left inguinal region of the mouse (section 3), the donor-derived tumor tissue was transplanted onto the left flank of the recipient in this step. The transplantation site can be adapted to the first-implanted tumor site.
- Anesthetize a tumor-matched recipient mouse with 250 mg/kg of 2,2,2-tribromoethanol via intraperitoneal injection. Pinch the toe of an extensor limb of the mouse to assess the level of anesthesia and wait for lack of pain reflex, which indicates the proper depth of anesthesia for performing the surgery. If vocalization or hind limb withdrawal is observed, further inject 0.01−0.03 mL of 2,2,2-tribromoethanol.
NOTE: The tumor-matched recipient mouse should be the same sex as the donor mouse that provides the allograft to avoid rejection issues. - Use veterinary ointment on eyes to prevent dryness. Shave the left flank of the mouse with an electric shaver. Apply a depilatory cream to remove the remaining hair.
NOTE: Avoid abrading the skin, which may increase the risk of contamination and infection. - Place the mouse in the biosafety cabinet. Place it in the prone position on a dissection board covered with clean absorbent paper, with the mouse's vertical axis parallel to and its head to the right side of the experimenter.
NOTE: The following procedures in this step must be performed in a biosafety cabinet to maintain strict asepsis. - Rub the skin of the shaved area with cotton soaked in povidone-iodine.
NOTE: Use povidone-iodine instead of 75% ethanol for sterilization to prevent loss of body heat. - Lift the skin at the center point between the mouse hip joints with surgical tweezers. Use the scissors to make a 5 mm-long vertical excision. Extend the cut rostrally along the dorsal midline to ~10-15 mm.
- Perform a sharp dissection by inserting the closed tips of the scissors into the incision and then opening to separate the peritoneum of the left flank from the skin and soft tissue.
NOTE: To avoid causing damage to the subcutaneous tissue and peritoneum, lift the skin at the center of the incision, and then insert the closed scissors as close to the skin as possible. - Make a skin pocket at the left flank by performing sharp dissection several times. Deposit the encapsulated, intact donor-derived tumor mass into the capsule.
NOTE: Mice in the sham-operated control group receive the same surgery operation without the donor-derived tumor transplantation. - Close the incision by interrupted suture (see Materials List). Place 2-3 sutures for each incision. Disinfect the skin around the cut with cotton soaked in povidone-iodine.
NOTE: There should be 5 mm between two consecutive stitches and a 3 mm distance from the incision. - Place the mouse in the lateral position in a clean and warm cage. Monitor it continuously until it has regained sufficient consciousness to maintain sternal recumbency.
- Administer buprenorphine subcutaneously at a dose of 0.1 mg/kg body weight every 8 h three times after surgery to alleviate the pain. Monitor the mouse's eating, drinking, moving, and the area operated on. Return the transplant recipient to the company of other animals only after it has fully recovered.
NOTE: The mouse typically recovers from the trauma of the surgery within 3 days. If the mouse is not back to normal feeding and mobility and shows any manifestations of infection, consult a veterinarian for interventions or euthanize it. - Sacrifice (euthanize the animals as in step 4.3.2) the mice at the indicated time points, and recover the cells of interest for flow cytometric analysis.
Representative Results
The schematic of this protocol is shown in Figure 1. Eight days after tumor inoculation, CD45.1+ and CD45.1+CD45.2+ OT-I cells were injected into B16F10-OVA tumor-bearing C57BL/6 mice. The tumor was surgically dissected from CD45.1+ OT-I cell-implanted mice (donor) on day 8 post-transfer and transplanted into tumor-matched CD45.1+CD45.2+ OT-I cell-implanted mice (recipient) in the dorsal flank on the same side as the implanted tumor. Through flow cytometry (gating strategy shown in Figure 2) analysis, two populations of CD44+CD8+ tumor antigen-specific T cells can be easily identified in the TME, including CD45.1+ donor-derived and CD45.1+CD45.2+ recipient-derived TILs. Subsequently, the proportions of these two populations within the allografts were analyzed at indicated time points to study the dynamics of the antigen-specific CD8+ T cells. At day 2 post-transplantation, there were ~83% of donor-derived antigen-specific CD8+ T cells within the transplanted tumor, more predominant than their recipient-derived counterparts. However, the proportion of recipient-derived OT-I cells was elevated in the late stage of tumorigenesis, exceeding tumor-inherent OT-I cells derived from the donor. (Figure 3).
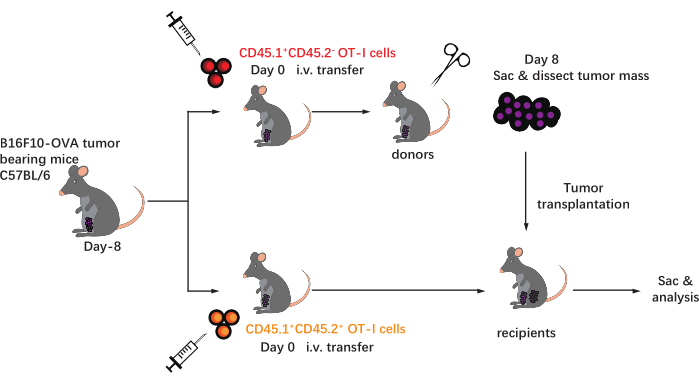
Figure 1: Schematic of the experimental design. C57BL/6mice are challenged with B16F10-OVA tumor on the inguinal area. Eight days later, different congenically marked (CD45.1+ or CD45.1+CD45.2+) OT-I cells are transferred into tumor-bearing mice. On day 8 post-transfer, the tumor on the CD45.1+ OT-I cell-implanted mice is surgically dissected and subcutaneously transplanted into tumor-matched CD45.1+CD45.2+ OT-I cell-implanted recipients in the flank on the same side as the existing tumor. Then, the mice are sacrificed, and antigen-specific T cells (OT-I cells) within the allografts are analyzed at the indicated time points. Abbreviations: CD = cluster of differentiation; i.v. = intravenous; Sac = sacrifice. Please click here to view a larger version of this figure.
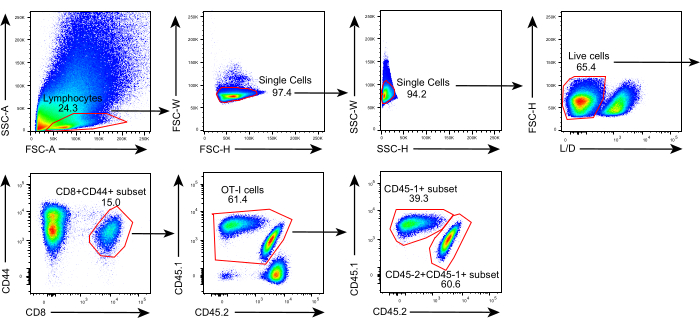
Figure 2: Gating strategy of flow cytometry analysis. Gating strategy used to identify donor-derived (CD45.1+) and recipient-derived (CD45.1+CD45.2+) antigen-specific CD44+CD8+ T cells within allografts. Abbreviations: SSC-A = side scattering-area; FSC-A = forward scattering-area; FSC-W = forward scattering-width; FSC-H = forward scattering-height; SSC-W = side scattering-width; SSC-H = side scattering-height; L/D = live/dead; CD = cluster of differentiation. Please click here to view a larger version of this figure.
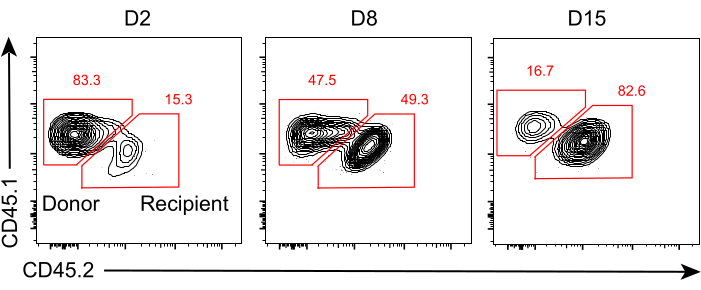
Figure 3: The ratio of donor- and recipient-derived antigen-specific CD8+ T cells within tumor allografts. Representative flow cytometry plots showing expression of the congenic markers CD45.1 and CD45.2 used to identify donor-derived and recipient-derived OT-I cells within tumor allografts at days 2, 8, and 15 after transplantation. The numbers represent the percentages of the two subsets in the CD44+CD8+ T cell population. Please click here to view a larger version of this figure.
Discussion
T cell-mediated immunity plays a crucial role in immune responses against tumors, with CTLs playing the leading role in eradicating cancerous cells. However, the origins of tumor antigen-specific CTLs within TME have not been elucidated30. The use of this tumor transplantation protocol has provided an important clue that intratumoral antigen-specific CD8+ T cells may not persist for a long time, despite the existence of stem-like TCF1+ progenitor CD8+ T cells. Notably, there is a continuous influx of periphery-derived tumor-specific CD8+ T cells into the tumor mass.
To our knowledge, this is a relatively convenient and convincing method confirming that the maintenance of antigen-specific CD8+ T cells within the TME predominantly depends on the replenishment of periphery-derived tumor-specific CD8+ T cells instead of the self-renewal of tumor-resident TILs. Although the protocol presented here only focuses on the proportions of donor-derived and recipient-derived TILs, the phenotypic, functional, and transcriptional properties of these two populations can be readily examined with flow cytometry. Moreover, it is feasible to combine ICB antibodies to investigate the responses of a specific cell subset to ICB therapy.
In this protocol, donor-derived tumor tissue is transplanted onto the recipient mouse with an existing original tumor. Two tumors in a recipient mouse will lead to the distribution of periphery-generated T cells into two tumor masses. Moreover, the tumor burden will be nearly doubled compared to animals without transplants. In pilot experiments, we attempted to excise the original tumor on recipient mice before transplantation; however, it was technically challenging to eliminate all tumor cells by surgery thoroughly. The residual tumor cells would rapidly and form a new tumor tissue soon. Thus, there is a limitation for this system when comparing T cell immune responses with those in non-transplanted mice. However, this system is still useful for the comparison of recently migrated and existing T cells within the same TME that is transplanted from donor tumor-bearing mice. Besides, there is no denying that the transplantation of tumor tissue may lead to inflammation, which might influence immune cell dynamics within the tumor. Though the impact of surgery on OT-I cell infiltration could be excluded through non-operated and sham-operated controls, we did not assess the effects of local inflammatory responses to OT-I cell dynamics.
Some considerations should be taken into account, one of which is the usage of cyclophosphamide. Cyclophosphamide31 is an alkylating agent widely used to treat solid organ malignancies and lymphoproliferative and autoimmune disorders. Six to eight days after B16F10-OVA inoculation, cyclophosphamide is administered before adoptive transfer to induce the lymphodepletion of host mice and enhance the activity of the transferred OT-I cells29. Although melanoma is not sensitive to this reagent, some tumor cell lines, such as EG732, a murine thymic lymphoma cell line, respond to cyclophosphamide. Treatment of EG7-bearing mice with cyclophosphamide results in the eradication of tumors, which suggests that cyclophosphamide must be carefully used or titrated for sensitive tumor models. The recommended alternative method is a single sublethal dose of radiation (4.5-5.5 Gy) one day before the transfer, and the optimal choice depends on the characteristic of tumor cell lines.
Other steps need to be taken cautiously, including the careful selection of tumor-bearing donor mice and the delicate surgical operation during tumor transplantation. Implanted tumors would be surgically removed and transplanted into tumor-matched recipient mice 8-10 days post-transfer. Before transplantation, a comparable size of tumor mass of ~5 mm diameter is to be chosen as an allograft to reduce discrepancies between individual mice and make acquired data more reliable. Moreover, during surgery, the incision should be near the midline of the mouse back to keep the allograft at a distance from the tumor already existing in the recipient mouse. Gentle dissection is also suggested to prevent injuries on the inguinal lymph node and surrounding tissues.
The effective killing of cancerous cells requires the coordination of various components within the TME33. The protocol presented here can be extended to the investigation of adaptive and innate immune cells such as natural killer cells, tumor-associated macrophages, and dendritic cells. Furthermore, in addition to the B16F10-OVA utilized here, this protocol can be applied to other subcutaneous tumor models. To conclude, the aforementioned tumor transplantation assay offers a new approach for the study of interactive transitions of certain types of immune cells during antitumor responses and is useful for researchers in tumor immunology.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was supported by grants from the National Natural Science Fund for Distinguished Young Scholars (No. 31825011 to LY) and the National Natural Science Foundation of China (No. 31900643 to QH, No. 31900656 to ZW).
Materials
| 0.22 μm filter | Millipore | SLGPR33RB | |
| 1 mL tuberculin syringe | KDL | BB000925 | |
| 1.5 mL centrifuge tube | KIRGEN | KG2211 | |
| 100 U insulin syringe | BD Biosciences | 320310 | |
| 15 mL conical tube | BEAVER | 43008 | |
| 2,2,2-Tribromoethanol (Avertin) | Sigma | T48402-25G | |
| 2-Methyl-2-butanol | Sigma | 240486-100ML | |
| 70 μm nylon cell strainer | BD Falcon | 352350 | |
| APC anti-mouse CD45.1 | BioLegend | 110714 | Clone:A20 |
| B16F10-OVA cell line | bluefbio | BFN607200447 | |
| BSA-V (bovine serum albumin) | Bioss | bs-0292P | |
| BV421 Mouse Anti-Mouse CD45.2 | BD Horizon | 562895 | Clone:104 |
| cell culture dish | BEAVER | 43701/43702/43703 | |
| centrifuge | Eppendorf | 5810R-A462/5424R | |
| cyclophosphamide | Sigma | C0768-25G | |
| Dulbecco's Modified Eagle Medium | Gibco | C11995500BT | |
| EasySep Mouse CD8+ T Cell Isolation Kit | Stemcell Technologies | 19853 | |
| EDTA | Sigma | EDS-500g | |
| FACS tubes | BD Falcon | 352052 | |
| fetal bovine serum | Gibco | 10270-106 | |
| flow cytometer | BD | FACSCanto II | |
| hemocytometer | PorLab Scientific | HM330 | |
| isoflurane | RWD life science | R510-22-16 | |
| KHCO3 | Sangon Biotech | A501195-0500 | |
| LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit, for 633 or 635 nm excitation | Life Technologies | L10199 | |
| needle carrier | RWD Life Science | F31034-14 | |
| NH4Cl | Sangon Biotech | A501569-0500 | |
| paraformaldehyde | Beyotime | P0099-500ml | |
| PE anti-mouse TCR Vα2 | BioLegend | 127808 | Clone:B20.1 |
| Pen Strep Glutamine (100x) | Gibco | 10378-016 | |
| PerCP/Cy5.5 anti-mouse CD8a | BioLegend | 100734 | Clone:53-6.7 |
| RPMI-1640 | Sigma | R8758-500ML | |
| sodium azide | Sigma | S2002 | |
| surgical forceps | RWD Life Science | F12005-10 | |
| surgical scissors | RWD Life Science | S12003-09 | |
| suture thread | RWD Life Science | F34004-30 | |
| trypsin-EDTA | Sigma | T4049-100ml |
Referências
- Blank, C. U., et al. Defining ‘T cell exhaustion. Nature Reviews Immunology. 19 (11), 665-674 (2019).
- Leko, V., Rosenberg, S. A. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell. 38 (4), 454-472 (2020).
- McLane, L. M., Abdel-Hakeem, M. S., Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annual Review of Immunology. 37, 457-495 (2019).
- Davis, M. M., Brodin, P. Rebooting human immunology. Annual Review of Immunology. 36, 843-864 (2018).
- Sharma, P., Allison, J. P. The future of immune checkpoint therapy. Science. 348 (6230), 56-61 (2015).
- Littman, D. R. Releasing the brakes on cancer immunotherapy. Cell. 373 (16), 1490-1492 (2015).
- Verma, V., et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1(+)CD38(hi) cells and anti-PD-1 resistance. Nature Immunology. 20, 1231-1243 (2019).
- Hashimoto, M., et al. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annual Review of Medicine. 69, 301-318 (2018).
- Dammeijer, F., et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell. 38 (5), 685-700 (2020).
- Buchwald, Z. S., et al. Tumor-draining lymph node is important for a robust abscopal effect stimulated by radiotherapy. Journal for ImmunoTherapy of Cancer. 8 (2), 000867 (2020).
- Philip, M., Schietinger, A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Current Opinion in Immunology. 58, 98-103 (2019).
- Miller, B. C., et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nature Immunology. 20, 326-336 (2019).
- Wu, T. D., et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 579, 274-278 (2020).
- Im, S. J., Konieczny, B. T., Hudson, W. H., Masopust, D., Ahmed, R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proceedings of the National Academy of Sciences of the United State of America. 117 (8), 4292-4299 (2020).
- Beltra, J. C., et al. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 52 (5), 825-841 (2020).
- Myers, L. M., et al. A functional subset of CD8(+) T cells during chronic exhaustion is defined by SIRPalpha expression. Nature Communications. 10 (1), 794 (2019).
- Jansen, C. S., et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 576, 465-470 (2019).
- Jadhav, R. R., et al. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proceedings of the National Academy of Sciences of the United State of America. 116 (28), 14113-14118 (2019).
- Li, H., et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. 176 (4), 775-789 (2018).
- Kurtulus, S., et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) tumor-infiltrating T cells. Immunity. 50 (1), 181-194 (2019).
- Fransen, M. F., et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight. 3 (23), 124507 (2018).
- E, J. F., et al. CD8(+)CXCR5(+) T cells in tumor-draining lymph nodes are highly activated and predict better prognosis in colorectal cancer. Human Immunology. 79 (6), 446-452 (2018).
- Snell, L. M., et al. CD8(+) T cell priming in established chronic viral infection preferentially directs differentiation of memory-like cells for sustained immunity. Immunity. 49 (4), 678-694 (2018).
- Siddiqui, I., et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 50 (1), 195-211 (2019).
- Wang, Y., et al. The transcription factor TCF1 preserves the effector function of exhausted CD8 T cells during chronic viral infection. Frontiers in Immunology. 10, 169 (2019).
- Krishna, S., et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science. 370 (6522), 1328-1334 (2020).
- Yost, K. E., et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nature Medicine. 25, 1251-1259 (2019).
- Zitvogel, L., Pitt, J. M., Daillere, R., Smyth, M. J., Kroemer, G. Mouse models in oncoimmunology. Nature Reviews Cancer. 16 (12), 759-773 (2016).
- Li, Y., et al. Bcl6 preserves the suppressive function of regulatory T cells during tumorigenesis. Frontiers in Immunology. 11, 806 (2020).
- Yu, D., Ye, L. A portrait of CXCR5(+) follicular cytotoxic CD8(+) T cells. Trends in Immunology. 39 (12), 965-979 (2018).
- Bracci, L., et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clinical Cancer Research. 13 (2), 644-653 (2007).
- Salem, M. L., El-Naggar, S. A., Mahmoud, H. A., Elgharabawy, R. M., Bader, A. M. Cyclophosphamide eradicates murine immunogenic tumor coding for a non-self-antigen and induces antitumor immunity. International Journal of Immunopathology and Pharmacology. 32, 1-5 (2018).
- Thorsson, V., et al. The Immune landscape of cancer. Immunity. 48 (4), 812-830 (2018).

