Microfluidics-Assisted Selective Depolarization of Axonal Mitochondria
Summary
The present protocol describes the seeding and staining of neuronal mitochondria in microfluidic chambers. The fluidic pressure gradient in these chambers allows for the selective treatment of mitochondria in axons to analyze their properties in response to pharmacological challenges without affecting the cell body compartment.
Abstract
Mitochondria are the primary suppliers of ATP (adenosine triphosphate) in neurons. Mitochondrial dysfunction is a common phenotype in many neurodegenerative diseases. Given some axons’ elaborate architecture and extreme length, it is not surprising that mitochondria in axons can experience different environments compared to their cell body counterparts. Interestingly, dysfunction of axonal mitochondria often precedes effects on the cell body. To model axonal mitochondrial dysfunction in vitro, microfluidic devices allow treatment of axonal mitochondria without affecting the somal mitochondria. The fluidic pressure gradient in these chambers prevents diffusion of molecules against the gradient, thus allowing for analysis of mitochondrial properties in response to local pharmacological challenges within axons. The current protocol describes the seeding of dissociated hippocampal neurons in microfluidic devices, staining with a membrane-potential sensitive dye, treatment with a mitochondrial toxin, and the subsequent microscopic analysis. This versatile method to study axonal biology can be applied to many pharmacological perturbations and imaging readouts, and is suitable for several neuronal subtypes.
Introduction
Mitochondria are the main suppliers of ATP (adenosine triphosphate) in neurons. As neuronal health is intimately linked to mitochondrial function, it is not surprising that dysfunctional regulation of these organelles has been associated with the onset of various neurodegenerative diseases, including Parkinson's disease1. Furthermore, mitochondrial intoxication has successfully been used to model Parkinsonian symptoms in animals2. In both animal models and human disease, the demise of neurons starts at the distal parts3,4, hinting that axonal mitochondria might be more susceptible to insults. However, the biology of mitochondria in axons is not well understood due to the difficulties associated with targeted treatment and analysis of axonal mitochondria without simultaneous disturbance of cell body processes.
Recent advances in culturing techniques of dissociated neurons in vitro now allow the fluidic separation of axons and cell bodies through microfluidic devices5. As depicted in Figure 1A, these devices feature four access wells (a/h and c/i), with two channels connecting each pair (d and f). The large channels are connected with each other by a series of 450 µm long microchannels (e). Intentional differences in the fill levels between the two chambers create a fluid pressure gradient (Figure 1B) that prevents the diffusion of small molecules from the channel with a lower fluid level to the other side (Figure 1C, illustrated with Trypan blue dye).
We recently used microfluidic devices to study local translation requirements in axonal mitophagy, the selective removal of damaged mitochondria6. In the present protocol, different steps are presented to induce local mitochondrial damage through selective treatment of axons using the mitochondrial complex III inhibitor Antimycin A6,7.
Protocol
All animal experiments were performed following the relevant guidelines and regulations of the Government of Upper Bavaria. The primary neurons were prepared from E16.5 C57BL/6 wild-type mouse embryos of both sexes following standard methods as previously described6.
1. Assembly of the microfluidic device
- Coat one six-well glass-bottom tissue culture plate with a final concentration of 20 µg/mL of Poly-D-Lysine and 3.4 µg/mL of Laminin in PBS (phosphate-buffered saline) (see Table of Materials).
- Incubate the coated plate overnight in the dark at room temperature.
NOTE: Coating can also be performed at 37 °C for 1-2 h. The choice of the coating depends on the cell type used. - After coating, bring the six-well plates to the sterile hood and wash them twice with sterile ddH2O.
NOTE: Do not wash with salt-containing buffers such as PBS, as salt crystals will interfere with seal formation. - Allow the plate to dry in a tilted position for 3-5 min. Remove excess water by vacuum suction or pipetting.
- Soak the microfluidic chamber in 80% ethanol.
NOTE: The microfluidic chamber was obtained from commercial sources (see Table of Materials). - Allow the microfluidic chamber to dry for 3-5 min in a tilted position. Remove excess ethanol by vacuum, suction, or pipetting.
- When completely dry, place the microfluidic chamber in the center of the well.
NOTE: The formation of the seal can be observed as a change in reflective properties upon exclusion of air from the interface between the plate and the silicone device. Both the plate and microfluidic chamber must be completely dry before assembly to create a good seal. However, drying time needs to be minimized as much as possible to ensure proper adherence to the cells. Therefore, the wells and chambers must be visually inspected for remaining liquid droplets. If no droplets are visible, immediately assemble the chambers. - Gently tap the microfluidic chamber at its borders and microgroove section in the middle for proper attachment to the glass plate.
2. Seeding and maintaining of neurons
- Collect the desired number of dissociated hippocampal neurons per chamber in a 1.5 mL reaction tube.
NOTE: For the present study, 1.5 x 105 neurons were seeded per device for imaging-based applications. - Centrifuge neurons at 1000 x g for 4 min at room temperature.
- Discard the supernatant with a pipette and resuspend the pellet in 8 µL of B27-Neurobasal media (see Table of Materials).
- Pipette the cell solution into the channel entrance of the microfluidic chamber (b de Figure 1A).
- Tap on the back of the plate to assist flow through the channel.
NOTE: Tapping can be done quite forcefully without worry that the seal will break. - Aspirate with a pipette any remaining cell suspension at the exit of the channel (g de Figure 1A) to decrease the number of cells outside the channel.
- Incubate the microfluidic chamber with cells for 15-20 min at 37 °C and 5% CO2.
- Fill the top axonal well with 50 µL of B27-Neurobasal media in (c de Figure 1A).
- Tap on the back of the plate to assist flow through the channel.
- Fill wells on the soma side (a and h de Figure 1A) with 150 µL of B27-Neurobasal media each, creating a tension bubble on top. The volume of the bubble is about 20 µL.
NOTE: Creating a tension bubble is not necessary to achieve fluidic isolation, but it provides a clear visual of the higher volume on the soma side. - Fill both wells of the axonal side (c and i de Figure 1A) with 100 µL of B27-Neurobasal media each.
NOTE: To promote axonal growth through the microgrooves, it is recommended that the wells on the soma side contain more media than the wells on the axon side. However, axons will by chance grow through the microgrooves even without volume differences. - Incubate the microfluidic chamber at 37 °C and 5% CO2 for 7-8 days.
NOTE: Growth of axons into the axonal chamber can usually be observed starting at day in vitro (DIV) 5. Longer culturing times are possible if more mature cultures are desired. - Feed neurons every 2-3 days by removing the medium from the two upper wells (a and c) and replacing it with fresh B27-Neurobasal medium until a tension bubble is formed.
3. Staining with mitochondrial membrane potential sensitive dye
- At DIV 7-8, assess that the axons have grown through the microgrooves and extend into the axonal compartment underneath a light microscope.
NOTE: Different DIV stages are also possible depending on the desired age. - Perform two washes with pre-warmed imaging medium (37 °C) by removing the B27-Neurobasal medium from all wells and adding about 100 µL of the imaging medium to the top wells of both the axonal and soma channels (a and c). Let the medium flow through to the lower wells (h and i).
- Remove the medium from the lower wells and any leftover medium in the top wells, and repeat with fresh medium, leaving the wells empty at the end of the wash.
NOTE: Due to the high capillary force in the channels (d and f), there will be a remaining wash medium in the channels that should not be removed to prevent the neurons from drying. Any phenol-red-free imaging medium can be used here, for instance, Hibernate E (see Table of Materials). If the microscope is not set up with a CO2 supply, ensure that the imaging medium uses an alternative buffering system to carbonate/CO2 (e.g., HEPES)8.
- Remove the medium from the lower wells and any leftover medium in the top wells, and repeat with fresh medium, leaving the wells empty at the end of the wash.
- Dilute 1 mM tetramethylrhodamine ethyl ester (TMRE, red, see Table of Materials) stock to 5 nM in the imaging medium.
NOTE: Do not exceed 1% DMSO in the final dilution to avoid toxicity. - Add 100 µL of the 5 nM TMRE dilution to both the somatic and axonal top wells (a and c), and let the medium flow through both the somatic and axonal compartments until there is an equal volume in all wells. Fill up with the TMRE-containing medium.
- Place the neurons back into a controlled environment chamber (37 °C and 5% CO2) for 25 min.
- Perform two washes with the pre-warmed imaging medium (37 °C) as described before (step 3.2). On the last wash, fill up with 100 µL in each well and create a tension bubble of the medium on the somatic wells (a and c).
4. Live-cell imaging
NOTE: Still images shown were acquired on a spinning disk confocal microscope, using a 40x NA 1.25 immersion objective (see Table of Materials). 200 ms exposure time and 10% laser power for the red channel and 500 ms exposure time for brightfield were chosen. However, regular confocal or widefield inverted microscopes can also be used to study TMRE intensity.
- Image the cells with an inverted microscope.
- Ensure that the microscope is equipped with a stage incubator to maintain the neuronal culture at 37 °C throughout the experiment.
- Select a region of interest in the somatic compartment, and follow it through the microgrooves into the axonal compartment. Ensure that the microgrooves imaged match those in the somatic and the axonal compartments (for easier baseline and post-treatment comparison).
- Capture fluorescence images using excitation at 561 nm and emission at 625 nm for red fluorescence signal.
- Acquire the images.
- To induce mitochondrial depolarization, add 20 µM Antimycin A (see Table of Materials) in the imaging medium to the axonal compartment. To ensure a volume difference between the soma and axonal chambers, check the presence of a tension bubble on the somatic side (equals volume >110 µL).
- Remove all imaging medium from the lower well of the axonal side (i), except for the medium inside the channel (f). Add 160 µL of 20 µM Antimycin A to the top axonal well (c) and let it flow through the channel until there is an equal volume in both axonal wells. Volumes in axonal wells are roughly 80 µL per well.
NOTE: Ensure that the volume in the axonal wells is smaller than in the somatic wells to ensure proper fluid pressure. A difference of at least 10 µL (10% of the well volume) is recommended, but also higher differences are possible.
- Remove all imaging medium from the lower well of the axonal side (i), except for the medium inside the channel (f). Add 160 µL of 20 µM Antimycin A to the top axonal well (c) and let it flow through the channel until there is an equal volume in both axonal wells. Volumes in axonal wells are roughly 80 µL per well.
- Incubate neurons in the controlled environment chamber (37 °C) for 20-30 min.
- Repeat imaging as described above (steps 4.3-4.5). Ensure that the same positions are imaged (easily identified by the microgrooves) as during the baseline acquisition.
Representative Results
Primary hippocampal neurons were grown in microfluidic devices for 7-8 days before mitochondria were stained with the membrane-sensitive dye (TMRE) for 25 min in both the channels. As shown in Figure 2A, this yielded homogenous staining of mitochondria on both sides of the microgrooves, yet it was insufficient to equilibrate the staining into the middle of the microgrooves. Upon addition of Antimycin A to the axonal side, somal mitochondria retained the TMRE signal (Figure 2B and Video 1), whereas TMRE fluorescence was lost from axonal mitochondria (Figure 2C and Video 1). The video was captured using a fully integrated digital widefield microscope (see Table of Materials).
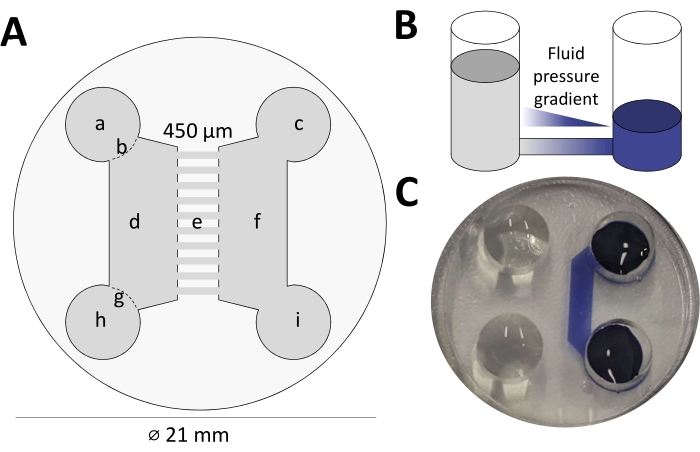
Figure 1: Microfluidic devices allow the fluidic isolation of axons. (A) Schematic of the microfluidic device used in this study. The silicone disk (diameter 21 mm) fits easily into a six-well plate. a) Well on soma side, b) Entrance of channel on soma side, c) Well on axon side, d) Channel on soma side, e) Microgrooves, f) Channel on axon side, g) Exit of the channel on soma side, h) Well on soma side, i) Well on axon side. (B) Schematic detailing how the different fluid levels create a fluidic pressure gradient across the microchannels. (C) Demonstration of the fluidic isolation by addition of Trypan blue to one side of the chamber. Note that due to the fluidic pressure gradient, the blue dye does not equilibrate across the microchannels. Please click here to view a larger version of this figure.
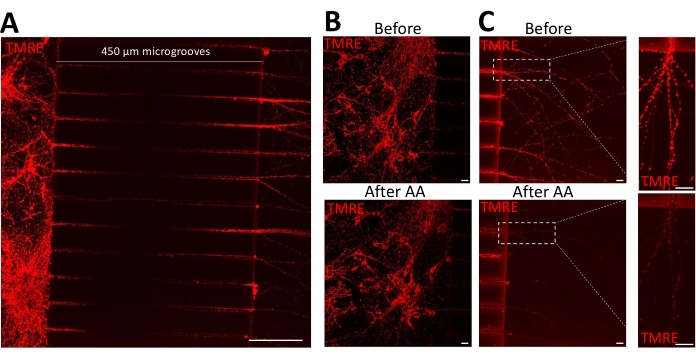
Figure 2: Selective treatment of axons depolarizes axonal but not cell body mitochondria. (A) Representative micrograph presenting an overview of the effectivity of TMRE staining in microfluidic devices. Scale bar = 100 µm. (B–C) The representative micrograph shows the same area in the cell body and the axonal compartment before and after adding 20 µM Antimycin A (AA) to the axonal compartment. Scale bar = 20 µm. Please click here to view a larger version of this figure.
Video 1: Effect of Antimycin A treatment on mitochondria in axons and cell bodies. Live cell imaging of the loss of TMRE fluorescence upon adding Antimycin A. Please click here to download this Video.
Discussion
The present protocol describes a method to seed and culture dissociated hippocampal neurons in a microfluidic device to treat axonal mitochondria separately. The utility of this approach with the membrane-sensitive dye TMRE and the complex III inhibitor Antimycin A (as previously demonstrated7) is demonstrated here, but this method can be easily adapted to other mitochondrial dyes or genetically encoded sensors of mitochondrial functions that allow local, microscopy-based readouts9. Other neuronal cell types can also be grown in microfluidic chambers, such as primary cortical neurons10 or induced pluripotent stem cell (ipSC)-derived motorneurons11, making this platform a versatile tool to study mitochondrial function in neurodegeneration in the neuronal cell type of interest. The assembly of microfluidic devices is crucial to achieving an efficient seal and is most easily explained by watching an experienced researcher perform the assembly. The downstream labeling and treatment procedure described here are meant to be exemplary and may be adjusted to fit the protocols currently established in the respective labs while maintaining the fluidic pressure gradient.
The seeding technique described here differs from published protocols and the manufacturer’s description, as we skip the suggested washes of the assembled device and instead directly seed the dissociated neurons into the dry chamber (section 2). It has been observed that this reduces the number of neurons needed, as it increases the density of neurons within the channel (e) and limits the spread of neurons into the wells far away from the microchannels. The dry seeding is aided by tapping the bottom of the plate (step 2.5), which may or may not be necessary depending on the force applied previously when assembling the device.
However, certain limitations exist in this procedure. There is some variability in the tightness of the seal due to differences in the force applied during assembly that may lead to restricted growth through the microgrooves. Also, remaining moisture can disturb the seal formation and allow axonal growth or even cell migration underneath the device. Both problems can easily be spotted prior to staining, leading to the exclusion of faulty chambers from the experiment.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was supported by the German Research Foundation (HA 7728/2-1 and EXC2145 Project ID 390857198) and the Max Planck Society.
Materials
| 6-well Glass bottom plate | Cellvis | P06.1.5H-N | Silicone device |
| Antimycin A | Sigma | A8674 | |
| B27 | Gibco | 17504044 | |
| EVOS M5000 widefield microscope | Thermofischer Scientific | EVOS M5000 | fully integrated digital widefield microscope |
| Hibernate E | BrainBits | HE500 | |
| Inverted spinning disk confocal | Nikon | TI2-E + CSU-W1 | With incubator chamber |
| Laminin | Invitrogen | L2020 | |
| Microfluidic devices | XONA microfluidics | RD450 | |
| Neurobasal medium | Gibco | 21103049 | |
| Poly-D-Lysine | Sigma | P2636 | |
| TMRE | Sigma | 87917 |
Referências
- Murali Mahadevan, H., Hashemiaghdam, A., Ashrafi, G., Harbauer, A. B. Mitochondria in neuronal health: from energy metabolism to Parkinson’s disease. Advanced Biology. 5 (9), 2100663 (2021).
- Dauer, W., Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron. 39 (6), 889-909 (2003).
- Moratalla, R., et al. Differential vulnerability of primate caudate-putamen and striosome-matrix dopamine systems to the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine. Proceedings of the National Academy of Sciences. 89 (9), 3859-3863 (1992).
- Cheng, H. -. C., Ulane, C. M., Burke, R. E. Clinical progression in Parkinson disease and the neurobiology of axons. Annals of Neurology. 67 (6), 715-725 (2010).
- Taylor, A. M., et al. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature Methods. 2 (8), 599-605 (2005).
- Harbauer, A. B., et al. Neuronal mitochondria transport Pink1 mRNA via synaptojanin 2 to support local mitophagy. Neuron. 110 (9), 1516-1531 (2022).
- Ashrafi, G., Schlehe, J. S., LaVoie, M. J., Schwarz, T. L. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. Journal of Cell Biology. 206 (5), 655-670 (2014).
- Shipman, C. Evaluation of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) as a tissue culture buffer. Proceedings of the Society for Experimental Biology and Medicine. 130 (1), 305-310 (1969).
- Harbauer, A. B., Schneider, A., Wohlleber, D. Analysis of mitochondria by single-organelle resolution. Annual Review of Analytical Chemistry. 15, 1-16 (2022).
- Taylor, A. M., et al. Axonal mRNA in uninjured and regenerating cortical mammalian axons. The Journal of Neuroscience. 29 (15), 4697-4707 (2009).
- Altman, T., et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nature Communications. 12 (1), 1-17 (2021).

