Genetic Study of Axon Regeneration with Cultured Adult Dorsal Root Ganglion Neurons
Summary
An in vitro model for genetic study of axon regeneration using cultured adult mouse dorsal root ganglion neurons is described. The method includes a re-suspension/re-plating step to allow axon re-growth from neurons undergoing genetic manipulation. This approach is especially useful for loss-of-function studies of axon regeneration using RNAi-based protein knockdown.
Abstract
It is well known that mature neurons in the central nervous system (CNS) cannot regenerate their axons after injuries due to diminished intrinsic ability to support axon growth and a hostile environment in the mature CNS1,2. In contrast, mature neurons in the peripheral nervous system (PNS) regenerate readily after injuries3. Adult dorsal root ganglion (DRG) neurons are well known to regenerate robustly after peripheral nerve injuries. Each DRG neuron grows one axon from the cell soma, which branches into two axonal branches: a peripheral branch innervating peripheral targets and a central branch extending into the spinal cord. Injury of the DRG peripheral axons results in substantial axon regeneration, whereas central axons in the spinal cord regenerate poorly after the injury. However, if the peripheral axonal injury occurs prior to the spinal cord injury (a process called the conditioning lesion), regeneration of central axons is greatly improved4. Moreover, the central axons of DRG neurons share the same hostile environment as descending corticospinal axons in the spinal cord. Together, it is hypothesized that the molecular mechanisms controlling axon regeneration of adult DRG neurons can be harnessed to enhance CNS axon regeneration. As a result, adult DRG neurons are now widely used as a model system to study regenerative axon growth5-7.
Here we describe a method of adult DRG neuron culture that can be used for genetic study of axon regeneration in vitro. In this model adult DRG neurons are genetically manipulated via electroporation-mediated gene transfection6,8. By transfecting neurons with DNA plasmid or si/shRNA, this approach enables both gain- and loss-of-function experiments to investigate the role of any gene-of-interest in axon growth from adult DRG neurons. When neurons are transfected with si/shRNA, the targeted endogenous protein is usually depleted after 3-4 days in culture, during which time robust axon growth has already occurred, making the loss-of-function studies less effective. To solve this problem, the method described here includes a re-suspension and re-plating step after transfection, which allows axons to re-grow from neurons in the absence of the targeted protein. Finally, we provide an example of using this in vitro model to study the role of an axon regeneration-associated gene, c-Jun, in mediating axon growth from adult DRG neurons9.
Protocol
1. Preparation of Coverslips, Culture Medium, and Digestion Enzymes
- The 12-mm round #1 glass coverslips are used for the neuronal culture. The coverslips are cleaned with 10% HCL overnight followed by ultrasonic wash with distilled and deionized water for 3 times (20 min/time). The cleaned coverslips are stored in 70% ethanol for future use. Before each experiments, the coverslips are air dried and placed into the culture plate.
- To coat the dried coverslips, 100 μl of coating solution containing 100 μg/ml Poly-D-Lysine and 10 μg/ml Laminin working solution is added onto each coverslip and the culture plate is transferred into a 37 °C incubator. After 1-2 hr, the coating solution is removed and the coverslips are washed 3 times with sterile 1X PBS.
- To prepare the culture medium, the Minimum Essential Medium (MEM) is supplemented with 5% fetal bovine serum (FBS), 1X Penicillin-Streptomycin solution (500 units of penicillin and 500 μg of streptomycin), 1X GlutaMAX-I supplement, and the antimitotic reagents containing 20 μM 5-fluoro-2-deoxyuridine and 20 μM uridine (FDU/R). For the serum-free medium, the FBS is replaced with the supplement B27.
- The collagenase A solution is prepared by dissolving collagenase A powder with MEM to make a working concentration of 1 mg/ml. For the trypsin, the 1X TrypLE Express is used.
2. Dissection and Harvest of Adult Mouse DRG Neurons
- After euthanizing the 6- to 10-weeks old adult mouse, remove the dorsal skin and cut off the whole spinal column. Wash the removed spinal column with 1 X PBS 2-3 times.
- Pin the removed spinal column to the dissecting plate with ventral side up, and carefully removes muscles to expose the sensory nerves under a dissecting microscope. The nerves connected to DRGs at the lumber level are the thickest and easy to locate. Therefore, for most experiments only the lumbar DRGs are harvested.
- Cut through each vertebra along the centerline with small scissors and carefully remove the intervertebral discs. Using forceps to split each vertebra to expose the spinal cord.
- To dissect out the lumber DRGs, lift up each lumber nerve with forceps and trace toward the spinal cord to locate the associated lumbar DRGs (from L1 to L6).
- Cut off each DRG from attached peripheral nerve, dorsal and ventral roots with spring scissors, and store it in microfuge tube with MEM medium placed on ice. More DRGs at different spinal levels (e.g. thoracic DRGs) can be dissected out in a similar way when necessary.
3. Digestion and Dissociation of Adult Mouse DRG Neurons
- After collecting all the dissected DRGs, replace the MEM medium with 1 ml collagenase A solution and incubate the microfuge tube at 37 °C for 90 min.
- Replace collagenase A solution with 500 μl 1X TrypLE Express solution and incubate at 37 °C for 15-20 min.
- Remove the TrypLE Express solution and wash the DRGs with 1 ml prepared culture medium (containing 5% FBS) for 3 times.
- Add 600 μl culture medium and gently pipette up and down to triturate the tissues for 20-30 times using a 1 ml blue, graduated pipette tip.
- After trituration, allow the non-dissociated tissues to settle down to the bottom of the microfuge and transfer the cell suspension to a 10 ml sterile tube. Add another 600 μl culture medium and repeat the trituration step until most tissues are dissociated. The obtained cell suspension contains both neurons and non-neuronal cells. In most cases, cells from 6 DRGs (~5 x104) are used for one electroporation reaction.
4. Genetic Manipulation of Neurons via Electroporation
- Prepare the transfection solution by mixing the Amaxa nucleofection solution for mouse neurons with DNA plasmids (~10 μg) or siRNA oligos (~0.2 nmol) to make a final volume of 100 μl for each transfection.
- Centrifuge the dissociated cells at 680 rpm for 7 min at room temperature, and discard supernatant as much as possible. Add the prepared transfection solution and gently pipette up and down 3-4 times with 200 μl pipette tips to re-suspend the cells.
- Transfer the cell suspension mixed with the transfection solution to the electroporation cuvette and electroporate the cells using the Amaxa Nucleofector system program G-013.
- After electroporation, immediately add 500 μl of pre-warmed (37 °C) culture medium containing FBS to the cuvette and transfer all the solution (~600 μl) into the coated culture plate at desired cell densities. For experiment that requires re-suspension and re-plating, neurons are cultured directly on the plastic culture dish at high density (10000-20000 cells/well). For experiment that directly examines axon growth, neurons are plated onto coated glass coverslips at lower density (3000-5000 cells/well). Place the culture plate into the incubator (37 °C, 5% CO2).
- Four hr after plating when neurons have attached to the substrates, gently replace the culture medium (which contains the Amaxa nucleofection solution) with 500 μl fresh and pre-warmed culture medium, and return the plate to the incubator for additional culture (37 °C, 5% CO2). Both culture medium containing FBS or the serum-free medium can be used at this step.
5. Culturing Adult DRG Neurons for Axon Growth Analysis
- For neurons transfected with DNA plasmids, the expression of gene-of-interest (e.g. EGFP) can be observed as early as a few hr after electroporation. For neurons transfected with siRNAs, we usually wait 3-4 days to allow sufficient depletion of the endogenous proteins. The cultured neurons can be either directly fixed for axon growth analysis at various time points (1-4 days after electroporation) or re-suspended and re-plated to analyze axon regrowth (see below).
- For RNAi-mediated loss-of-function studies, the cultured neurons can be re-suspended and re-plated to allow axons to regrow from neurons, in which the targeted proteins are already depleted. To do so, replace the old culture medium of the high-density cultured neurons with 1 ml pre-warmed fresh medium and pipette gently up and down for 6-10 times to re-suspend the attached neurons from the culture plate. Because non-neuronal cells (e.g. Schwann cells) attach much tighter to the culture dish than neurons, the re-suspended cells are mostly neurons.
- Transfer the re-suspended neurons into a microfuge tube and gently triturate 10-15 times to re-dissociate the cell clumps into the single cell suspension.
- Re-plate the re-dissociated neurons onto newly prepared coverslips at low density (3000-5000 cells/well) and culture overnight (16-24 hr).
6. Fixation, Immuno-staining, and Fluorescence Imaging
- Aspirate the medium and add 4% paraformaldehyde (PFA) (200 μl/well) to fix the cells for 15-20 min at room temperature. The fixed cells are then washed with 1X PBS for 3 times.
- Aspirate the PBS and add the blocking solution (1% bovine serum albumin, 0.1% Triton X-100, and 2.0% normal goat serum in 1X PBS) to the fixed neurons for 60 min at room temperature.
- To label axons, the neurons are immuno-stained with anti-neurofilaments or anti-βIII tubulin antibody. To do so, place a 30μl drop of primary antibody solution (1:1200 dilution for the -βIII tubulin antibody) on a parafilm for each coverslip. Invert the coverslips and place them onto the primary antibody solution for 60 min at room temperature.
- Return coverslips to the original culture plate and wash them with 1X PBS for 3 times.
- Repeat the same procedure for the secondary antibody.
- Wash the coverslips with distilled water for 3 times, and then mount coverslips on glass slides with the mounting solution (e.g. ProLong Gold Antifade).
- The stained neurons can be imaged with any fluorescence microscopy system equipped with a digital camera. The axon lengths are measured and analyzed with imaging analysis software.
7. Representative Results
In the absence of any added extracellular growth factors, the adult DRG neurons usually start to grow axons 48 hr after first plating. The axons often show branched morphologies (Figure 1). In contrast, the re-plated neurons start to extend axons only a few hr after plating, and the axons elongate with much reduced branching (Figure 2). These results suggest that re-plated neurons share similar properties to those of conditioning lesioned neurons. By using this approach, we have recently performed loss-of-function studies to examine the role of axon regeneration associated transcription factor c-Jun in axon growth from adult DRG neurons in vitro. The results showed that electroporation of a group of 4 different siRNAs targeting different regions of c-Jun (ON-TARGETplus) markedly reduced the protein levels of c-Jun in adult DRG neurons 3 days after transfection (Figure 3)9. When the neurons were re-plated and cultured overnight, axon growth from c-Jun knockdown neurons was significantly reduced (Control: 348.37± 16.21mm; si-c-Jun: 262.32±15.69 μm, Figure 3)9. These results indicate that cultured adult DRG neurons provide a useful model system to study axon growth from adult neurons.
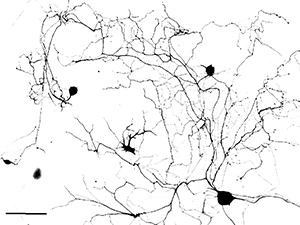
Figure 1. Adult mouse DRG neurons cultured at low density for 3 days. The neurons were stained with anti–βIII tubulin antibody. Note that most axons show branched morphologies. Scale bar: 125 μm.
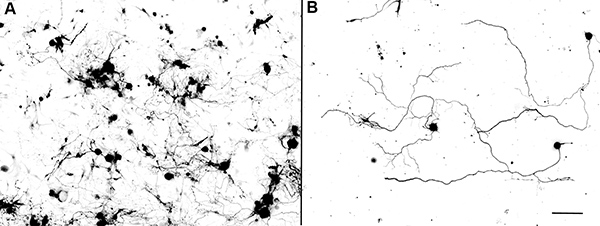
Figure 2. Re-plating adult mouse DRG neurons after 3 days in culture. (A) Adult DRG neurons cultured at high density for 3 days. (B) Re-plated adult DRG neurons were cultured at low density for overnight. Note that most axon show elongated morphologies with little axon branching. Scale bar: 250 μm in A and 125 μm in B.
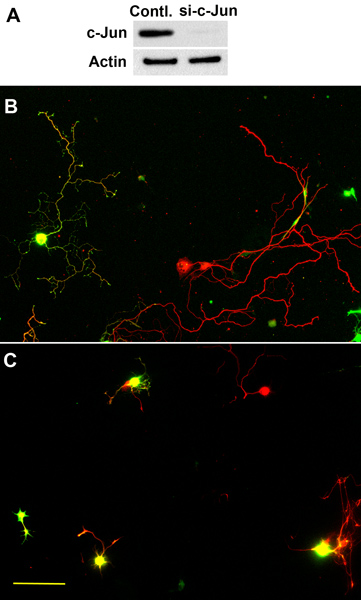
Figure 3. Role of c-Jun in axon growth from adult DRG neurons in vitro. (A) Western blot analysis of c-Jun in adult mouse DRG neurons after electroporation of c-Jun siRNAs. The result shows markedly reduced level of c-Jun. (B) Control neurons transfected with EGFP grew long axons after re-plating and overnight culture. (C) Co-transfection of c-Jun siRNAs and EGFP impaired axon growth from adult DRG neurons after re-plating and overnight culture. Red: Tuj-1 staining; Green: EGFP. Scale bar: 125 μm. These results have been published in Saijilafu et al.9.
Discussion
Adult DRG neurons regenerate their axons robustly after peripheral nerve injury in vivo and in vitro, thus providing a useful system to study axon regeneration in adult animals. In vitro culture of adult DRG neurons is becoming a widely used method to investigate the molecular mechanisms by which axon regeneration is regulated. The in vitro procedure of culturing adult mouse DRG neurons presented here enables rapid and effective genetic study of regenerative axon growth. The re-suspension and re-plating procedure is particularly useful for loss-of-function studies because it allows axons re-grow from neurons in which the targeted protein has already been depleted. Moreover, re-plating cultured DRG neurons mimics an in vivo conditioning lesion effect, thus providing a more physiological relevant approach to study axon regeneration in vitro.
The transfection efficiency of adult DRG neurons with the described electroporation approach is about 20-50% for DNA plasmids depending on the sizes of the constructs, which is sufficient for morphological analysis of axon growth. Comparing to electroporation, the viral-mediated gene transfer has much higher efficiency, which is suitable for biochemical analysis using DRG neurons. However, constructing and producing virus for each gene-of-interest is much more labor intensive and time consuming. For siRNA oligos, the transfection efficiency can reach nearly 90% based on the knocking down efficiency of the targeted proteins (see Figure 3A), which makes biochemical analysis of adult DRG neurons possible. However, the effect of siRNAs reduces after longer period due to the degradation of the siRNA oligos.
When neurons are co-transfected with 2 plasmids mixed at 1:1 ratio, the plasmid with smaller size usually has higher transfection efficiency than the plasmid with bigger size. As a result, adjusting the ratio of the two plasmids will change the co-transfection efficiency accordingly. For siRNA transfection, we often co-transfect the neurons with EGFP to label neurons. Because the siRNA oligos are much smaller than EGFP, their transfection efficiency is much higher. Therefore, in our experiments we generally think that all EGFP positive neurons are also positive for siRNAs.
Many genetic profiling studies of adult DRG neurons after peripheral axotomy have identified a large number of regeneration-associated genes (RAGs)10-12, which are believed to underlie the regeneration ability of adult DRG neurons. However, the functions of these RAGs in mediating axon growth of adult DRG neurons have not been well characterized. Here we examined the role of a well-known RAG, c-Jun, in mediating axon growth from adult DRG neurons via RNAi-mediated loss-of-function approach. Such method provides a valuable in vitro tool to investigate the roles of other RAGs in regulation of axon regeneration.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants to F. Z. from NIH (R01NS064288) and the Craig H. Neilsen Foundation.
Materials
| Name of the reagent | Company | Catalogue number |
| MEM | Invitrogen | 11090-081 |
| Poly-D-Lysine hydrobromide | Sigma -Aldrich | P6407 |
| Laminin | Invitrogen | 23017-015 |
| 5-fluoro-2-deoxyuridine | Sigma -Aldrich | F0503 |
| Uridine | Sigma -Aldrich | U3003 |
| Collagenase A | Roche | 10103578001 |
| TrypLE Express | Invitrogen | 12604-013 |
| Fetal bovine serum | Invitrogen | 10270-098 |
| Penicillin-streptomycin (100X) | Invitrogen | 15140-122 |
| GlutaMAX-I (100X) | Invitrogen | 35050-038 |
| Glass coverslips (#1) | Electron Microscopy sciences | 72196-12 |
| 24 well cell culture plate | Becton Dickinson | 35-3047 |
| 1X PBS | Mediatech | 21-040-CV |
| Sterile, distilled and deionized water | Mediatech | 25-055-CV |
| Nucleofector and electroporation Kits for Mouse Neurons | Lonza | VPG-1001 |
| ON-TARGETplus siRNA against c-Jun | Dharmacon | L-043776 |
| Anti–βIII tubulin antibody (Tuj-1) | Covance | MMS-435P |
| ProLong Gold Antifade mounting solution | Invitrogen | P36930 |
References
- Yiu, G., He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7, 617-627 (2006).
- Liu, K., Tedeschi, A., Park, K. K., He, Z. Neuronal Intrinsic Mechanisms of Axon Regeneration. Annu. Rev. Neurosci. , (2010).
- Zhou, F. Q., Snider, W. D. Intracellular control of developmental and regenerative axon growth. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361, 1575-1592 (2006).
- Neumann, S., Woolf, C. J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 23, 83-91 (1999).
- Liu, R. Y., Snider, W. D. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J. Neurosci. 21, RC164 (2001).
- Zhou, F. Q. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. J. Cell Sci. 119, 2787-2796 (2006).
- Zou, H., Ho, C., Wong, K., Tessier-Lavigne, M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J. Neurosci. 29, 7116-7123 (2009).
- Zhou, F. Q., Zhou, J., Dedhar, S., Wu, Y. H., Snider, W. D. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 42, 897-912 (2004).
- Saijilafu, E. M., Hur, F. Q., Zhou, Genetic dissection of axon regeneration via in vivo electroporation of adult mouse sensory neurons. Nat. Commun. 2, 543-54 (2011).
- Costigan, M. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 3, 16 (2002).
- Xiao, H. S. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc. Natl. Acad. Sci. U.S.A. 99, 8360-8365 (2002).
- Michaelevski, I. Signaling to transcription networks in the neuronal retrograde injury response. Sci. Signal. 3, ra53 (2010).

