Establishment of a Clinically Relevant Ex Vivo Mock Cataract Surgery Model for Investigating Epithelial Wound Repair in a Native Microenvironment
Summary
Described here is the establishment of a clinically relevant ex vivo mock cataract surgery model that can be used to investigate mechanisms of the injury response of epithelial tissues within their native microenvironment.
Abstract
The major impediment to understanding how an epithelial tissue executes wound repair is the limited availability of models in which it is possible to follow and manipulate the wound response ex vivo in an environment that closely mimics that of epithelial tissue injury in vivo. This issue was addressed by creating a clinically relevant epithelial ex vivo injury-repair model based on cataract surgery. In this culture model, the response of the lens epithelium to wounding can be followed live in the cells’ native microenvironment, and the molecular mediators of wound repair easily manipulated during the repair process. To prepare the cultures, lenses are removed from the eye and a small incision is made in the anterior of the lens from which the inner mass of lens fiber cells is removed. This procedure creates a circular wound on the posterior lens capsule, the thick basement membrane that surrounds the lens. This wound area where the fiber cells were attached is located just adjacent to a continuous monolayer of lens epithelial cells that remains linked to the lens capsule during the surgical procedure. The wounded epithelium, the cell type from which fiber cells are derived during development, responds to the injury of fiber cell removal by moving collectively across the wound area, led by a population of vimentin-rich repair cells whose mesenchymal progenitors are endogenous to the lens1. These properties are typical of a normal epithelial wound healing response. In this model, as in vivo, wound repair is dependent on signals supplied by the endogenous environment that is uniquely maintained in this ex vivo culture system, providing an ideal opportunity for discovery of the mechanisms that regulate repair of an epithelium following wounding.
Introduction
The clinically relevant, mock cataract surgery, ex vivo epithelial wound healing model described here was developed to provide a tool for investigating the mechanisms that regulate repair of epithelial tissues in response to an injury. Key features that were aimed for in creating this model included 1) providing conditions that closely replicated the in vivo response to wounding in a culture setting, 2) ease of modulating the regulatory elements of repair, and 3) ability to image the repair process, in its entirety, in real time. The challenge, therefore, was to create a culture model in which it was possible to study, and manipulate, epithelial wound repair in the cells’ native microenvironment. The availability of this wound-repair model opens new possibilities for identifying the endogenous signaling cues from matrix proteins, cytokines and chemokines that regulate the repair process. In addition, the model is ideal for examining how an epithelium is able to move as a collective sheet to re-epithelialize the wound area 2,3, and for determining the lineage of mesenchymal leader cells at the wound edge that function in directing the collective migration of the injured epithelium 4. This model also provides a platform with which to identify therapeutics that could promote effective wound healing and prevent aberrant wound repair5.
There are already a number of available wound-repair models, both in culture and in vivo, which have provided most of what is known about the wound repair process today. In animal injury models, such as cornea 6-12 and skin13-17, there is the opportunity to study the response of the tissue to wounding in the context of all the repair mediators that could be involved in the process, including contributions from the vasculature and nervous system. However, there are limitations to manipulating the experimental conditions in vivo, and it is not yet possible to conduct imaging studies of the repair response in vivo, continuously over time. In contrast, most in vitro wound-repair culture models, such as the scratch wound, can be easily manipulated and followed over time but lack the environmental context of studying wound healing in the in vivo tissue. While ex vivo models offer the advantage of studying the injury repair process continuously over time in the context of the cells’ microenvironment coupled with the ability to modulate the molecular regulators of repair at any time point in the process, there are few models that fit these parameters.
Here is described a procedure to generate highly reproducible ex vivo epithelial wound healing cultures that reproduce an epithelial tissue’s response to a physiological wounding. Using the chick embryo lens as a tissue source, an ex vivo mock cataract surgery is performed. The lens is an ideal tissue to use for these studies since it is self-contained within a thick basement membrane capsule, avascular, not innervated, and free of any associated stroma18,19. In the human disease, cataract surgery addresses vision loss due to opacification of the lens, and involves removal of the lens fiber cell mass, which comprises the bulk of the lens. Following cataract surgery vision is restored through the insertion of an artificial intraocular lens. The cataract surgery procedure, through removal of fiber cells, induces an injury response in the adjacent lens epithelium, which responds by re-epithelialization of the posterior area of the lens capsule that had been occupied by the fiber cells. In cataract surgery, as in most wound repair responses, there sometimes occurs an aberrant fibrotic outcome to the wound healing response, associated with the emergence of myofibroblasts, which in the lens is known as Posterior Capsule Opacification20-22. To generate the cataract surgery wound healing model, a cataract surgery procedure is mimicked in lenses removed from the chick embryo eye to produce a physiological injury. Microsurgical removal of lens fiber cells results in a very consistent circular wound area surrounded by the lens epithelial cells. This cell population remains firmly attached to the lens basement membrane capsule and is injured by the surgical procedure. The epithelial cells migrate onto the denuded area of the endogenous basement membrane to heal the wound, led by a population of vimentin-rich mesenchymal cells known in the repair process as leader cells1. With this model the response of an epithelium to injury can be easily visualized and followed with time in the context of the cells’ microenvironment. The cells are readily accessible to modifications of the expression or activation of molecules expected to play a role in wound repair. A powerful feature of this model is the ability to isolate and study migration-specific changes in the framework of wound healing. The ability to prepare large numbers of aged matched ex vivo wound healing cultures for studies is another advantage of this model. Thus, this model system provides a unique opportunity to tease apart mechanisms of wound repair and test therapeutics for their effect on the wound healing process. The ex vivo mock cataract surgery model is expected to have wide applicability, providing a critical resource for studying mechanisms of injury repair.
Protocol
The following protocol complies with the Thomas Jefferson University Institutional Animal Care and Use Committee guidelines and with the ARVO Statement for the Use of Animals in Vision Research.
1. Setup and Preparation of Lenses for Ex Vivo Wound Culture
- Place three 100 mm petri dishes in a sterile, laminar flow hood. Fill two of the petri dishes halfway with Tris/Dextrose buffer (TD buffer; 140 mM NaCl, 5 mM KCl, 0.7 mM Na2PO4, 5 mM D-glucose, 8.25 mM Tris Base, pH to 7.4 with HCl) at RT, leaving the third empty. Pre-warm culture media (Media 199 supplemented with, 1% L-glutamine and 1% penicillin/streptomycin) to 37oC.
Note: The standard wound healing culture media is serum-free, as occurs in vivo; however, the wound-repair cultures can be grown successfully in defined media conditions that include serum or other factors. - Remove fertile embryonic day 15 white leghorn chick egg from incubator (held at 37.7° C with gentle rocking)
- Place selected egg in the laminar flow hood and clean outside of shell with 70% ethanol from a wash bottle. Conduct all procedures below under aseptic conditions in the laminar flow hood, using sterile solutions and instruments.
- Crack egg and place contents into the empty 100 mm petri dish. Decapitate embryo using standard forceps and fine scissors. Place the chick embryo head in a petri dish containing TD buffer and properly dispose of the remainder of the embryo. Optionally keep the chick embryo heads in TD buffer for a short period of time, no longer than 15 min.
- Place the chick embryo head on a petri dish lid. Using high precision forceps, remove the lens along with its attached vitreous humor from the eye in the following sequence. Pinch the back of the eye with the forceps to create a small opening in the back of the eye.
- Then, grasp the vitreous humor with forceps and gently tug on the vitreous with a rolling motion, the vitreous with the lens attached will be dislodged from the eye. Place lens/vitreous in the remaining petri dish containing TD buffer. Allow the lenses to remain in TD buffer for no longer than 30 min.
- Move the lens to a new petri dish lid under a dissecting microscope. From this point on perform all steps under a dissecting microscope. With high precision forceps carefully brush away any ciliary body (pigmented cells) that were dislodged with the lens using the edge of the forceps, taking caution not to damage the lens tissue.
Note: Removing the ciliary body ensures that cell types that are not endogenous to the lens are not included in the wound repair culture. - Separate the lens from the vitreous humor with high precision forceps by pinching off the vitreous body from its association with the posterior lens capsule.
- Using high precision forceps transfer the lens into a small drop of TD buffer (about 200 µl) in a 35mm tissue culture dish.
2. Performing Mock Cataract Surgery
- Orient the lens in the drop of TD buffer in the 35 mm dish with the anterior aspect of the lens facing up.
Note: The anterior of the lens is easily identified by the presence of a dense ring in the tissue that notes the border between the anterior and equatorial region of the lens epithelium. In contrast, there is an absence of markings on the posterior of the lens capsule to which the lens fiber cells are attached. - Using two high precision forceps make a small incision (approximately 850µm) in the center of the anterior lens capsule, the thick basement membrane that surrounds the lens tissue, and its associated anterior lens epithelium, by grasping the tissue with one forceps in each hand and gently tugging in opposing directions.
- Remove the fiber cell mass, which makes up the bulk of the lens tissue, dislodging it from its attachments to the lens epithelium and surrounding lens capsule by hydro-elution (an approach used in classic cataract surgery, modeled in Figure 1A).
- Fill a 1 ml syringe with a 27.5 G needle tip with 300 µl of TD buffer. Insert the needle tip into the incision made in the anterior lens capsule, and about halfway into the lens.
- Gently depress the syringe injecting the TD buffer into the lens fiber cell mass. Inject between 50 and 200 µl TD, and never more than 300 µl. Observe the fiber cell mass loosening itself from the epithelium and lens capsule.
- Using high precision forceps, remove the loosened fiber cell mass from the lens through the anterior incision site.
Note: This procedure leaves the posterior lens basement membrane capsule to which the fiber cells had been attached denuded of cells, and an injured lens epithelium just adjacent to this site.
3. Preparing the Wounded Lens for Ex Vivo Culture
- Flatten the lens capsular bag that results from the cataract surgery described above on the culture dish, cell side up, by making five cuts in the anterior aspect of the capsular bag.
- Cut perpendicular to the original incision site through to the equator of the lens. Flatten the resultant five “flaps” of lens capsule with attached epithelium on the culture dish capsule side down, cell side up. Note the ex vivo wounded lens now should take on a star or flowerlike shape (see Figure 1B).
- To secure the capsule to the dish, press softly down with the forceps at each point of the star. This will make a small indentation at the five most outside tips of the explant and result in sustained attachment to the dish.
Note: It is possible to damage the capsule during this procedure and so it is important to secure the capsule to the dish as close to the tips of the flaps as possible, as well as to make the least amount of securing points as possible (generally two, maximum of three per flap). - Remove the TD buffer from the 35mm dish and replace it with 1.5 ml of pre-warmed media. Cover the 35mm dish with its lid and place in the incubator (37°C, 5% CO2).
4. Separation of the Central Migration Zone (CMZ), where Re-epithelialization of the Wounded Area of the Posterior Capsule Occurs, from the Original Attachment Zone (OAZ) of Lens Epithelial Cells, for Quantitative Analysis.
Note: The cells begin to move into the CMZ region immediately in response to injury. By day one in culture enough cells are migrating across the CMZ for molecular and biochemical analysis, following separation of the CMZ and the OAZ by micro-dissection23. This protocol involves removal of one flap (OAZ) at a time from the wounded area of the capsule.
- Observe a demarcation, clearly visible under the dissecting microscope, between the OAZ and CMZ (see Figure 2A). Using two high precision forceps, grasp with both forceps at the edge of the OAZ/CMZ line, one just adjacent to the other, on either side of the line (see Figure 2A, B, arrow).
- Using one hand/one forceps on the CMZ side continue to hold onto the wounded culture, while with the other hand/forceps, gently pull the OAZ along the OAZ/CMZ line. The CMZ easily separates from the OAZ along this line. Continue along this line around the entire culture until the two regions are completely separated.
- Study the separated OAZ and CMZ fractions for molecular analysis such as RNA-Seq 24 or biochemical analysis such as western blot or co-immunoprecipitation 23,25.
Representative Results
Ex Vivo Model created to study the wound healing process in the cells’ native microenvironment
To investigate mechanisms involved in regulating wound healing of an epithelium within the cells’ native microenvironment, a clinically relevant ex vivo mock cataract surgery model was created. This model is created from lens tissue which offers many advantages due to its intrinsic properties: 1) the lens is a self-contained organ surrounded by a thick basement membrane called the lens capsule; 2) it is avascular, 3) not innervated and 4) free of associated stroma. Therefore, the repair process examined is limited to cells that are innate to the lens proper. To create this model, lenses are removed from an embryonic (E) day 15 chick embryo (Figure 1A). A small incision is made in the anterior lens capsule and its associated lens epithelial cells through which the lens fiber cell mass is removed by hydro-elution, a classic cataract procedure that creates a physiologically relevant wounding (Figure 1A). The lens epithelium, which is present as a continuous monolayer of cells along the anterior and equatorial aspects of the lens capsule surrounding the fiber cell mass, along with its endogenous population of vimentin-rich mesenchymal repair cell progenitors1, remain in the lens during the extraction of fiber cells (Figure 1A). Following removal of the fiber cell mass, five cuts are made in the anterior aspect of the lens capsule and the epithelium flattened cell-side up, facing the medium. This procedure enables imaging of the response of the injured epithelium by various microscopic approaches, including time-lapse microscopy (Video 1). These additional cuts in the anterior lens capsule create a star-shape explant of the wounded lens with the circular wound created by removing the fiber cell mass in the center of the explant (Figure 1B). Post-cataract surgery and flattening of the tissue, the wounded epithelium is located in the points of the star, which is referred to as the Original Attachment Zone (OAZ) (Figure 1A, B).
The removal of the fiber cell mass from its attachment site on the posterior lens capsule creates a highly reproducible wound area on the basement membrane, surrounded by the wounded epithelium (Figure 1B, C). The exposed edge of the lens equatorial epithelium, just adjacent to where the fiber cells were attached, is the leading edge of the wound. Immediately following injury, a subpopulation of vimentin-rich mesenchymal repair cells is activated and migrates to the wound edge of the epithelium1. The lens epithelium, with these mesenchymal repair cells at their leading edge, rapidly moves onto the cell-free region of the endogenous basement membrane capsule, the Central Migration Zone (CMZ), to begin healing the wound. Lens epithelial cells move collectively, as a sheet, into the CMZ led by the mesenchymal leader cells1, which extend protrusions along the substrate and direct the wound healing process (Figure 1D, Video 1). Wound healing progresses, covering a significant area of the wound (67%) by day 1 in culture (Figure 1C), and is typically completed within 3 days in culture (Figure 1B, C).
A clear physical distinction can be made between the OAZ and CMZ regions as early as day 1, which is demarcated as a crease or wrinkle between these two zones (Figure 2A, arrow). This phenomenon provides a guideline by which to separate these areas at any point during the wound healing process. Using a fine tipped forceps, the cultures can be micro-dissected to separate the OAZ and CMZ regions in order to analyze molecular differences between these distinct zones (Figure 2B). This is a powerful approach that has been used to identify migration-specific changes associated with wound-repair. Previously, it was found that there is an increase in Focal Adhesion Kinase (FAK) activation in the ex vivo wounded cultures during the wound healing process5. FAK has a well-established role in cell migration26-29. The ability to enrich for the OAZ vs. CMZ now made it possible to examine whether this increase in FAK activation is specific to the migration-specific CMZ region. For this study, the OAZ and CMZ regions were separated on day 1 – 3 (throughout the wound healing process) and analyzed for biochemical changes in FAK activation (FAK pY397). The results demonstrated that the increase in FAK activation was associated with the migration-specific CMZ region (Figure 2C). The ex vivo model system described here provides a unique and invaluable opportunity in which to investigate the molecular programs involved in coordinating the wound repair process.
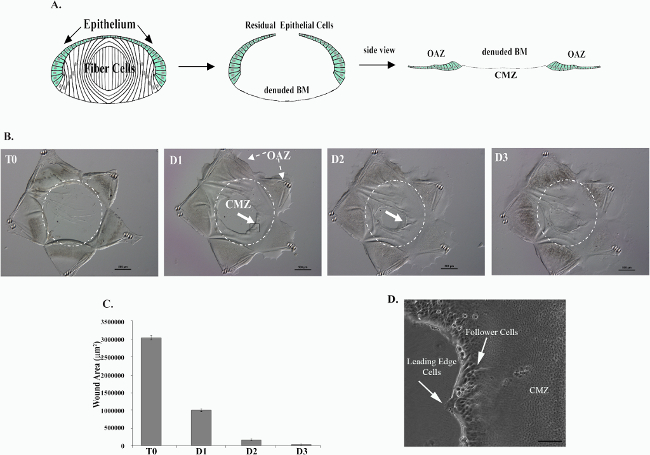
Figure 1. Creation of an ex vivo model in which to study the wound healing process within the cells’ native microenvironment in response to a physiological wounding. Mock cataract surgery was performed on E15 chick lenses. The lens fiber cell mass (white) is removed through an incision in the anterior capsule by hydro-elution. This process leaves behind epithelial cells (green) that remain tightly adherent to the lens capsule and a cell-denuded basement membrane (BM) onto which cells will migrate to heal the wound (A). Five cuts are made to the epithelium to flatten out and create a star shaped ex vivo culture (B). The residual lens epithelial cells that fill the points of the star are referred to as the Original Attachment Zone (OAZ) (A, B). The denuded BM onto which cells will migrate is referred to as the Central Migration Zone (CMZ) (A, B). Immediately in response to injury, cells begin to move into the CMZ region on the cell-denuded BM (B). The open wound area is quantified over time in (C). Wound healing is typically completed by D3 in culture (B, C). In (B, C) T0 denotes time of wounding, D1-3 denotes Days 1-3. Within the CMZ, two populations of cells can be distinguished, the lens epithelial cells and the mesenchymal leader cells that localize to the wound edge, extending protrusions along the substrate (D). This figure is reprinted from Menko et al 23.Please click here to view a larger version of this figure.
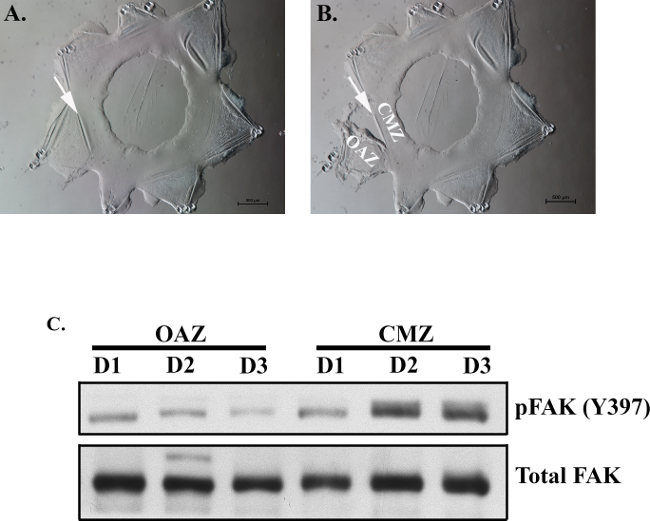
Figure 2. Separation of OAZ and CMZ regions to identify migration-specific changes associated with wound healing. By day 1, OAZ and CMZ regions can be distinguished from one another by a crease (arrow), which can be used as a guide to separate these zones (A). At this crease, fine tipped forceps can be used to grip the edge of the OAZ/CMZ line. The culture can be separated along this line allowing isolation of the OAZ and CMZ regions (B). Micro-dissection of the OAZ and CMZ daily, from day 1 (D1) – day 3 (D3) was performed to determine if migration-specific changes in FAK activation occur in the region of wound repair. Lysates from each region were examined by Western blot analysis for either FAK activation (pFAK Y397) or Total FAK expression (C). While little change in total FAK levels was observed, an increase in FAK activation was associated with the migration-specific CMZ region (C).
Video 1. Wound healing in the ex vivo mock cataract surgery model is followed by time-lapse microscopy from time 0 after injury through to day 3. Wound closure is viewed from the center of the wound area.
Discussion
Here is described a technique for preparing a culture model of wound repair that involves performing an ex vivo cataract surgery on chick embryo lenses after their removal from the eye. The lens epithelium responds to this clinically relevant wounding with a repair process that closely mimics that which occurs in vivo, and shares features with wound repair in other epithelial tissues2,4. While the protocol is straightforward and simple to follow, performing mock cataract surgery with embryonic lenses requires developing skills in the handling of small tissues that can be acquired with practice, as the embryonic lenses are only about 2mm in diameter. The cataract surgery procedure is performed under the dissecting microscope, and the critical technical steps include making a small incision in the anterior capsule, removing the fiber cell mass by hydro-elution, and making additional cuts in the anterior epithelium that make it possible to flatten the ex vivo post-cataract surgery lens tissue on the substrate to which it is pinned for study in culture. The microsurgical cataract surgery procedure produces a highly reproducible wound area, exposing a circular region of the tissue’s endogenous basement membrane onto which the injured epithelium migrates as it closes the wound. This model has been used to study mechanisms regulating wound repair of epithelia, including how the epithelial cells are able to move collectively into the wound area 30 and the leader cell function of an endogenous vimentin-rich repair cell population of mesenchymal lineage at the wound edge1,23.
This ex vivo wound model lends itself readily to live microscopic observation throughout the repair process including time-lapse imaging, and confocal image analysis following immuno-localization of proteins of interest. Making cuts in the anterior lens capsule so that the capsule with its attached injured epithelium can be flattened on the culture substrate makes such ease of observation possible. Including this critical step is essential for viewing the behavior of the response of the epithelial cells in the original zone of attachment and the movement of the injured epithelium onto the wounded area of the posterior capsule from the time of injury. A great advantage for biochemical or molecular biology analyses is that the cells that are associated with the basement membrane in the original attachment zone (OAZ) can be separated by micro-dissection from those cells migrating across the wounded area of the basement membrane (CMZ) at any time during the wound repair process.
The mock cataract surgery wound repair culture model was established using lenses from E15 chick embryos. Alternatively, wound repair cultures can be successfully prepared using this protocol from lenses removed at other stages of chick embryonic development, and from lenses isolated from both adult mice and rats. While studies to date have focused on wound repair in the chick embryo lens model, in part because of the ease in obtaining large numbers of age-matched tissue for experimental analysis, there is no reason that would prevent this protocol from being used with lenses isolated from any species. However, it is widely recognized in the literature that wound repair in the embryo often occurs more quickly and with less scarring than in the adult 31-35. The wound repair cultures presented here are typically grown in the absence of serum to maintain conditions close to the in vivo microenvironment, suggesting that the factors that promote wound healing are provided by components of that environment including ones produced by the cells themselves. However, wound repair in this culture model also occurs effectively in defined media conditions, and can be modulated by including specific pharmaceutical inhibitors in the culture media 5,23,30, or knocking down expression of proteins through an siRNA approach 23. Note that inclusion of serum in the culture media induces movement of the cells from the cut edges on the outside of the flattened explant onto the tissue culture substrate, while in serum-free medium the cells remain associated with the capsule and migrate only onto the wounded area of the basement membrane of the lens capsule. The approach of adding serum to the media can be an advantage if one desires to examine the behavior of the wounded epithelium as it moves onto different substrates. To that end, there are other options that can be taken to alter the environment for wound repair that includes use of alternate tissue culture substrates on which to pin the tissue following the mock cataract surgery, such as substrates with defined rigidities as can be provided by collagen or acrylamide gels. While the ex vivo mock cataract surgery cultures can be facilely pinned to tissue culture plastic, collagen gels, or acrylamide gels, affixing the cultures to glass substrates has proven difficult. An important adaptation of this wound repair model to consider is its usefulness for re-injury studies. In this approach a scrape wound is inflicted on the healed lens epithelium after wound repair is completed, which removes an area of the lens epithelium from its underlying basement membrane capsule. This re-injury can be performed in either the original zone of attachment of the epithelium to the lens capsule or in the central migration zone where the epithelium has healed the initial wound area.
A great advantage to this wound-repair model is that the lens is an avascular tissue, not innervated without an associated stromal compartment, thus creating an ideal reductionist model of wound repair. As such, this model allows for examination of the native wound repair process under conditions in which only cells and microenvironment that are intrinsic to this epithelial tissue can modulate the repair process. In order to examine the role of cells that could be recruited following injury from neighboring vasculature or stromal compartments, it would be necessary to add such cells to the culture environment.
In previous culture wound models, the major limitation to investigating how an epithelial tissue responds to wounding is that most are limited to examining cells, often cell lines, plated on tissue culture plastic. Popular models using this approach, such as the scratch wound assay, have provided significant insight into how cells migrate to fill an area of the culture dish from where the cells have been removed. However, in these scratch wound studies the movement of cells onto the exposed area of the culture substrate occurs in the absence of many of the dynamic wound response signals that regulate wound healing in vivo. Therefore, the most important advantage of the mock cataract surgery culture model is that wound repair can be studied ex vivo within the cells’ native microenvironment and the endogenous signals originating from the underlying matrix, growth factors, cytokines and repair cell subpopulations. As this new wound repair model makes it possible to examine and manipulate the response of an epithelium to wounding in its native microenvironment, it is likely to have many applications, and holds great promise as an ideal system for revealing the molecular-mediators of wound-repair.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Institutes of Health Grant to A.S.M. (EY021784).
Materials
| Sodium Chloride (NaCl) | Fisher Scientific | S271-3 | Use at 140mM in TD Buffer |
| Potassium Chloride (KCl) | Fisher Scientific | P217-500 | Use at 5mM in TD Buffer |
| Sodium Phosphate (Na2HPO4) | Sigma | S0876 | Use at .7mM in TD Buffer |
| D-glucose (Dextrose) | Fisher Scientific | D16-500 | Use at 0.5mM in TD Buffer |
| Tris Base | Fisher Scientific | BP152-1 | Use at 8.25mM in TD Buffer |
| Hydrochloric acid | Fisher Scientific | A144-500 | Use to pH TD buffer to 7.4 |
| Media 199 | GIBCO | 11150-059 | |
| L-glutamine | Corning/CellGro | 25-005-CI | Use at 1% in Media199 |
| Penicillin/streptomycin | Corning/CellGro | 30-002-CI | Use at 1% in Media199 |
| 100mm petri dishes | Fisher Scientific | FB0875711Z | |
| Stericup Filter Unit | Millipore | SCGPU01RE | Use to filter sterilize Media |
| Dumont #5 forceps (need 2) | Fine Science Tools | 11251-20 | |
| 35mm Cell Culture Dish | Corning | 430165 | |
| 27 Gauge 1mL SlipTip with precision glide needle | BD | 309623 | |
| Fine Scissors | Fine Science Tools | 14058-11 | |
| Standard Forceps | Fine Science Tools | 91100-12 | |
| Other Items Needed: General dissection instruments, fertile white leghorn chicken eggs, | |||
| check egg incubator (humidified, 37.7°C), laminar flow hood, binocular stereovision dissecting | |||
| microscope | |||
References
- Walker, J. L., et al. Unique precursors for the mesenchymal cells involved in injury response and fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 107, 13730-13735 (2010).
- Friedl, P., Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nature reviews. Molecular cell biology. 10, 445-457 (2009).
- Riahi, R., Yang, Y., Zhang, D. D., Wong, P. K. Advances in wound-healing assays for probing collective cell migration. Journal of laboratory automation. 17, 59-65 (2012).
- Khalil, A. A., Friedl, P. Determinants of leader cells in collective cell migration. Integrative biology : quantitative biosciences from nano to macro. 2, 568-574 (2010).
- Walker, J. L., Wolff, I. M., Zhang, L., Menko, A. S. Activation of SRC kinases signals induction of posterior capsule opacification. Investigative ophthalmology & visual science. 48, 2214-2223 (2007).
- Sta Iglesia, D. D., Stepp, M. A. Disruption of the basement membrane after corneal debridement. Investigative ophthalmology & visual science. 41, 1045-1053 (2000).
- Pal-Ghosh, S., Pajoohesh-Ganji, A., Brown, M., Stepp, M. A. A mouse model for the study of recurrent corneal epithelial erosions: alpha9beta1 integrin implicated in progression of the disease. Investigative ophthalmology & visual science. 45, 1775-1788 (2004).
- Pal-Ghosh, S., Pajoohesh-Ganji, A., Tadvalkar, G., Stepp, M. A. Removal of the basement membrane enhances corneal wound healing. Experimental eye research. 93, 927-936 (2011).
- Stepp, M. A., et al. Wounding the cornea to learn how it heals. Experimental eye research. 121, 178-193 (2014).
- Kuwabara, T., Perkins, D. G., Cogan, D. G. Sliding of the epithelium in experimental corneal wounds. Investigative ophthalmology. 15, 4-14 (1976).
- Sherrard, E. S. The corneal endothelium in vivo: its response to mild trauma. Experimental eye research. 22, 347-357 (1976).
- Stramer, B. M., Zieske, J. D., Jung, J. C., Austin, J. S., Fini, M. E. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Investigative ophthalmology & visual science. 44, 4237-4246 (2003).
- Escamez, M. J., et al. An in vivo model of wound healing in genetically modified skin-humanized mice. The Journal of investigative dermatology. 123, 1182-1191 (2004).
- Werner, S., Breeden, M., Hubner, G., Greenhalgh, D. G., Longaker, M. T. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. The Journal of investigative dermatology. 103, 469-473 (1994).
- Tarin, D., Croft, C. B. Ultrastructural studies of wound healing in mouse skin. II. Dermo-epidermal interrelationships. Journal of anatomy. 106, 79-91 (1970).
- Croft, C. B., Tarin, D. Ultrastructural studies of wound healing in mouse skin I. Epithelial behaviour. Journal of anatomy. 106, 63-77 (1970).
- Winstanley, E. W. The epithelial reaction in the healing of excised cutaneous wounds in the dog. Journal of comparative pathology. 85, 61-75 (1975).
- Wormstone, I. M., Wride, M. A. The ocular lens: a classic model for development, physiology and disease. Philosophical transactions of the Royal Society of London. Series B, Biological. 366, 1190-1192 (2011).
- Danysh, B. P., Duncan, M. K. The lens capsule. Experimental eye research. 88, 151-164 (2009).
- Awasthi, N., Guo, S., Wagner, B. J. Posterior capsular opacification: a problem reduced but not yet eradicated. Archives of ophthalmology. 127, 555-562 (2009).
- Walker, T. D. Pharmacological attempts to reduce posterior capsule opacification after cataract surgery–a review. Clinical & experimental ophthalmology. 36, 883-890 (2008).
- Schmidbauer, J. M., et al. Posterior capsule opacification. International ophthalmology clinics. 41, 109-131 (2001).
- Menko, A. S., et al. A central role for vimentin in regulating repair function during healing of the lens epithelium. Molecular biology of the cell. 25, 776-790 (2014).
- Chauss, D., et al. Differentiation state-specific mitochondrial dynamic regulatory networks are revealed by global transcriptional analysis of the developing chicken lens. G3 (Bethesda). 4, 1515-1527 (2014).
- Leonard, M., Zhang, L., Bleaken, B. M., Menko, A. S. Distinct roles for N-Cadherin linked c-Src and fyn kinases in lens development. Developmental dynamics : an official publication of the American Association of Anatomists. 242, 469-484 (2013).
- Sieg, D. J., et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nature cell biology. 2, 249-256 (2000).
- Sieg, D. J., Hauck, C. R., Schlaepfer, D. D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. Journal of cell science. 112 (Pt 16), 2677-2691 (1999).
- Hauck, C. R., Hsia, D. A., Schlaepfer, D. D. The focal adhesion kinase–a regulator of cell migration and invasion). IUBMB life. 53, 115-119 (2002).
- Zhao, X., Guan, J. L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Advanced drug delivery reviews. 63, 610-615 (2011).
- Menko, A. S., Bleaken, B. M., Walker, J. L. Regional-specific alterations in cell-cell junctions, cytoskeletal networks and myosin-mediated mechanical cues coordinate collectivity of movement of epithelial cells in response to injury. Experimental cell research. 322, 133-148 (2014).
- Martin, P. Wound healing–aiming for perfect skin regeneration. Science. 276, 75-81 (1997).
- Ferguson, M. W., O’Kane, S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philosophical transactions of the Royal Society of London. Series B, Biological. 359, 839-850 (2004).
- Redd, M. J., Cooper, L., Wood, W., Stramer, B., Martin, P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philosophical transactions of the Royal Society of London. Series B, Biological. 359, 777-784 (2004).
- Nodder, S., Martin, P. Wound healing in embryos: a review. Anatomy and embryology. 195, 215-228 (1997).
- Gurtner, G. C., Werner, S., Barrandon, Y., Longaker, M. T. Wound repair and regeneration. Nature. 453, 314-321 (2008).

